Abstract
Background
In tobacco-exposed persons with preserved spirometry (active smoking or secondhand smoke [SHS] exposure), air trapping can identify a subset with worse symptoms and exercise capacity. The physiologic nature of air trapping in the absence of spirometric airflow obstruction remains unclear. The aim of this study was to examine the underlying pathophysiology of air trapping in the context of preserved spirometry and to determine the utility of bronchodilators in SHS tobacco-exposed persons with preserved spirometry and air trapping.
Methods
We performed a double-blinded placebo-controlled crossover randomized clinical trial in nonsmoking individuals at risk for COPD due to exposure to occupational SHS who had preserved spirometry and air trapping defined as either a residual volume-to-total lung capacity ratio (RV/TLC) > 0.35 or presence of expiratory flow limitation (EFL, overlap of tidal breathing on maximum expiratory flow-volume loop) on spirometry at rest or during cardiopulmonary exercise testing (CPET). Those with asthma or obesity were excluded. Participants underwent CPET at baseline and after 4-week trials of twice daily inhalation of 180 mcg of albuterol or placebo separated by a 2-week washout period. The primary outcome was peak oxygen consumption (VO2) on CPET. Data was analyzed by both intention-to-treat and per-protocol based on adherence to treatment prescribed.
Results
Overall, 42 participants completed the entire study (66 ± 8 years old, 91% female; forced expiratory volume in 1 s [FEV1] = 103 ± 16% predicted; FEV1 to forced vital capacity [FVC] ratio = 0.75 ± 0.05; RV/TLC = 0.39 ± 0.07; 85.7% with EFL). Adherence was high with 87% and 93% of prescribed doses taken in the treatment and placebo arms of the study, respectively (P = 0.349 for comparison between the two arms). There was no significant improvement in the primary or secondary outcomes by intention-to-treat or per-protocol analysis. In per-protocol subgroup analysis of those with RV/TLC > 0.35 and ≥ 90% adherence (n = 27), albuterol caused an improvement in peak VO2 (parameter estimate [95% confidence interval] = 0.108 [0.014, 0.202]; P = 0.037), tidal volume, minute ventilation, dynamic hyperinflation, and oxygen-pulse (all P < 0.05), but no change in symptoms or physical activity.
Conclusions
Albuterol may improve exercise capacity in the subgroup of SHS tobacco-exposed persons with preserved spirometry and substantial air trapping. These findings suggest that air trapping in pre-COPD may be related to small airway disease that is not considered significant by spirometric indices of airflow obstruction.
Key messages
What is already known on this topic?
Many people who have been exposed to tobacco smoke (direct or indirect) but have preserved spirometry show abnormal lung volumes suggestive of presence of air trapping. The physiologic nature of air trapping in the absence of spirometric airflow obstruction remains unclear.
What this study adds?
Albuterol may improve ventilation and exercise capacity in secondhand tobacco-exposed persons with preserved spirometry and lung volumes suggestive of air trapping.
How this study might affect research, practice, or policy?
Air trapping in pre-COPD may be related to small airway disease that is not considered significant by spirometric indices of airflow obstruction. Stratification of the tobacco-exposed persons with preserved spirometry by lung volumes and air trapping may help in identifying a subset who do benefit from the use of bronchodilators.
Similar content being viewed by others
Background
Chronic exposure to secondhand tobacco smoke (SHS) is a risk factor for development of chronic obstructive pulmonary disease (COPD) [1,2,3]. Lung function studies in people with history of long-term occupational exposure to SHS have shown the majority of those exposed to have preserved spirometry but also to have a remarkably wide distribution of lung volume indices that represent air trapping, specifically the ratio of residual volume or functional residual capacity to total lung capacity (RV/TLC or FRC/TLC) [4, 5]. Wide ranges of RV/TLC and FRC/TLC distributions have also been reported among people with history of direct smoking and preserved spirometry [6,7,8]. The significance of these observations continues to be a topic of investigation, though several recent studies have shown that in these unobstructed tobacco smoke-exposed people, lung volumes that represent air trapping (elevated RV/TLC and FRC/TLC) can identify a subset with worse symptoms and lower exercise capacity who, at least in the case of those with history of active smoking, will progress to develop overt airflow obstruction and spirometric COPD [5,6,7].
The physiologic nature of air trapping in the absence of spirometric airflow obstruction remains unclear, and a question remains on whether elevated RV/TLC or FRC/TLC is a consequence of an airway obstructive process that has not yet resulted in spirometric airflow obstruction by formal criteria, or the result of other yet unexplained lung parenchymal changes that cause an elevated RV/TLC and FRC/TLC without airway obstructive disease. A possible approach to investigate this question is to determine whether air trapping and its associated diminished exercise capacity and worse respiratory symptoms improve with the use of bronchodilators in this tobacco-exposed population with air trapping but preserved spirometry, based on the presumption that the main effect of bronchodilators is through its relief of airflow obstruction at the level of airways.
The main goal of this study was to understand the nature of air trapping in the setting of no spirometric obstruction in people at risk for COPD (pre-COPD) by investigating whether administration of bronchodilators in that setting could improve the adverse outcomes associated with air trapping including exercise tolerance. To investigate this, a clinical trial approach was taken, not to necessarily prove a recommendation for routine use of bronchodilators in pre-COPD, but rather to determine whether bronchodilation improves air trapping and its outcomes in that setting. We hypothesized that the exercise capacity and respiratory symptoms associated with air trapping in the absence of spirometric airflow obstruction (preserved spirometry) improve with administration of bronchodilators. To examine this hypothesis, we performed a clinical trial investigating the effectiveness of albuterol, a selective β2-adrenergic receptor agonist that relaxes airway smooth muscle and causes bronchodilation, in nonsmoking individuals at risk for COPD due to occupational exposure to SHS with preserved spirometry and air trapping, and assessed whether administration of albuterol improves their peak oxygen consumption (VO2) and other performance indices on cardiopulmonary exercise testing (CPET) as well as their level of symptoms, quality of life, and daily physical activity.
Methods
Study design
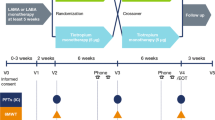
This was a single-center, double-blinded, randomized, crossover, placebo-controlled trial investigating the effectiveness of albuterol in improving exercise capacity and respiratory symptoms in nonsmoking SHS tobacco-exposed individuals at risk for COPD due to occupational exposure to SHS with preserved spirometry and physiologic evidence of air trapping. Air trapping was defined as either (1) an absolute RV/TLC value > 0.35 on plethysmography or (2) presence of expiratory flow limitation (EFL) as defined by the presence of graphic overlap of tidal breathing on maximum expiratory flow-volume loop on spirometry at rest or during maximum effort exercise testing, regardless of their RV/TLC value. Potential participants underwent baseline characterization with questionnaire administration, pulmonary function testing (PFT), and CPET to determine eligibility. Eligible participants were then randomized to take twice daily inhalation of either albuterol (2 inhalations; 180 mcg) or placebo for 4 weeks before coming back in for a repeat evaluation. The assigned inhaler was taken up to and on the morning of evaluation. The dose on the morning of evaluation was a supervised administration about 30 to 60 min before the exercise testing. The participants subsequently underwent a washout period of at least 2 weeks duration, after which they were crossover assigned to take the alternate treatment (albuterol or placebo) for 4 weeks followed by repeat evaluation. Participants also wore an activity monitor during the last week of each treatment period (Fig. 1).
The primary outcome of the study was peak VO2 on CPET. The secondary outcomes included improvement in other CPET indices including EFL and dynamic hyperinflation as well as symptoms, quality of life, and daily level of physical activity. Data was analyzed by both intention-to-treat and per-protocol approaches. The per-protocol approach was based on adherence defined as the number of albuterol or placebo inhalations that the participants actually took as a fraction of the total number of inhalations that they were instructed to take.
Trial regulatory matters
The University of California San Francisco (UCSF) Institutional Review Board (IRB) and the San Francisco Veterans Affairs Health Care System (SFVAHCS) Committee on Research and Development approved the study protocols. Written IRB-approved informed consent and Health Insurance Portability and Accountability Act (HIPAA) were obtained from all study participants. All participants received monetary compensation for their participation in the study. The study was registered with the United States (U.S.) National Library of Medicine (Secondhand Smoke Respiratory Health Study; ClinicalTrials.gov identifier NCT02797275; date first posted in the trial registration 13/06/2016 [June 13, 2016]).
Study population
The study population consisted of past or current U.S. commercial airline flight crewmembers who had previously participated in an observational study of the potential adverse health effects of the cabin environment including health effects of exposure to occupational SHS in the aircraft cabin [5, 9, 10]. Crewmembers were eligible to participate in the study if they were between 40 and 80 years of age, nonsmokers, had worked onboard of planes for at least one year before enactment of tobacco smoking ban in aircraft, and had preserved spirometry (defined as a ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC) ≥ 0.70 and greater than lower limit of normal value (LLN). The participants also had to have evidence of air trapping as determined by either (1) an absolute RV/TLC ratio of > 0.35 by plethysmography (this threshold was used based on previous work by our group showing this RV/TLC level to be a statistically optimal cutoff point for RV/TLC association with presence of exercise limitation and symptoms) [5]; or (2) EFL defined as the presence of overlap of tidal breathing on maximum expiratory flow-volume loop graph on spirometry at rest or during their baseline maximum effort exercise testing, regardless of their RV/TLC value (EFL with exercise is suggestive of progressive air trapping and dynamic hyperinflation, which correlate better with symptoms and functional capacity than spirometric indices) [11, 12].
Those with history of asthma, COPD, interstitial lung diseases, active cardiovascular disease, uncontrolled hypertension, auto-immune diseases, or other known conditions affecting lung function (e.g., history of radiation therapy to chest in the setting of history of breast cancer), obesity (defined as body mass index or BMI > 30 kg/m2), or history of cannabis or other recreational drug use were excluded.
Randomization
Randomization of the participants occurred between June 6, 2016 and February 27, 2020. Randomization for each individual participant to receive albuterol or placebo as their first inhaler was done using a random number generator. A random number between 0 and 1 was generated for each eligible participant. Participants with a random number ≥ 0.5 received albuterol as their first inhaler while participants with a random number < 0.5 received placebo as their first inhaler. The randomization was blinded from the participants and from the study team. A staff member, who was otherwise not involved in the study, performed the randomization and then delivered the assigned inhalers to the participants.
Study medication
Albuterol is a selective β2-adrenergic receptor agonist, inhalation of which causes relaxation of airway smooth muscle and bronchodilation [13, 14]. Albuterol has a terminal half-life of 3 to 8 h and was considered to be a “long-acting” agent in 1980s [14], but with the advent of other agents with much longer duration of action (long-acting and ultra-long-acting agents) [15,16,17], albuterol is now considered to be a “short-acting” β2-agonist. Both short-, long-, and ultra-long-acting β2-agonists can interact with adrenergic receptors on other cells and organ systems and cause potentially unwanted effects including plasma electrolytes derangement, cardiovascular effects such as tachycardia, and skeletal muscle tremor [17, 18], although these effects are most common with systemic (oral or parenteral) rather than inhalational routes of administration. In lungs, inhalation of albuterol and other β2-agonists are thought to mainly affect airway smooth muscle cells and have a bronchodilatory effect, although effects on other lung cells (such as airway and alveolar epithelial cells as well as airway immune cells) have also been documented [19,20,21,22,23,24].
Administration of albuterol for the study was performed using ProAir RespiClick metered inhalation powder (albuterol sulfate 90 mcg per inhalation) (Teva Respiratory, LLC., Parsippany, NJ, USA), as the intervention drug and the Demo version of RespiClick (obtained from ProAir manufacturer) that contained no active medication, as the placebo. The participants were instructed to take two inhalations of either albuterol (2 inhalations; 180 mcg) or placebo twice daily for 4 weeks before coming back in for a repeat evaluation. The assigned inhaler was taken up to and on the morning of evaluation. The dose on the morning of evaluation was a supervised administration about 30 to 60 min before the exercise testing. The 4-week duration of albuterol administration was chosen based on the previous literature showing that despite normalization of any spirometric airflow obstruction, reversal of air trapping and hyperinflation may require weeks of therapy to resolve [25].
The inhalers were provided to participants after randomization in their first visit by a staff member who was otherwise not involved with the study. The same staff member reviewed the correct use of the inhalers with the participants. To avoid any possible adrenergic adverse effects at night, the participants were instructed to use two inhalations of the ProAir RespiClick in the morning upon awakening and two more inhalations about six hours later in the early afternoon, with no further usage after that throughout the evening and night. The participants were contacted by the study staff at one week and three weeks after the initiation of medication to assess their usage and any potential adverse effects. The participants were also asked to use the study medication on the day they returned to the laboratory for their follow-up visits (V2 and V3).
During the participants’ follow-up visits (V2 and V3), the same staff member who dispensed the inhaler to the participants collected the inhalers from them, and noted and recorded the number of inhalations that was used from the built-in use counter for later calculation of the rate of adherence to the protocol. The data on adherence to inhaler use was unblinded and calculated after the end of the study on September 9, 2022.
SHS exposure characterization
Details of SHS exposure characterization and quantification are available in the Supplemental Appendix and have been described previously [4].
Pulmonary function and cardiopulmonary exercise testing
Participants performed physician-supervised, symptom-limited, progressively increasing stepwise maximal exercise tests in the seated position on an electromagnetically braked, upright cycle ergometer with continuous monitoring of their heart rate (HR), blood pressure (BP), electrocardiogram (ECG), and breath-by-breath gas exchange. Approximately 45 min after completing their maximum effort exercise testing, the participants underwent a dynamic hyperinflation exercise testing protocol. This protocol included tidal volume (VT), inspiratory capacity (IC), and maximal expiratory flow (MEF) measurements in seated position at increasing work rates corresponding to 20%, 40%, 60%, and 80% of the peak VO2 they attained during the maximum effort exercise testing, as described by O’Donnell et al [26, 27]. Details of pulmonary function and maximum effort cardiopulmonary function testing are available in the Supplemental Appendix and have been described previously [4, 5].
Physical activity monitoring using actigraphy
Physical activity was monitored using a triaxial accelerometer-based activity monitor (ActiGraph GT3X; Actigraph Corporations, Pensacola, FL). Technical details of the device, data processing, and data analysis are available in the Supplemental Appendix and have been described previously [28].
Respiratory symptom assessment
Patient-reported respiratory symptoms, physical activity, and quality of life assessments were conducted using the COPD Assessment Test (CAT) [29], modified Medical Research Council (mMRC) Dyspnea Scale [30], the Short Form 12-Item Health Survey (SF12) [31], International Physical Activity Questionnaire (IPAQ) [32], Airway Questionnaire 20 (AQ20) [33], and a self-reported questionnaire (UCSF Flight Attendant Medical Research Institute (FAMRI) SHS Questionnaire) that elicited symptoms of dyspnea, cough, and participants’ perception of a decreased level of exertion compared to peers over the year preceding enrollment [34], further details of which are available in the Supplemental Appendix.
Power and sample size calculations
The a priori power and sample size calculations were performed as described below. Previous studies have reported high level of correlation (coefficient of correlation ≥ 0.90 and coefficient of variation < 7%) between repeated measurements of peak VO2 in maximum effort exercise testing among healthy as well as diseased populations [35,36,37,38]. For the purpose of this study with a repeated measure design, we used a presumed correlation coefficient of 0.90. Moreover, previous CPET data from a relatively large cohort of the SHS-exposed flight attendants with preserved spirometry and air trapping have documented peak VO2 measures (mean ± standard deviation) of 1.496 ± 0.429 L/min [5]. Given the above, and assuming that some 80% of the participants would have a small airway disease process that is responsive to administration of bronchodilator, a sample size of 103 was determined to provide a power of 80% to detect 10% change in peak VO2 (or 0.15 L/min). In the end, we planned to recruit a total of 100 participants, although because of various impediments including research holds related to COVID-19 pandemic, the targeted sample size was not achieved.
Data analysis
Distributions of participants’ characteristics, pulmonary function, cardiopulmonary exercise, and SHS exposure quantification variables were examined. Rate of change in cardiopulmonary exercise measures during the exercise with respect to the workload were approximated by estimating the slopes from linear regression modeling of those measures over workload at each stage. Peak cardiopulmonary exercise variables were estimated using the last 30-s average values obtained during the highest stage of the exercise test as described in Supplemental Appendix. Cumulative work achieved throughout the exercise (WorkTotal), or the area under the curve of workload in Watts vs. time in minutes, was computed as the sum of the product of watts completed and time spent at each stage in the unit of Watts-Minute. Changes in the outcomes from baseline were calculated by subtracting the subsequent visits (V2 or V3) outcome values from those of baseline visit (V1).
Intention-to-treat analysis
The primary intention-to-treat analysis was performed as described below. The effect of albuterol on the outcome variables was examined with a repeated measure design using mixed-effect linear regression modeling with the random subject effect and fixed effect variables including age, sex, height, weight, and the corresponding baseline measure of the outcome variable. Participants who completed only one arm of the study were included in this analysis (intention-to-treat). The resulted coefficients representing the adjusted difference between the outcome variables measured in the albuterol visit and the placebo visit were reported.
The changes after administration of albuterol were also compared with those after administration of placebo using paired t-test. Changes that were significantly different between taking albuterol and taking placebo were reported.
Per-protocol and subgroup analyses
The a priori per-protocol analysis was performed as described below. To assess the effect of albuterol under different levels of adherence, per-protocol analyses using the same methodology as described above for intention-to-treat analysis were performed based on thresholds of adherence. Thresholds of adherence of 70%, 80%, and 90% were applied to each arm of the study. We then analyzed the subgroups of participants whose adherence data was equal or above the thresholds.
To assess whether the treatment was effective in the subgroup who had definitive air trapping (excluding those with EFL who did not have an RV/TLC > 0.35), post-hoc subgroup analyses were also performed based on the thresholds of adherence and RV/TLC. To evaluate any potential interaction between other lung function indices that represent small airway disease or air trapping and the effect of albuterol treatment on the study outcomes, we performed additional regression analyses with inclusion of these variables (forced expiratory flow [FEF] at 25% to 75% of FVC [FEF25-75], FEF at 75% of FVC [FEF75], ratio of alveolar volume [VA] to TLC) as an independent variable as well as an interaction term within the models.
For each analysis, the total number of participants included for that analysis were reported along with the results from the regression modeling and paired t-test. R software (version 4.2.2; R Foundation for Statistical Computing) was used for data management and statistical analysis. Figures were generated by GraphPad (Prism version 9.0).
Results
Participant characteristics
Between June 6, 2016 and February 27, 2020, 378 potential participants were identified and contacted. From those who responded and were interested, 141 met the initial eligibility criteria, and 82 ended up being enrolled in the study (Fig. 2). From the 61 participants randomized, six withdrew, two lost to follow-up, and eleven were not able to complete the study due to institutional holds on clinical research during the COVD-19 pandemic. Overall, 42 completed the entire study. In addition, seven participants who completed the second study visit were also included in the analysis. The characteristics of the 49 participants with available data for analysis are shown in Table 1. From the 49 participants, 27 had air trapping by RV/TLC > 0.35 at baseline and ≥ 90% adherence to inhaler use and were examined in a subgroup analysis. The characteristics of the 42 participants who completed the entire study and the 27 participants in the subgroup analysis are shown in Supplemental Table S1. The participants were predominantly women (43 out of 49 or 88%), 66 ± 8 years of age, and all nonsmokers with history of past exposure to SHS.
Adherence and safety
Adherence to treatment protocol for ≥ 70%, ≥ 80%, and ≥ 90% of doses taken was 95 ± 7%, 96 ± 5%, and 98 ± 3%, respectively. Adherence in the treatment (albuterol) and control (placebo) arms were 87% and 93% of prescribed doses, respectively (P = 0.349 for comparison between the two arms). No serious adverse events were reported by the participants. Two participants stop their participation, one while in the treatment arm and another while in the placebo arm, due to non-serious adverse events, which were deemed unlikely to be related to treatment or placebo (knee pain and high blood pressure for the participant in the treatment arm and diarrhea for the participant in the placebo arm).
Baseline lung function and exercise data
All participants had preserved spirometry as defined by FEV1 to FVC ratio ≥ 0.7 and greater than lower limit of predicted normal values (LLN) (Table 1). While the average diffusing capacity was within the normal limits, 12 of the 49 participants had diffusing capacities below LLN. Similarly, while the average FRC, RV, and TLC values were within the normal limits, two, one, and three of the 49 participants had FRC, RV, and TLC above the upper limit of normal values (ULN), respectively. Of note, 4 of the 12 participants with diffusing capacity < LLN had RV/TLC ≤ 0.35 but had EFL both at rest and during exercise.
The volume of oxygen consumption at peak exercise (VO2) was 1,335 ± 414 mL/min (99 ± 21% predicted). Thirteen participants (27%) had a VO2Peak < 84% predicted, a presumed threshold for abnormal results. The peak VO2 was achieved at a peak workload of 113 ± 36 watts (115 ± 28% predicted) (Table 1) and over a period of 9.6 ± 1.8 min of loaded exercise.
None of the participants reported adverse effects during the CPET, including no chest pain, chest tightness, lightheadedness, or dizziness. Furthermore, no participants had any clinically significant ECG changes or arrhythmias, with the exception of occasional premature ventricular contractions that were not exercise dependent. The nadir of the ratio of oxy-hemoglobin to total hemoglobin (oxygen saturation or SpO2) at peak exercise was 97 ± 2% with all participants having an SpO2 of ≥ 95%. Thirty-four participants (72%) showed a hypertensive response to exercise. At peak exercise, the heart rate and oxygen-pulse (O2-Pulse) were 141 ± 22 beat/min (92 ± 14% predicted) and 9.7 ± 3.8 mL/beat (106 ± 30% predicted), respectively. Eight (16.3%) and six (12.2%) participants did not achieve their 80% (a presumed threshold for abnormal results) predicted normal values of their heart rate and O2-Pulse, respectively.
The pulmonary response to exercise was remarkable for a minute ventilation (VE) of 53.0 ± 16.5 L/min (56 ± 12% predicted) at peak exercise; only seven participants (14.3%) exceeded the 70% threshold for inappropriate ventilatory response to maximum effort exercise. The respiratory rate (RR) at peak exercise was below the 60 breaths/minute threshold (for possible concern for interstitial lung disease) in all participants. All participants achieved their anaerobic threshold (AT) as determined by V slope method on VO2. Additionally, ventilatory efficiency (ratio of VE to carbon dioxide production [VCO2]) at peak exercise was 34.4 ± 9.8 (86 ± 20% predicted) with lowest VE/VCO2 at 31.5 ± 10.0 (79 ± 25% predicted). More comprehensive CPET data is available in Supplemental Table S2.
Thirty-five participants (71%) had RV/TLC > 0.35 and 43 participants (88%) had EFL at rest or during exercise (Table 2). Twenty-nine of the 35 participants with RV/TLC > 0.35 (83%) had EFL at rest or during exercise. Twenty-three of the 43 participants with EFL (53%) had EFL at rest and the remaining 20 (47%) developed EFL during exercise.
Efficacy of albuterol on exercise capacity, symptoms, quality of life, and physical activity
In the primary analysis with inclusion of data from all 49 participants, there was no significant change in primary or any of the secondary outcomes by intention-to-treat or per-protocol analysis (Fig. 3). Although VE/VCO2 and diastolic blood pressure (DBP) at peak exercise showed a significant improvement with albuterol, these variables were not a priori included as a secondary outcome. Analysis including only those participants who completed the entire study (n = 42) showed similar result with no significant changes in primary or a priori secondary outcomes (Supplemental Fig. S1).
Associations of Albuterol and outcomes in all participants. The effect of Albuterol on the outcome variables was examined with a repeated measure design using mixed-effect linear regression modeling with the random subject effect and fixed effect variables including age, sex, height, weight, and the corresponding baseline measure of the outcome variable. The number of participants (N), the summary statistics (mean ± standard deviation) for each outcome variables measured in the placebo visit and the albuterol visit as well as the resulted parameter estimate (PE) representing the adjusted difference and the corresponding 95% confidence interval (CI) are shown. In this intention-to-treat analysis, N represents the number of participants who completed each (albuterol or placebo) arm of the study. The dot-and-whisker plots represent the PE and 95% CI with colors black (scaled on the top) and red (scaled on the bottom) to distinguish outcomes in which higher versus lower values are preferable. The PE and 95% CI for the statistically significant associations were shown in bold. Abbreviations: VO2: oxygen uptake; Watts: work stage completed in watts; VE: minute ventilation value; RR: respiratory rate; VT: tidal volume; VCO2: carbon dioxide production; SpO2: oxygen saturation; O2-Pulse: oxygen uptake per heartbeat; EELV: end-expiratory lung volume; VFL: volume of the tidal breath that is flow limited on expiration; EFL: expiratory flow limitation; SF12: Short Form 12-Item Health Survey; IPAQ: International Physical Activity Questionnaire; MET: metabolic equivalent: MVPA: moderate to vigorous physical activities; PE: parameter estimate; CI: confidence interval
Subgroup analysis based on presence of RV/TLC > 0.35 and/or level of adherence to treatment protocol showed a gradually larger improvement in primary outcome (peak VO2 response) that became statistically significant with increasing adherence to assigned treatment (Fig. 4). Similar gradual improvement in some of the secondary outcomes was also observed (Fig. 4).
Associations of Albuterol and outcomes in subgroup analysis. The effect of Albuterol on the outcome variables was examined with a repeated measure design using mixed-effect linear regression modeling with the random subject effect and fixed effect variables including age, sex, height, weight, and the corresponding baseline measure of the outcome variable. The analysis was separately done within the whole group of participants and the subgroups based on RV/TLC and/or adherence. The number of participants (N), the summary statistics (mean ± standard deviation) for each outcome variables measured in the placebo visit and the albuterol visit as well as the resulted parameter estimate (PE) representing the adjusted difference and the corresponding 95% confidence interval (CI) are shown. In these subgroup analyses, N represents the number of participants in both arms of the study who met the air trapping and/or adherence criteria. The dot-and-whisker plots represent the PE and 95% CI with colors black (scaled on the top) and red (scaled on the bottom) to distinguish outcomes in which higher versus lower values are preferable. Abbreviations: VO2Peak: oxygen uptake in peak exercise; VTPeak: tidal volume in peak exercise; VFL: volume of the tidal breath that is flow limited on expiration; PE: parameter estimate; CI: confidence interval
In per-protocol subgroup analysis of participants with RV/TLC > 0.35 and ≥ 90% adherence (n = 27), albuterol inhalation caused a significant improvement in primary outcome (peak VO2) (parameter estimate [95% confidence interval] = 0.108 [0.014, 0.202]) as well as some of the secondary outcomes: tidal volume (VT) (0.084 [0.028, 0.140]), minute ventilation (VE) (4.242 [0.983, 7.500]), oxygen-pulse (O2-Pulse) (0.688 [0.337, 1.039]), and some of the dynamic hyperinflation indices (Fig. 5). However, no significant changes in physical activity, symptoms, or quality of life scores were observed (Fig. 5).
Associations of Albuterol and outcomes in the subgroup with RV/TLC > 0.35 and ≥ 90% adherence. The effect of Albuterol on the outcome variables was examined with a repeated measure design using mixed-effect linear regression modeling with the random subject effect and fixed effect variables including age, sex, height, weight, and the corresponding baseline measure of the outcome variable. The number of participants (N), the summary statistics (mean ± standard deviation) for each outcome variables measured in the placebo visit and the albuterol visit as well as the resulted parameter estimate (PE) representing the adjusted difference and the corresponding 95% confidence interval (CI) are shown. In this subgroup analysis, N represents the number of participants in each arm of the study who met the adherence criteria of ≥ 90%. The dot-and-whisker plots represent the PE and 95% CI with colors black (scaled on the top) and red (scaled on the bottom) to distinguish outcomes in which higher versus lower values are preferable. The PE and 95% CI for the statistically significant associations were shown in bold. Abbreviations: VO2: oxygen uptake; Watts: work stage completed in watts; VE: minute ventilation value; RR: respiratory rate; VT: tidal volume; VCO2: carbon dioxide production; SpO2: oxygen saturation; O2-Pulse: oxygen uptake per heartbeat; EELV: end-expiratory lung volume; VFL: volume of the tidal breath that is flow limited on expiration; EFL: expiratory flow limitation; SF12: Short Form 12-Item Health Survey; IPAQ: International Physical Activity Questionnaire; MET: metabolic equivalent: MVPA: moderate to vigorous physical activities; PE: parameter estimate; CI: confidence interval
Similar findings were observed for primary and subgroup analysis when paired t-test approach was used to examine the response among those participants who completed both arms of the study (Supplemental Table S3). Inclusion of other lung function indices representing small airway disease (FEF25-75 or FEF75) or air trapping did not show any significant interaction with the effect of albuterol on the study outcomes.
Discussion
In this clinical trial, we found that inhaled bronchodilator treatment with albuterol did not improve exercise capacity (VO2, primary outcome) or other exercise performance indices, respiratory symptoms, quality of life, or daily physical activity (secondary outcomes) in nonsmoking SHS tobacco-exposed individuals with preserved spirometry but with air trapping (defined by RV/TLC > 0.35 or presence of EFL). The lack of efficacy was present regardless of how well the participants adhered to the inhalational treatment protocol (per-protocol analysis by ≥ 70%, ≥ 80%, or ≥ 90% adherence). However, in subgroup analysis of those with air trapping defined by an RV/TLC > 0.35 who had ≥ 90% adherence to treatment, inhaled albuterol did cause an improvement in exercise capacity (VO2, primary outcome), some measures of dynamic hyperinflation, ventilation, and oxygen-pulse (a proxy of cardiac output); however, symptoms, quality of life, or daily physical activity level were not improved. Of note, inhaled albuterol also improved the diastolic blood pressure at peak exercise, an outcome that was not specified a priori in our study, but retrospectively was of interest given the consideration that air trapping may adversely contribute to cardiovascular diastolic dysfunction [9, 43, 44].
Occupational exposure to SHS, a risk factor for development of COPD [1,2,3], is associated with both reduced diffusing capacity [4, 45] and wide distributions of lung volume indices, such as RV/TLC or FRC/TLC, that represent air trapping despite preserved spirmtery [4, 5]. Studies have shown that even in the absence of spirometric airflow obstruction, elevated RV/TLC or FRC/TLC can identify a subset of individuals with worse exercise capacity and symptoms who may progress to develop overt airflow obstruction and spirometric COPD [5,6,7,8]. Thus, air trapping in the setting of preserved spirometry may represent an early small airway disease, which contributes to exercise limitation and symptoms, probably through dynamic hyperinflation, even in the absence of spirometric airflow obstruction [5, 45]. In such a case, bronchodilator treatment may be useful in relieving air trapping and dynamic hyperinflation and thus improving exercise capacity.
Although our findings in this study are not supportive of such hypothesis, in the subgroup with RV/TLC > 0.35 and high adherence to treatment, bronchodilator use with albuterol did improve exercise capacity and dynamic hyperinflation, suggesting that at least in those with significant air trapping, as measured by elevated RV/TLC, bronchodilator could be advantageous. However, further research, perhaps in people with preserved spirometry but more significant air trapping, is needed to confirm this possibility. Compared to people with history of occupational SHS exposure and no direct smoking, people with history of direct smoking have a much wider distribution of lung volumes representing air trapping with approximately a third showing abnormal RV/TLC greater than the upper limit of predicted normal values [5,6,7]. Thus, it is plausible that inhaled bronchodilator treatment could have a larger effect in those with history of direct smoking and preserved spirometry who have significant air trapping. A recent a randomized controlled trial on the utility of bronchodilators in direct tobacco-exposed persons with preserved spirometry who had respiratory symptoms found that bronchodilator treatment did not alleviate respiratory symptoms [46]. However, lung volumes were not assessed in that study, and thus stratification of the participants by their lung volumes or air trapping could not be done, making it impossible to distinguish smokers with preserved spirometry with abnormal/high RV/TLC or FRC/TLC who may have been more likely to benefit from bronchodilators.
During the course of this study, we recruited and enrolled nonsmoking participants with preserved spirometry and air trapping. To improve recruitment, we enrolled participants with air trapping defined as (1) those with RV/TLC > 0.35, a limit that we had previously determined as a reasonable cutoff for presence of exercise limitation and symptoms [5], but also (2) those with EFL as determined by presence of graphic overlap of tidal breathing on maximum expiratory flow-volume loop at rest or during exercise. EFL has been shown to be associated with air trapping and dynamic hyperinflation, both of which contribute to exercise limitation and worse respiratory symptoms [5, 47,48,49]. Two interesting observations from our study in this regard are that in this non-obese population at risk for COPD due to occupational SHS exposure but with preserved spirometry, about 20% of those with RV/TLC > 0.35 did not have any EFL at rest or during exercise, while some with RV/TLC ≤ 0.35 had EFL even at rest. Given the subgroup analysis finding of albuterol effectiveness in those with RV/TLC > 0.35 and high adherence to therapy, these observations suggest resting lung volumes that represent air trapping such as RV/TLC may be better predictors of performance response to bronchodilator treatment than the presence of EFL at rest or during exercise. These findings are consistent with our previous studies in implicating lung volumes as better prognostic indicators for lung disease presence in pre-COPD when flows are within normal limits.
One interesting finding in our study was the decline in diastolic blood pressure that was observed in response to albuterol administration. While change in diastolic blood pressure was not a priori hypothesized as an outcome of albuterol therapy, there are potential mechanistic rationales that could explain the observed effect. In patients with diastolic heart failure (heart failure with preserved ejection fraction or HFpEF), both intravenous administration of dobutamine (a β-agonist) and inhalational administration of albuterol (2.5 mg via nebulization, which is roughly equivalent to four 90 mcg inhalation via metered dose inhaler) have been shown to elicit pulmonary and systemic vasodilatory effects along with a decline in systolic and diastolic blood pressure [50,51,52]. These studies however did not include any characterization of pulmonary status of the participating subjects. On the other hand, HFpEF has been described in patients with COPD whether severe [53] or mild [54] and even in those with preserved spirometry (normal FEV1/FVC) with declining vital capacity, as it happens with higher RV and RV/TLC and air trapping [55]. Given the above, another potential explanation for these observations could be the presence of air trapping. Lungs occupy the same finite space (thoracic cavity) as heart and great vessels, and increased lung volumes as it happens with air trapping and dynamic hyperinflation could introduce space limitation and changes to intrathoracic pressure, which in turn could contribute to cardiovascular dysfunction [9, 56]. Studies have described arterial stiffness as well as abnormalities in myocardial wall motion and relaxation in presence of abnormal lung volumes and air trapping [55, 57], all of which could contribute to increased ventricular end-diastolic pressure and hence increased diastolic blood pressure. Therefore, the changes in diastolic blood pressure seen with albuterol treatment in our study may have their origin in the effects of albuterol on lung volumes in addition to any direct effects on vasculature.
Our study has limitations that should be kept in view. First, the study sample size was small and further suffered from the research holds applied during the COVID-19 pandemic. Given that the final number of participants was significantly fewer than what was proposed in the original sample size and power calculation, the study was underpowered to detect the changes in the primary outcome. However, while the main study findings were negative, the subgroup analyses did provide interesting evidence suggesting the usefulness of bronchodilator therapy in the context of preserved spirometry with air trapping in individuals at risk for COPD. The findings also suggest that the nature of lung volume indices representing air trapping in the setting of preserved spirometry may in fact be an airway process not captured by airflow measures but observed on lung volume measurements. Second, studying the nature of air trapping in the setting of preserved spirometry may have been better performed in a population with more severe air trapping but preserved spirometry. Such population may be studied by enrolling individuals with history of direct smoking who have preserved spirometry but significant air trapping. However, the purpose of the current study was to examine the nonsmoking population with history of occupational SHS exposure. Epidemiologic data suggest that nearly 21% of the US population continue to be exposed to tobacco smoke [58], and thus understanding the health effects associated with exposure to SHS, which is a COPD risk factor, is of significant public health importance. Understanding whether inhaled bronchodilators could have beneficial effects on performance of respiratory system and exercise capacity could provide rationale for such therapy for when the respiratory demands are similarly increased during diseases such as respiratory infections. Third, we used albuterol, a short-acting β-agonist bronchodilator (SABA), instead of a long-acting or an ultra-long-acting β-agonist bronchodilator (LABA or ULABA) to test our hypothesis. Routine use of albuterol at high doses (800 mcg per day on four times a day dosing) in people with asthma has been reported to be associated with increased airway hyperresponsiveness as well as tachyphylaxis [59,60,61,62], although other studies have also documented opposing results showing that the airway bronchodilator responses are maintained even with high doses of albuterol [63,64,65]. However, the overall evidence in the literature suggests that in those without history of asthma or COPD, the lower doses and frequencies utilized in our study should not dampen the bronchodilator response. Moreover, such dampening of response would have resulted in a bias towards null hypothesis. Furthermore, while using LABA or ULABA may have been a more superior choice for the purpose of this study, we settled on using a short-acting agent in this study population with no overt obstruction because of concerns about adherence to therapy as those with milder symptoms or lung function abnormalities might be more likely to discontinue the use of study medication with occurrence of any mild adverse effects.
Conclusions
In conclusion, we did not find a significant improvement from albuterol on exercise performance, respiratory symptoms, quality of life, or daily physical activity level in this population at risk for COPD due to occupational exposure to SHS who had preserved spirometry but also air trapping and/or expiratory airflow limitation. However, in the subset of those participants in whom air trapping was strictly defined by RV/TLC > 0.35, albuterol did improve exercise performance and some measures of dynamic hyperinflation although no other outcomes. This study suggests that air trapping in the setting of pre-COPD (tobacco-exposed persons with preserved spirometry) may be related to small airway disease that is not considered significant by spirometric indices of airflow obstruction. In addition, it suggests that stratification of the tobacco-exposed persons with preserved spirometry by lung volumes and air trapping may help in identifying a subset who do benefit from the use of bronchodilators. Further study of this approach in persons with history of direct smoking and preserved spirometry who may have more severe air trapping should be able to provide a clinically useful indication in that population.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AQ20:
-
Airway Questionnaire 20
- BP:
-
Blood pressure
- CAT:
-
COPD Assessment Test
- COPD:
-
Chronic obstructive pulmonary disease
- CPET:
-
Cardiopulmonary exercise testing
- DBP:
-
Diastolic blood pressure
- EELV:
-
End-expiratory lung volume
- EFL:
-
Expiratory flow limitation
- FAMRI:
-
Flight Attendant Medical Research Institute
- FEF25-75 :
-
Forced expiratory flow at 25% to 75% of FVC
- FEF75 :
-
Forced expiratory flow at 75% of FVC
- FEV1 :
-
Forced expiratory volume in 1 s
- FRC:
-
Functional residual capacity
- FVC:
-
Forced vital capacity
- GLI:
-
Global Lung Function Initiative
- GOLD:
-
Global Initiative on Obstructive Lung Disease
- HR:
-
Heart rate
- IC:
-
Inspiratory capacity
- IPAQ:
-
International Physical Activity Questionnaire
- LLN:
-
Lower limit of normal
- MEF:
-
Maximum expiratory flow
- mMRC:
-
Modified Medical Research Council Dyspnea Scale
- O2-Pulse:
-
Oxygen-pulse
- PFT:
-
Pulmonary function test
- RR:
-
Respiratory rate
- RV:
-
Residual volume
- SF-12:
-
Short Form 12-Item Health Survey
- SHS:
-
Secondhand tobacco smoke
- SpO2 :
-
Oxygen saturation
- TLC:
-
Total lung capacity
- ULN:
-
Upper limit of normal
- VA:
-
Alveolar volume
- VCO2 :
-
Carbon dioxide production
- VE :
-
Minute ventilation
- VFL :
-
Volume of the tidal breath that is flow-limited on expiration
- VO2 :
-
Oxygen consumption
- VT :
-
Tidal volume
- WattsPeak :
-
Peak work achieved in watts
- WorkTotal :
-
Cumulative work achieved in Watts-Minutes throughout the exercise
References
Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693–718.
Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4(1):1–8.
van Koeverden I, Blanc PD, Bowler RP, Arjomandi M. Secondhand Tobacco Smoke and COPD Risk in Smokers: a COPDGene study cohort subgroup analysis. COPD. 2015;12(2):182–9.
Arjomandi M, Haight T, Redberg R, Gold WM. Pulmonary function abnormalities in never-smoking flight attendants exposed to secondhand tobacco smoke in the aircraft cabin. J Occup Environ Med. 2009;51(6):639–46.
Arjomandi M, Zeng S, Geerts J, et al. Lung volumes identify an at-risk group in persons with prolonged secondhand tobacco smoke exposure but without overt airflow obstruction. BMJ Open Respir Res. 2018;5(1):e000284.
Arjomandi M, Zeng S, Barjaktarevic I, et al. Radiographic Lung Volumes Predict Progression to COPD in Smokers with Preserved Spirometry in SPIROMICS. Eur Respir J. 2019;54:1802214.
Zeng S, Tham A, Bos B, Jin J, Giang B, Arjomandi M. Lung volume indices predict morbidity in smokers with preserved spirometry. Thorax. 2018;74:114–24.
Zeng S, Luo G, Lynch DA, Bowler RP, Arjomandi M. Lung volumes differentiate the predominance of emphysema versus airway disease phenotype in early COPD: an observational study of the COPDGene cohort. ERJ Open Res. 2023;9(5).
Zeng S, Dunn M, Gold WM, Kizer JR, Arjomandi M. Remote exposure to secondhand tobacco smoke is associated with lower exercise capacity through effects on oxygen pulse, a proxy of cardiac stroke volume. BMJ Open Respir Res. 2022;9(1):e001217.
Mustra Rakic J, Zeng S, Rohdin-Bibby L, et al. Elastin degradation and lung function deterioration with remote secondhand tobacco smoke exposure in never-smokers. Chronic Obstr Pulm Dis. 2022;9(3):377–93.
O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542–9.
The American Thoracic Society Board of Directors. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321–40.
Tabachnick II. A summary of the pharmacology and toxicology of albuterol (Proventil). Ann Allergy. 1981;47(5 Pt 2):379–83.
Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105–21.
Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11(1):149.
Burkes RM, Panos RJ. Ultra long-acting β-agonists in chronic obstructive pulmonary disease. J Exp Pharmacol. 2020;12:589–602.
Cazzola M, Page CP, Rogliani P, Matera MG. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187(7):690–6.
Lulich KM, Goldie RG, Ryan G, Paterson JW. Adverse reactions to beta 2-agonist bronchodilators. Med Toxicol. 1986;1(4):286–99.
Basran GS, Hardy JG, Woo SP, Ramasubramanian R, Byrne AJ. Beta-2-adrenoceptor agonists as inhibitors of lung vascular permeability to radiolabelled transferrin in the adult respiratory distress syndrome in man. Eur J Nucl Med. 1986;12(8):381–4.
Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L52–59.
McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32(7):1470–6.
Loza MJ, Foster S, Peters SP, Penn RB. Beta-agonists modulate T-cell functions via direct actions on type 1 and type 2 cells. Blood. 2006;107(5):2052–60.
Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol. 2002;110(6 Suppl):S282–290.
Perez-Garcia J, Pino-Yanes M, Plender EG, et al. Epigenomic response to albuterol treatment in asthma-relevant airway epithelial cells. Clin Epigenetics. 2023;15(1):156.
Gold WM, Kaufman HS, Nadel JA. Elastic recoil of the lungs in chronic asthmatic patients before and after therapy. J Appl Physiol. 1967;23(4):433–8.
O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1557–65.
O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–7.
Chen J, Weldemichael L, Zeng S, et al. Actigraphy informs distinct patient-centered outcomes in Pre-COPD. Respir Med. 2021;187:106543.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline LN. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–54.
Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257–66.
Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy. 1997;2(1):14–8.
Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Hajiro T, Nishimura K, Jones PW, et al. A novel, short, and simple questionnaire to measure health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(6):1874–8.
Eisner MD, Wang Y, Haight TJ, Balmes J, Hammond SK, Tager IB. Secondhand smoke exposure, pulmonary function, and cardiovascular mortality. Ann Epidemiol. 2007;17(5):364–73.
Katzel LI, Sorkin JD, Macko RF, Smith B, Ivey FM, Shulman LM. Repeatability of aerobic capacity measurements in Parkinson disease. Med Sci Sports Exerc. 2011;43(12):2381–7.
Koufaki P, Naish PF, Mercer TH. Reproducibility of exercise tolerance in patients with end-stage renal disease. Arch Phys Med Rehabil. 2001;82(10):1421–4.
Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of Peak Oxygen Uptake and Other Cardiopulmonary Exercise Testing Parameters in Patients With Heart Failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing). Am J Cardiol. 2008;102(6):712–7.
Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing). Am J Cardiol. 2008;102(6):712–7.
Wasserman K, Hansen J, Sue D, Stringer W, Whipp B. Principles of Exercise Testing and Interpretation. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506.
Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43.
Crapo RO, Morris AH, Gardner RM. Reference values for pulmonary tissue volume, membrane diffusing capacity, and pulmonary capillary blood volume. Bull Eur Physiopathol Respir. 1982;18(6):893–9.
Takamura T, Onishi K, Sugimoto T, et al. Patients with a hypertensive response to exercise have impaired left ventricular diastolic function. Hypertens Res. 2008;31(2):257–63.
Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. 2014;78(1):20–32.
Arjomandi M, Haight T, Sadeghi N, Redberg R, Gold WM. Reduced exercise tolerance and pulmonary capillary recruitment with remote secondhand smoke exposure. PLoS One. 2012;7(4):e34393.
Han MK, Ye W, Wang D, et al. Bronchodilators in Tobacco-Exposed Persons with Symptoms and Preserved Lung Function. N Engl J Med. 2022;387:1173–84.
Calverley PMA, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186.
O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–4.
O’Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3(4):219–32.
Andersen MJ, Hwang SJ, Kane GC, et al. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8(3):542–50.
Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B, Borlaug BA. The β-adrenergic agonist albuterol improves Pulmonary Vascular Reserve in Heart Failure With Preserved Ejection Fraction. Circ Res. 2019;124(2):306–14.
Snyder EM, Wong EC, Foxx-Lupo WT, Wheatley CM, Cassuto NA, Patanwala AE. Effects of an inhaled β2-agonist on cardiovascular function and sympathetic activity in healthy subjects. Pharmacotherapy. 2011;31(8):748–56.
Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14(4):414–22.
Cherneva Z, Valev D, Youroukova V, Cherneva R. Left ventricular diastolic dysfunction in non-severe chronic obstructive pulmonary disease - a step forward in cardiovascular comorbidome. PLoS One. 2021;16(3):e0247940.
Cuttica MJ, Colangelo LA, Shah SJ, et al. Loss of lung health from young adulthood and cardiac phenotypes in middle age. Am J Respir Crit Care Med. 2015;192(1):76–85.
Watz H. The lungs and the heart. Am J Respir Crit Care Med. 2015;192(1):7–8.
Luehrs RE, Newell JD Jr, Comellas AP, et al. CT-measured lung air-trapping is associated with higher carotid artery stiffness in individuals with chronic obstructive pulmonary disease. J Appl Physiol (1985). 2018;125(6):1760–6.
Brody DJ, Faust E, Tsai J. Secondhand Smoke Exposure Among Nonsmoking Adults: United States, 2015-2018. NCHS Data Brief. 2021;(369):1–8. PMID: 33541515.
Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet. 1993;342(8875):833–7.
Cockcroft DW, Swystun VA, Bhagat R. Interaction of inhaled beta 2 agonist and inhaled corticosteroid on airway responsiveness to allergen and methacholine. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1485–9.
Gauvreau GM, Jordana M, Watson RM, Cockroft DW, O’Byrne PM. Effect of regular inhaled albuterol on allergen-induced late responses and sputum eosinophils in asthmatic subjects. Am J Respir Crit Care Med. 1997;156(6):1738–45.
Wraight JM, Hancox RJ, Herbison GP, Cowan JO, Flannery EM, Taylor DR. Bronchodilator tolerance: the impact of increasing bronchoconstriction. Eur Respir J. 2003;21(5):810.
Lipworth BJ, Struthers AD, McDevitt DG. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high dose inhaled salbutamol in asthmatics. Am Rev Respir Dis. 1989;140(3):586–92.
Repsher LH, Anderson JA, Bush RK, et al. Assessment of tachyphylaxis following prolonged therapy of asthma with inhaled albuterol aerosol. Chest. 1984;85(1):34–8.
Haney S, Hancox RJ. Overcoming beta-agonist tolerance: high dose salbutamol and ipratropium bromide. Two randomised controlled trials. Respir Res. 2007;8(1):19.
Notation of prior abstract publication/presentation
Some of the results of this study have been previously reported in the form of an abstract (American Thoracic Society International Meeting 2023: Am J Respir Crit Care Med; 2023).
Funding
This work was supported by: The Flight Attendant Medical Research Institute (FAMRI) (012500WG and CIA190001 to Mehrdad Arjomandi) and National Library of Medicine Training Grant (NIH: T15LM007442 to Siyang Zeng). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The statements and conclusions in this publication are those of the authors and not necessarily those of the funding agencies. The mention of commercial products, their source, or their use in connection with the material reported herein is not to be construed as an actual or implied endorsement of such products.
Author information
Authors and Affiliations
Contributions
Conceived and designed the current manuscript study: WMG, MA; Developed study protocols: WMG, MA; Collected samples and data: SZ, MN, LW, HL, WMG, MA; Analyzed and interpreted data: SZ, MN, LM, MA; Prepared and edited the manuscript: SZ, HL, MA; Obtained funding: SZ, WMG, MA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. The University of California San Francisco (UCSF) Institutional Review Board (IRB) and the San Francisco Veterans Affairs Health Care System (SFVAHCS) Committee on Research and Development approved the study protocols. Written IRB-approved informed consent and Health Insurance Portability and Accountability Act (HIPAA) were obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, S., Nishihama, M., Weldemichael, L. et al. Effect of twice daily inhaled albuterol on cardiopulmonary exercise outcomes, dynamic hyperinflation, and symptoms in secondhand tobacco-exposed persons with preserved spirometry and air trapping: a randomized controlled trial. BMC Pulm Med 24, 44 (2024). https://doi.org/10.1186/s12890-023-02808-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02808-7