Abstract
Background
We present a case of an inflammatory myofibroblastic tumor cured with a short period of steroid administration, a treatment previously unreported for such cases.
Case presentation
A 49-year-old man had a chief complaint of chest pain for more than 3 days. Computed tomography (CT) revealed a tumoral lesion suspected to have infiltrated into the right first rib and intercostal muscles, with changes in lung parenchymal density around the lesion. The maximal standardized uptake value on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography was high (16.73), consistent with tumor presence. CT-guided biopsy revealed an inflammatory myofibroblastic tumor with no distant metastases. Surgery was indicated based on the disease course. However, he had received an oral steroid before the preoperative contrast-enhanced CT scan due to a history of bronchial asthma, and subsequent CT showed that the tumor shrank in size after administration; he has been recurrence-free for more than a year.
Conclusions
Surgery is still the first choice for inflammatory myofibroblastic tumors, as the disease can metastasize and relapse; however, this condition can also be cured with a short period of steroid therapy.
Similar content being viewed by others
Background
An inflammatory myofibroblastic tumor (IMT) is characterized by the proliferation of myofibroblasts. IMTs are rare, accounting for only approximately 0.04% of all lung tumors and occurring mainly during childhood and adolescence. Further, there is no sex-specific difference in incidence [1]. Hematological findings include increased hemopoiesis, increased platelets, increased polyclonal γ-globulin, and elevated levels of interleukin (IL)-6 and IL-1β; however, no specific markers have been reported [2].
While the exact etiology of this disease is unknown, it is a relatively rare condition defined as a benign-malignant intermediate tumor that exhibits characteristics such as metastasis, recurrence, and marked infiltration of inflammatory cells, mainly lymphocytes and plasma cells [3, 4]. IMTs are found in the abdominopelvic region, lungs, retroperitoneum, and other organs [5]. However, reports of IMT occurring subcutaneously in the chest wall and invading the ribs are rare [6], and the present case occurred as a subcutaneous tissue tumor, not a lung tumor. Furthermore, to the best of our knowledge, there are no reported cases of IMTs that resolved only with short-term steroid treatment rather than surgical treatment. Although surgery is considered the first-line treatment, we present a case of an IMT cured with a short period of steroid administration.
Case presentation
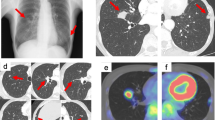
A 49-year-old man was diagnosed with asthma controlled without medication. Two months before presentation, the patient had no abnormal results during a health check. However, he experienced chest pain and visited our hospital’s outpatient department. The patient had no relevant family history, did not drink or smoke, and had no allergies. The laboratory data were unremarkable. Chest radiography revealed a mass at the proximal end of the right clavicle. Computed tomography (CT) showed an approximately 36-mm tumoral lesion suspicious of infiltration into the right first rib and intercostal muscles, with changes in the density of the lung parenchyma around the mass, making it difficult to distinguish whether the mass was intrapulmonary or extrapulmonary (Figs. 1A and 2A). The findings suggested an extension of the lesion along the lymphatic vessels and stroma. The pain consistent with the same area was so severe that a nonsteroidal anti-inflammatory drug failed to control the pain, and opioids were administered. A bronchoscopy performed on the 21st day post-admission failed to reach a diagnosis, and a computed tomography (CT)-guided biopsy was performed on the 25th day post-admission.
(A) Computed tomography on the 12th day post-admission shows a tumoral lesion suspicious of infiltration into the right first rib and intercostal muscles, with changes in the density of the lung parenchyma around the tumor. These findings are suggestive of extension of the lesion along the lymphatic vessels and stroma. (B) 8 F-Fluorodeoxyglucose positron emission tomography-computed tomography on the 17th day post-admission shows a tumoral lesion with a high degree of accumulation with a maximum standardized uptake value = 16.73
Clinical course. Computed tomography on the 12th day post-admission shows a tumoral lesion suspicious of infiltration into the right first rib and intercostal muscles (A). On the 47th day post-admission, computed tomography shows that the mass had shrunk and flattened since the previous 8 F-Fluorodeoxyglucose positron emission tomography-computed tomography (B). On the 176th day post-admission, computed tomography shows that the right lung S1 segment has a scar-like appearance (C)
Hematoxylin and eosin staining revealed a partially hyalinized collagenous fibrous tissue containing abundant inflammatory cells, including neutrophils and lymphocytes. In areas with abundant fibroblast-like spindle-shaped cells, storiform fibrosis, and no cellular atypia were observed (Fig. 3A).
Photomicrographs of a computed tomography-guided percutaneous lung biopsy specimen. (A) Hematoxylin and eosin staining. The main component is collagenous fibrous tissue, which contains abundant inflammatory cells. Inflammatory cells are diverse, including neutrophils and lymphocytes. The fibrous tissue is partially vitiligo-like. In the areas with abundant fibroblast-like spindle-shaped cells, there is a mat-like appearance and no atypia. Immunostaining for vimentin (B) and α-smooth muscle actin (C) were positive, and immunostaining for Anaplastic lymphoma kinase with a D5F3 antibody reveals a faint positive image in half of the spindle-shaped cells (D)
Immunostaining was positive for vimentin (Fig. 3B) and α-smooth muscle actin (Fig. 3C) and negative for anti-cytokeratin (AE)1/AE3, cytokeratin 7, cluster of differentiation (CD) 34, desmin, soluble 100 protein (S-100), and B-cell lymphoma protein 2. CD3 and CD20 showed positive staining in background small lymphocytes, and the number of CD3-positive lymphocytes was higher than that of CD20-positive lymphocytes. CD138-positive plasma cells remained in nonspecific clusters. Ki67 positivity was approximately 5%. 18 F-fluorodeoxyglucose positron emission tomography/CT (18 F-FDG PET/CT) performed 4 days after CT-guided biopsy showed a tumoral lesion with a maximum standardized uptake value of 16.73 in the same area as the CT image, with no abnormal accumulation in other organs (Fig. 1B).
Based on these results, pathological malignant lymphoma was ruled out, and the patient was referred to the Respiratory Surgery Department in another hospital for surgical treatment. Additional immunostaining performed at the hospital showed that the samples were positive for nuclear BRCA1-associated protein-1 and methylthioadenosine phosphorylase; partially positive for D2-40; equivocal for protein HEG homolog 1; and negative for Calretinin, Epstein-Barr virus-encoded RNA (using in situ hybridization), Periodic acid–Schiff, Grocott, and Ziehl-Neelsen staining. No pathogens (Epstein–Barr virus-infected cells, fungi, or mycobacteria) were detected. Histopathological examination revealed that the tumor was predominantly fibroblastic, and there were no obvious malignant findings. Additional immunostaining for anaplastic lymphoma kinase (ALK) with a D5F3 antibody revealed a very faint positive image in half of the spindle-shaped cells (Fig. 3D).
Contrast-enhanced CT was scheduled as a preoperative examination; however, due to the patient’s pre-existing bronchial asthma, the decision was made to perform CT after administering steroids. Oral prednisolone (30 mg) was administered 12 and 2 h before contrast administration. The contrast-enhanced CT showed that the mass-like structure in S1b of the right lung had shrunk and flattened compared to the previous 18 F-FDG PET/CT findings. Further, the lesion appeared continuous from the lung parenchyma to the right first rib costochondral transition area (Fig. 2B), suggesting inflammatory changes and the presence of costochondritis. Thus, the lesion was suspected to be caused by an inflammatory change, such as costochondritis. The adjacent right first rib showed sclerotic changes and reactive changes suggestive of osteomyelitis or inflammation. The parenchymal side of the lung showed a discoid shadow along the bronchovascular side, indicating atelectasis.
Therefore, surgery was avoided, and CT imaging obtained 4 months later showed that the lesion in the right lung S1b subsegment had further shrunk and became scar-like (Fig. 2C).
After oral steroid administration, the tumor, which showed bone invasion, shrank in size. As for the cause of the shrinkage of the lesions, spontaneous remission was considered a possibility; however, it was suggested that the administration of corticosteroids may have been involved as a trigger.
Based on the pathological findings and disease course, the diagnosis of IMT was made. More than 1 year later, the patient has had no symptoms and no recurrence.
Discussion and conclusions
Pathologically, IMTs show dense proliferation of various spindle-shaped cells (myofibroblasts) against a background of myxoedematous collagenous stroma and inflammatory cell infiltration, mainly plasma cells and lymphocytes [2]. Immunostaining has been reported to be positive for mesenchymal markers, such as vimentin (99%), smooth muscle actin (92%), and desmin (69%), but negative for cytokeratin, CD34, S-100, and epithelial membrane antigen [6]. Most reports suggest that surgical resection is the only effective treatment for IMT, while other studies report the efficacy of steroid therapy and nonsteroidal anti-inflammatory drugs. However, the latter has not yet been established as a standard treatment [1]. Carboplatin and Taxol; Bleomycin, Etoposide, and Cisplatin; vincristine/methotrexate; and Ifosfamide, Carboplatin, Etoposide, and Taxol regimens have been used in inoperable cases but have not been established as standard treatments [5, 7]. Reports of improvement with oral steroids alone, as shown in the present case, are rare. Conversely, the possibility that corticosteroids may promote IMT proliferation and relapse after steroid therapy has been reported [8, 9]. Although the response of IMTs to steroid therapy in combination with antibiotics is well known, some conclusions have not yet been reached [9]. The efficacy of combination therapy with radiation and glucocorticoids or methotrexate and glucocorticoids has been documented, but these case reports are rare [10, 11]. However, there are no reports of resolution of IMT with glucocorticoids alone or after a very short period of treatment. Moreover, some groups of patients with IMTs may be more susceptible to steroid therapy, and some may be more likely to experience subsequent relapses. However, since this is a rare disease, research has not been extensive. Several inducers have been hypothesized to increase the response to tissue injury in the formation of IMTs [2]. Previous case reports have demonstrated the involvement of local inflammation in the progression of IMTs, supporting a potential role for anti-inflammatory therapy. Adjuvant therapy with steroids, radiation therapy, chemotherapy, and nonsteroidal anti-inflammatory drugs has rarely been reported. Currently, there is no consensus regarding the most effective nonsurgical course of treatment [12].
Further, crizotinib use in an anaplastic lymphoma kinase-positive patient has been reported (not covered by insurance in Japan but approved by the US Food and Drug Administration in 2021) [13]. Gambacorti-Passerini et al. reported that almost two-thirds of objective responses to crizotinib in patients with IMTs persisted for more than 2 years, with a 67% progression-free survival at this time point. Despite the small number of patients enrolled, the findings support the National Comprehensive Cancer Network’s recommendation for crizotinib as a treatment for IMTs with ALK rearrangements and suggest that ALK testing should be performed routinely in all patients with IMTs [14]. In these patients, ALK expression detected by immunohistological staining can reach approximately 50% and correlates with gene rearrangement [2], which may be a reason for choosing crizotinib as the treatment option. Moreover, if the patient shows recurrence, additional immunostaining may expand treatment options.
Some cases of IMTs may metastasize or recur, and patients should be regularly monitored in anticipation of this possibility. If recurrence is observed after follow-up with glucocorticoid monotherapy, re-administration of glucocorticoids might be considered one of the effective treatments. However, since this disease has no established treatment, surgery should be considered if the lesions are resectable; if they are extensive, crizotinib or chemotherapy should be considered.
Data availability
All data and materials are available for sharing if needed.
Abbreviations
- IMT:
-
Inflammatory myofibroblastic tumor
- IL:
-
Interleukin
- CT:
-
Computed tomography
- AE:
-
Anti-cytokeratin
- CD:
-
Cluster of differentiationS-100:Soluble 100 protein
- 18F-FDG PET/CT:
-
18 F-Fluorodeoxyglucose positron emission tomography-computed tomography
- ALK:
-
Anaplastic lymphoma kinase
References
Khatri A, Agrawal A, Sikachi RR, Mehta D, Sahni S, Meena N. Inflammatory myofibroblastic tumor of the lung. Eur J Cardiothorac Surg. 2004;25:155–9.
Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–37.
Cantera JE, Alfaro MP, Rafart DC, Zalazar R, Muruzabal MM, Barquín PG, et al. Inflammatory myofibroblastic tumours: a pictorial review. Insights Imaging. 2015;6:85–96.
Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour. Ed. by Fletcher CDM, Unni KK, Mertens F, World Health Organization Classification of tumours of Soft Tissue and Bone. IARC Press. Lyon. 2002;91–93.
Gros L, Dei Tos AP, Jones RL, Digklia A. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385–91.
Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–72.
Suetsugu S, Yamamoto H, Izumi M, Takayama K, Inoue H, Nakanishi Y. [A case of rapidly growing inflammatory myofibroblastic tumor in the lung]. Nihon Kokyuki Gakkai Zasshi. 2009;47:1156–60.
Panigada S, Sacco O, Girosi D, Magnano GM, Tuo P, Tarantino V, et al. Corticosteroids may favor proliferation of thoracic inflammatory myofibroblastic tumors. Pediatr Pulmonol. 2014;49:E109–11.
Jiang JY, Comsa M, Wong VCK, Mansberg R. Steroid responsive inflammatory myofibroblastic tumor of the lung evaluated by FDG PET/CT imaging. Radiol Case Rep. 2022;17:907–10.
Biswas R, Halder A, Gangopadhyay M, Biswas D. Inflammatory myofibroblastic tumor of maxillary sinus successfully treated with radiotherapy and corticosteroid: report of a rare case. J Egypt Natl Canc Inst. 2020;32:26.
Navinan MR, Liyanage I, Herath S, Yudhishdran J, Shivanthan C, Beneragama D, et al. Inoperable inflammatory myofibroblastic tumour of the para-nasal sinuses and orbit with recurrence responding to methotrexate and prednisolone: a case report. BMC Res Notes. 2015;8:27.
Shatzel J, Wooten K, Ankola A, Cheney RT, Morrison CD, Skitzki JJ. Inflammatory myofibroblastic tumor of the mesentery: a clinical dilemma. Int J Clin Oncol. 2012;17:380–4.
Pearson ADJ, Barry E, Mossé YP, Ligas F, Bird N, de Rojas T, et al. Second paediatric Strategy Forum for anaplastic lymphoma kinase (ALK) inhibition in paediatric malignancies: ACCELERATE in collaboration with the European Medicines Agency with the participation of the Food and Drug Administration. Eur J Cancer. 2021;157:198–213.
Gambacorti-Passerini C, Orlov S, Zhang L, Braiteh F, Huang H, Esaki T, et al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): a phase 1b open-label study. Am J Hematol. 2018;93:607–14.
Acknowledgements
We would like to thank Editage (https://www.editage.com) for English language editing.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Author information
Authors and Affiliations
Contributions
RW, SA, MS, and RS collected data. RW and SA analyzed the data. RW and SA prepared the manuscript. YK made the diagnosis and prepared the pathological images. NK and NH supervised and validated the study. All authors have approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study conformed to the Ethical Guidelines for Clinical Studies issued by the Ministry of Health, Labour and Welfare of Japan.
Consent for publication
Informed consent for the publication of this report was obtained from the patient.
Competing interests
The authors declare that they have no conflicts of interest concerning the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Watanabe, R., Ano, S., Kikuchi, N. et al. Inflammatory myofibroblastic tumor directly invading the right first rib treated with oral steroids: a case report. BMC Pulm Med 24, 67 (2024). https://doi.org/10.1186/s12890-024-02873-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-02873-6