Abstract
Background
Coronavirus disease 2019 (COVID-19) has posed increasing challenges to global health systems. We aimed to understand the effects of pulmonary air leak (PAL), including pneumothorax, pneumomediastinum and subcutaneous emphysema, on patients with COVID-19.
Methods
We searched PubMed, Embase and Web of Science for data and performed a meta-analysis with a random-effects model using Stata 14.0. This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Thirty-five articles were included in the meta-analysis. The data came from 14 countries and included 3,047 COVID-19 patients with PAL, 11,3679 COVID-19 patients without PAL and 361 non-COVID-19 patients with PAL. We found that the incidence of PAL was much higher in COVID-19 patients than in non-COVID-19 patients (odds ratio (OR) = 6.13, 95% CI: 2.09–18.00). We found that the group of COVID-19 patients with PAL had a longer hospital stay (standardized mean difference (SMD) = 0.79, 95% CI: 0.27–1.30) and intensive care unit (ICU) stay (SMD = 0.51, 95% CI: 0.19–0.83) and comprised more ICU (OR = 15.16, 95% CI: 6.51–35.29) and mechanical ventilation patients (OR = 5.52, 95% CI: 1.69–17.99); furthermore, the mortality rate was also higher (OR = 2.62, 95% CI: 1.80–3.82).
Conclusions
Patients with lung injuries caused by COVID-19 may develop PAL. COVID-19 patients with PAL require more medical resources, have more serious conditions and have worse clinical outcomes.
PROSPERO registration number
CRD42022365047.
Similar content being viewed by others
Introduction
In December 2019, the first case of coronavirus disease 2019 (COVID-19) was diagnosed in Wuhan, China, and quickly spread worldwide [1]. As of December 19, 2022, there were a total of 653 million confirmed COVID-19 cases worldwide, of which 6.7 million were fatal cases [2]. COVID-19 is caused by SARS-CoV-2. Coronaviruses caused severe acute respiratory syndrome (SARS) in 2003 and Middle East respiratory syndrome (MERS) in 2012 [3, 4]. The source of SARS was civet cats, that of MERS was camels, and coronaviruses are transferred from bats to these animals [4, 5]; however, the source of COVID-19 is not yet clear. COVID-19 is a multisystem disease, and the lung is the most affected organ [6]. Pulmonary air leak (PAL), including pneumothorax, pneumomediastinum and subcutaneous emphysema, is a pulmonary complication of COVID-19 [7], and similar conditions occur in patients with SARS and MERS [8,9,10]. According to the study conducted by Shrestha et al., the incidence of PAL in patients with COVID-19 increases with the severity of the disease, ranging from 4.2 to 18.4% [11]. Patients with COVID-19 usually present with symptoms similar to those of patients with PAL, including tachycardia, tachypnoea, hypoxia and decreased breath sounds by auscultation, which are likely to result in a delayed diagnosis or misdiagnosis of PAL [12]. As COVID-19 is still a relatively new disease, the effects of PAL on COVID-19 patients are not completely clear. Therefore, we conducted a meta-analysis on data from COVID-19 patients with PAL to study the clinical features of these patients. This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Our study was registered with the International Prospective Register of Systematic Reviews.
Materials and methods
Eligibility criteria
Studies that met the following criteria were included in the meta-analysis: (1) studies mainly including COVID-19 patients with PAL; (2) studies of patients with at least one type of PAL, including pneumothorax, pneumomediastinum and subcutaneous emphysema; (3) case‒control studies; and (4) studies describing the clinical characteristics or outcomes of patients.
The exclusion criteria were as follows: (1) nonhuman studies, (2) case reports, reviews and commentaries, (3) studies without control group data, (4) studies with duplicated data, and (5) studies with a sample size less than five.
Information sources
We searched PubMed, Embase and Web of Science for studies published before December 19, 2022, that included COVID-19 patients with PAL. Language was not limited to identify more useful articles worldwide.
Search strategy
For the term COVID-19, the search was limited to the title, and for the term PAL, it was limited to the title and abstract. The detailed search strategy is provided in Additional file 1.
Study selection process
We imported all the literature retrieved from the databases into NoteExpress software. After removing duplicate articles, we conducted an initial screening by reading titles and abstracts to remove articles that were not relevant to our study. Then, by reading the full texts, articles for which data could not be extracted were further removed.
Data selection process and items
Data extraction was completed by two authors. They determined the data items for meta-analysis through discussion, extracted the data independently by reading the full texts, and then compared the results. If the data were consistent, they were included in the meta-analysis. If discrepancies occurred, they were resolved through discussion and a third author made the final decision.
The following information was extracted from the literature and ultimately included in the meta-analysis: study design, diagnosis method, PAL type, author, country, publication year, study period, sample size, age, sex, the number of patients with diabetes, hypertension, chronic obstructive pulmonary disease (COPD), asthma, cancer and a history of smoking, the number of patients in the intensive care unit (ICU), length of ICU stay, length of hospital stay, the number of deaths, the number of patients with mechanical ventilation (MV), positive end-expiratory pressure (PEEP), peak inspiratory pressure, PaO2/FiO2 ratio, tidal volume, and various laboratory findings, including leucocyte, neutrophil, and lymphocyte counts and C-reactive protein, ferritin, platelet, D-dimer, aspartate aminotransferase and lactate dehydrogenase levels.
Study risk of bias assessment
The Newcastle–Ottawa Quality Assessment Scale was used to independently assess the quality and risk of bias of the included studies. If the total score was equal to or higher than seven points, the included article was considered to have a low risk of bias and be of high quality. This assessment was performed by one author and checked by another.
Reporting bias assessment
We used funnel plots (number of events ≥ 10) and Egger’s test for reporting bias assessment, and a p value < 0.05 indicated the presence of bias.
Statistical analysis
Odds ratios (ORs) and standardized mean differences (SMDs) were used for data analysis and evaluation, in which ORs were used for dichotomous variables, SMDs were used for continuous variables, and the confidence intervals (CIs) were set at 95%. For data including only the sample size and quartile, we used the transformation formula to determine the mean and standard deviation [13]. The I2 statistic was used to quantify heterogeneity among studies, with I2 ≤ 50% indicating low heterogeneity, 50%<I2 ≤ 75% indicating moderate heterogeneity, and I2 > 75% indicating high heterogeneity [14]. A random-effects model was used to estimate the effect value. The statistical software was Stata 14.0, and a z test p value < 0.05 was considered to indicate statistical significance.
Results
Study selection
A total of 2,184 articles were retrieved from the databases, including 668 from PubMed, 858 from Embase, and 658 from Web of Science. After importing the data into NoteExpress software, 724 duplicate articles were deleted. By reading titles and abstracts, 1,352 irrelevant articles were further eliminated, and of the remaining 108 articles, 73 articles were removed after reading the full text. The flow diagram of the study selection is shown in Fig. 1.
Risk of bias in the studies
According to the Newcastle–Ottawa Quality Assessment Scale, we found that most of the literature included in the meta-analysis was of high quality and had a low risk of bias (Additional file 3: Table S1).
Characteristics and results of individual studies
Thirty-five articles were included in the meta-analysis. The data came from 14 countries and included 3,047 COVID-19 patients with PAL, 11,3679 COVID-19 patients without PAL and 361 non-COVID-19 patients with PAL. Information on the characteristics and results of the individual studies is detailed in Table 1.
Results of syntheses
The incidence of PAL
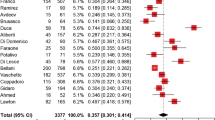
A total of five articles were used to study the effect of COVID-19 infection on the incidence of PAL (Table 2). The participants included in the experimental group were all COVID-19 patients, while none of the patients in the control group were infected with SARS-CoV-2. We found that the incidence of PAL was much higher in COVID-19 patients than in non-COVID-19 patients (OR = 6.13, 95% CI: 2.09–18.00, I2 = 88.9%, p = 0.001, Fig. 2).
Clinical characteristics
A total of 33 articles were included to study the clinical characteristics of patients with PAL. All patients in the experimental group had COVID-19 with PAL, while the control group had COVID-19 but did not suffer from the corresponding type of PAL (Additional file 3: Tables S2–S5).
Age, sex and hospital admission information
The data of control patients in four articles were matched according to age [36,37,38, 47], those of control patients in three articles were matched according to sex [36, 37, 47], and another article included data on the age and sex characteristics of patients with haemothorax [45]. Therefore, we excluded information from the above literature when studying the effect of age and sex on the development of PAL in COVID-19 patients. We found that males (OR = 1.38, 95% CI: 1.10–1.74, I2 = 54.1%, p = 0.005, Additional file 3: Figure S1) and younger individuals (SMD=-0.20, 95% CI: -0.32–0.07, I2 = 73.1%, p = 0.002, Additional file 3: Figure S2) were more likely to develop PAL. If all patients in a study were from ICU wards, their data were not included in the study of the ICU admission rate. After screening, six articles were included, which showed that the ICU admission rate of the experimental group was significantly higher than that of the control group (OR = 15.16, 95% CI: 6.51–35.29, I2 = 75.5%, p < 0.001, Additional file 3: Figure S3). At the same time, we also found that COVID-19 patients with PAL had a longer length of ICU stay (SMD = 0.51, 95% CI: 0.19–0.83, I2 = 85.5%, p = 0.002, Additional file 3: Figure S4) and hospital stay (SMD = 0.79, 95% CI: 0.27–1.30, I2 = 97.7%, p = 0.003, Additional file 3: Figure S5).
Comorbidities
In terms of comorbidities, our study showed that the prevalence of diabetes (OR = 0.67, 95% CI: 0.80–0.67, I2 = 22.2%, p = 0.014, Additional file 3: Figure S6) and hypertension (OR = 0.69, 95% CI: 0.56–0.84, I2 = 38.7%, p < 0.001, Additional file 3: Figure S7) was lower in COVID-19 patients with PAL; however, COPD (OR = 0.85, 95% CI: 0.53–1.35, I2 = 33.3%, p = 0.486, Additional file 3: Figure S8), asthma (OR = 1.76, 95% CI: 0.95–3.27, I2 = 75.0%, p = 0.072, Additional file 3: Figure S9) and cancer (OR = 1.16, 95% CI: 0.90–1.15, I2 = 1.8%, p = 0.254, Additional file 3: Figure S10) did not affect the development of PAL in COVID-19 patients. We also compared the differences in smoking between the experimental group and the control group and found that smoking (OR = 1.00, 95% CI: 0.88–1.14, I2 = 2.9%, p = 0.974, Additional file 3: Figure S11) did not increase the risk of PAL in COVID-19 patients.
Laboratory findings
Through the analysis of a large number of patients with laboratory results, we found that the presence of PAL was associated with increased D-dimer levels (SMD = 0.74, 95% CI: 0.34–1.14, I2 = 83.2%, p < 0.001, Additional file 3: Figure S12), leucocyte counts (SMD = 0.57, 95% CI: 0.26–0.87, I2 = 67.2%, p < 0.001, Additional file 3: Figure S13), aspartate aminotransferase levels (SMD = 0.57, 95% CI: 0.31–0.84, I2 = 0, p < 0.001, Additional file 3: Figure S14) and lactate dehydrogenase levels (SMD = 0.35, 95% CI: 0.03–0.67, I2 = 73.6%, p = 0.031, Additional file 3: Figure S15) in patients with COVID-19. However, for neutrophil counts (SMD = 0.19, 95% CI: 0.00–0.39, I2 = 0, p = 0.05, Additional file 3: Figure S16), lymphocyte counts (SMD = 0.07, 95% CI: -0.33–0.47, I2 = 84.6%, p = 0.727, Additional file 3: Figure S17), C-reactive protein levels (SMD = 0.21, 95% CI: 0.05–0.47, I2 = 74%, p = 0.109, Additional file 3: Figure S18), ferritin levels (SMD = 0.59, 95% CI: 0.11–1.28, I2 = 89.7%, p = 0.099, Additional file 3: Figure S19), platelet counts (SMD = 0.13, 95% CI: 0.40–0.15, I2 = 74.0%, p = 0.376, Additional file 3: Figure S20) and creatinine levels (SMD = 0.59, 95% CI: 1.47–0.29, I2 = 83.2%, p = 0.187, Additional file 3: Figure S21), there was no significant impact.
MV
When studying whether PAL affected the number of COVID-19 patients who needed MV, we excluded two types of studies: (1) studies in which all patients had MV [20,21,22,23, 26, 30, 32, 34, 35, 38, 41, 42, 44,45,46,47,48,49], and (2) studies in which the number of patients with MV in the experimental and control groups was matched [24]. A total of five studies were included, and it was found that a higher number of COVID-19 patients with PAL required MV (OR = 5.52, 95% CI: 1.69–17.99, I2 = 89.5%, p = 0.005, Additional file 3: Figure S22). After excluding studies in which all patients needed invasive MV [21, 22, 41] or noninvasive MV [38], six articles were included, and the analysis showed that among COVID-19 patients with MV, those with PAL did not require more invasive MV (OR = 0.93, 95% CI: 0.43–1.99, I2 = 62.6%, p = 0.850, Additional file 3: Figure S23). With regard to ventilator parameters, we found that COVID-19 patients with PAL had higher PEEP values (SMD = 0.25, 95% CI: 0.03–0.47, I2 = 61.6%, p = 0.026, Additional file 3: Figure S24), but there were no significant differences in peak inspiratory pressure (SMD = 0.53, 95% CI: -0.08–1.14, I2 = 85.7%, p = 0.086, Additional file 3: Figure S25), PaO2/FiO2 ratio (SMD=-0.30, 95% CI: -0.64–0.04, I2 = 77.9%, p = 0.084, Additional file 3: Figure S26) and tidal volume (SMD = 0.14, 95% CI: -0.11–0.39, I2 = 66.4%, p = 0.264, Additional file 3: Figure S27) values.
Mortality
Our study showed that PAL contributed to an increased mortality rate in patients with COVID-19 (OR = 2.62, 95% CI: 1.80–3.82, I2 = 90.1%, p < 0.001, Fig. 3). Since mortality is a very important clinical index, we performed a subgroup analysis of the mortality rate in COVID-19 patients with different types of PAL and found that COVID-19 patients with pneumothorax had a higher mortality rate than those without pneumothorax (OR = 3.25, 95% CI: 1.82–5.80, I2 = 92.1%, p < 0.001, Fig. 3), and the same conclusion was obtained for COVID-19 patients with pneumomediastinum (OR = 2.22, 95% CI: 1.08–4.58, I2 = 48.1%, p = 0.031, Fig. 3). A subgroup analysis of the mortality rate in COVID-19 patients with subcutaneous emphysema was not performed because no data were available.
We also compared the mortality rate in patients with COVID-19 who had multiple types of PAL at the same time and those who had only one type (Additional file 3: Table S6). The results showed that there was no significant difference between the two groups (OR = 2.07, 95% CI: 0.69–6.21, I2 = 41.7%, p = 0.194, Additional file 3: Figure S28).
Reporting biases
We used funnel plots and Egger’s test for the analysis of reporting bias. The results showed that there was no reporting bias in most of the conclusions (Additional file 2).
Discussion
The incidence of PAL in patients with COVID-19 is much higher than that in patients without COVID-19, which may be due to various reasons. The primary target of SARS-CoV-2 is the angiotensin-converting enzyme-2 (ACE-2) receptor, which is more frequently expressed in type II pneumocytes. Previous studies have shown that ACE-2 has a protective effect against pulmonary inflammation, pulmonary fibrosis, and pulmonary hypertension. However, the interaction between ACE-2 and SARS-CoV-2 may lead to downregulation [49]. A host-triggered dysregulated immune response causes lung injury in COVID-19 patients, leading to extensive inflammation and eventual fibrosis, which may lead to dysregulated surfactant production, impaired lung compliance, and increased alveolar air space surface tension, thus predisposing individuals with COVID-19 to PAL [50]. COVID-19 also causes the development of nonhomogeneous pulmonary parenchyma, which can result in maldistribution of ventilatory stress due to different local stresses in two consecutive lung regions with different compliance and lead to PAL [7]. The increased probability of PAL in COVID-19 patients may also be related to the Macklin effect, in which the rupture of the alveolar tree is related to the increase in pressure in the alveoli, which may release air and subsequently dissect the peribronchial sheath [51]. These alveoli rupture, isolate or merge and then cause pulmonary lacerations. Belletti et al. reported that almost all patients with pneumothorax/pneumomediastinum showed the Macklin effect on chest CT [35]. Another reason for the high incidence of PAL in patients with COVID-19 may be coughing, a common symptom of COVID-19. The increased intrathoracic pressure caused by coughing can cause the alveolar walls to rupture, leading to air leakage from damaged alveoli and separating the interstitial tissue around the bronchus [52, 53].
Noppen et al. showed that before the pandemic, the incidence of pneumothorax was higher in men (7.4 to 18 cases per 100,000 people) than in women (1.2 to 6 cases per 100,000 people) [54]. In 2015, Kouritas et al. indicated that men account for approximately 76% of patients with pneumomediastinum [55]. We obtained a similar conclusion that male COVID-19 patients are more likely to develop PAL. Bwire et al. explained the biological differences between the male and female immune systems, noting that men have higher ACE-2 expression due to different sex hormones and are less resistant to SARS-CoV-2 infection than women [56]. Chen et al. believed that among COVID-19 patients, the conditions of men were often more serious [57], which might lead to more air leak events in male patients. Our study found that in COVID-19 patients, younger individuals were more likely to develop PAL, which is consistent with findings in non-COVID-19 patients [55, 58]. Diabetes and hypertension are the most common comorbidities in COVID-19 patients with PAL because the expression of ACE-2 receptors is increased in patients with these conditions [7, 59]. Our study showed that among COVID-19 patients, patients with PAL had a lower risk of developing diabetes and hypertension. This finding may be because the incidence of diabetes and hypertension is positively correlated with age, while COVID-19 patients develop PAL at a younger age.
Infection with SARS-CoV-2 results in an insufficient respiratory capacity, and patients often require prolonged MV [60]. Our study found that COVID-19 patients with PAL had higher rates of MV and higher PEEP values. These higher rates are mainly because pulmonary barotrauma is a potential complication of MV, and it occurs as a result of excessive volume of lung parenchyma, which can cause alveolar rupture, leading to pneumothorax, pneumomediastinum and subcutaneous emphysema [61]. Gidaro et al. conducted a case‒control study and divided COVID-19 patients into two groups according to the levels of PEEP used (≤ 10 cmH2O and > 10 cmH2O). The number of patients in the high-PEEP group who developed PAL was much higher than that in the low-PEEP group [62]. Although a high PEEP maintains oxygenation and prevents repetitive alveolar collapse, it also partially overexpands lungs with normal compliance and then increases the risk of barotrauma [63, 64].
The COVID-19 pandemic rapidly saturated health care services, especially in the early stage. Some medical institutions suspended the services of other departments and converted their wards into COVID-19 wards to cope with the rapid increase in the number of patients [65]. The challenge was equally acute in the ICU, with some patients with severe COVID-19 admitted to “nonconventional” temporary ICUs, such as operating theatres or postanaesthesia care units [66]. Our research showed that the occurrence of PAL in COVID-19 patients aggravated this tense situation. These patients needed more ICU, longer hospital stays and ICU stays and required more medical resources. Therefore, it is necessary to reduce the incidence of PAL in COVID-19 patients to relieve the enormous pressure placed on the health care system.
SARS-CoV-2 infection has caused millions of deaths worldwide [2], and severe PAL can also pose a great threat to patients’ lives [67]. Although the presence of multiple types of PAL did not increase the mortality risk among COVID-19 patients with PAL, the finding that PAL contributed to an increased mortality risk among COVID-19 patients is still of concern. The combined effects of COVID-19 and PAL may cause more damage to patients’ lungs, which leads to more severe illness and worse clinical outcomes.
Limitations
Our study has certain limitations. The type of PAL was not completely consistent across the included studies, which might lead to overestimation or underestimation of events when studies were included in the pooled analysis. Publication bias and high heterogeneity existed in a small part of our study results, which can be solved by incorporating more high-quality studies in the future. Since November 2021, the Omicron variant has rapidly spread worldwide [68]. In this meta-analysis, all the patients included had been infected prior to the Omicron outbreak. The effect of PAL on patients infected with the Omicron variant requires further study.
Conclusions
In this systematic review and meta-analysis, we studied and discussed the effects of PAL on patients with COVID-19 in detail. Patients with lung injuries caused by COVID-19 may develop PAL. COVID-19 patients with PAL require more medical resources, have more serious conditions and have worse clinical outcomes.
Data Availability
All data relevant to the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Abbreviations
- ACE-2:
-
Angiotensin-converting enzyme-2
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Confidence intervals
- COPD:
-
Chronic obstructive pulmonary disease
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- MERS:
-
Middle East respiratory syndrome
- MV:
-
Mechanical ventilation
- OR:
-
Odds ratio
- PAL:
-
Pulmonary air leak
- PEEP:
-
Positive end-expiratory pressure
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- SARS:
-
Severe acute respiratory syndrome
- SMD:
-
Standardized mean difference
References
Sim MR. The COVID-19 pandemic: major risks to Healthcare and other workers on the Front line. Occup Environ Med. 2020;77(5):281–2.
COVID-19 Data in Motion. : Monday, December 19, 2022. Available from: https://coronavirus.jhu.edu/map.html.
Stockman LJ, Bellamy R, Garner P. SARS: systematic review of Treatment effects. PLoS Med. 2006;3(9):e343.
Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370(26):2499–505.
Wang LF, Eaton BT. Bats civets and the emergence of SARS. Curr Top Microbiol Immunol. 2007;315:325–44.
Ding X, Xu J, Zhou J, Long Q. Chest CT findings of COVID-19 Pneumonia by duration of symptoms. Eur J Radiol. 2020;127:109009.
Singh A, Singh Y, Pangasa N, Khanna P, Trikha A, Risk, Factors. Clinical characteristics, and Outcome of Air Leak Syndrome in COVID-19: a systematic review. Indian J Crit Care Med. 2021;25(12):1434–45.
Chu CM, Leung YY, Hui JYH, Hung IFN, Chan VL, Leung WS, et al. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23(6):802–4.
Das KM, Lee EY, Jawder SEA, Enani MA, Singh R, Skakni L, et al. Acute Middle East Respiratory Syndrome Coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. AJR Am J Roentgenol. 2015;205(3):W267–74.
Kao HK, Wang JH, Sung CS, Huang YC, Lien TC. Pneumothorax and mortality in the mechanically ventilated SARS patients: a prospective clinical study. Crit Care. 2005;9(4):R440–5.
Shrestha DB, Sedhai YR, Budhathoki P, Adhikari A, Pokharel N, Dhakal R, et al. Pulmonary barotrauma in COVID-19: a systematic review and meta-analysis. Ann Med Surg (Lond). 2022;73:103221.
Geraci TC, Williams D, Chen S, Grossi E, Chang S, Cerfolio RJ, et al. Incidence, management, and outcomes of patients with COVID-19 and Pneumothorax. Ann Thorac Surg. 2022;114(2):401–7.
Estimating the sample mean and standard. deviation (SD) from the five-number summary and its application in meta-analysis. https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html?sr=1&nm=wechat.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Malik S, Kaushik C, Kaushik E, Polychronopoulou E, Kuo YF, Sharma G, et al. Characteristics and factors Associated with mortality in patients with Coronavirus Disease 2019 and Pneumothorax. Mayo Clin Proc Innov Qual Outcomes. 2022;6(3):257–68.
Pierre L, Rieu LPJ, Lemmet T, Ion C, Gravier S, Mohseni-Zadeh M, et al. Characteristic outcomes and risk assessment of pneumothorax in 21 patients with COVID-19. Infect Dis Now. 2022;52(5):321–3.
Akram J, Yousaf Z, Alabbas Y, Almoyaaf MIA, Ibrahim ASS, Kharma N. Epidemiological and outcome analysis of COVID-19-associated pneumothorax: multicentre retrospective critical care experience from Qatar. BMJ Open. 2022;12(2):e053398.
Miro O, Llorens P, Jimenez S, Pinera P, Burillo-Putze G, Martin A, et al. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with Coronavirus Disease 2019: a Case-Control, Emergency Medicine-based Multicenter Study. Chest. 2021;159(3):1241–55.
Berg B, Pajak A, Gandhi P, Thakur S, Al-Mohamad T, Liou J, et al. Incidence, risk factors and impact on outcomes of pneumothorax in COVID-19 patients requiring ICU level of care-a single-center retrospective study. Am J Respir Crit Care Med. 2021;203:A2525.
Chopra A, Tarbsheh AHA, Shah NJ, Yaqoob H, Hu K, Feustel PJ, et al. Pneumothorax in critically ill patients with COVID-19 Infection: incidence, clinical characteristics and outcomes in a case control multicenter study. Respir Med. 2021;184:106464.
Ozdemir S, Bilgi DO, Kose S, Oya G. Pneumothorax in patients with coronavirus Disease 2019 Pneumonia with invasive mechanical ventilation. Interact Cardiovasc Thorac Surg. 2021;32(3):351–5.
Capaccione KM, D’souza B, Leb J, Luk L, Duong J, Tsai WY, et al. Pneumothorax rate in intubated patients with COVID-19. Acute Crit Care. 2021;36(1):81–4.
Taha M, Elahi M, Elahi K, Samavati L. Incidence and risk factors of COVID-19 associated pneumothorax. PLoS ONE. 2022;17(8):e0271964.
Reis AE, Emami N, Chand S, Ogundipe F, Belkin DL, Ye K, et al. Epidemiology, risk factors and outcomes of Pneumomediastinum in patients with Coronavirus Disease 2019: a case-control study. J Intensive Care Med. 2022;37(1):12–20.
Arciniega TGR, Diaz ES, Martinez JAF, Alvizo-Perez ME, Lopez-Leal IN, Corona-Nakamura AL, et al. Frequency and risk factors for spontaneous Pneumomediastinum in COVID-19 patients. Front Med (Lausanne). 2021;8:662358.
Ozdemir S, Bilgi DO, Hergunsel GO, Citak N. Incidence and risk factors for pneumomediastinum in COVID-19 patients in the intensive care unit. Interact Cardiovasc Thorac Surg. 2022;34(2):236–44.
Ozsoy IE, Tezcan MA, Guzeldag S, Ozdemir AT. Is spontaneous Pneumomediastinum a poor prognostic factor in Covid-19? J Coll Physicians Surg Pak. 2021;31(2):132–7.
Baslas R, Condurache DG, Jayal A, Colquhoun M, Wolff JF. Pneumomediastinum in patients with COVID-19 undergoing CT pulmonary angiography: a retrospective cohort study. Postgrad Med J. 2022;postgradmedj-2022-141642.
Loffi M, Regazzoni V, Sergio P, Martinelli E, Stifani I, Quinzani F et al. Spontaneous pneumomediastinum in COVID-19 Pneumonia. Monaldi Arch Chest Dis. 2020;90(4).
Righetti F, Colombaroli E. Pneumomediastinum in ARDS (acute respiratory distress syndrome) caused by COVID-19 (coronavirus Disease 2019): is protective lung ventilation really a weapon to our advantage? Crit Care. 2022;26(Suppl1):P179.
Muhammad AI, Shaw MMM, Hussain N, Joseph S, Vancheeswaran R. Incidence and clinical features of Pneumomediastinum and Pneumothorax in COVID-19 Pneumonia. J Intensive Care Med. 2022;37(8):1015–8.
Tetaj N, Garotto G, Albarello F, Mastrobattista A, Maritti M, Stazi GV, et al. Incidence of Pneumothorax and Pneumomediastinum in 497 COVID-19 patients with moderate-severe ARDS over a year of the pandemic: an observational study in an Italian third level COVID-19 hospital. J Clin Med. 2021;10(23):5608.
Udwadia ZF, Toraskar KK, Pinto L, Mullerpatan J, Wagh HD, Mascarenhas JM, et al. Increased frequency of pneumothorax and pneumomediastinum in COVID-19 patients admitted in the ICU: a multicentre study from Mumbai, India. Clin Med (Lond). 2021;21(6):e615–9.
Gazivoda VP, Ibrahim M, Dick AK, Sun A, Silver M, Wiesel O, et al. Outcomes of Barotrauma in critically Ill COVID-19 patients with severe Pneumonia. J Intensive Care Med. 2021;36(10):1176–83.
Belletti A, Palumbo D, Zangrillo A, Fominskiy EV, Franchini S, Acqua AD, et al. Predictors of Pneumothorax/Pneumomediastinum in mechanically ventilated COVID-19 patients. J Cardiothorac Vasc Anesth. 2021;35(12):3642–51.
Ernst EB, Corwin D, Stahlnecker DM, Marinescu AR, Dumache R, Tudoran M. Pneumothorax and pneumomediastinum in patients with COVID-19 Infection: a case-control study of risk factors and patient characteristics. Am J Respir Crit Care Med. 2021;203:A2648.
Bonato M, Fraccaro A, Landini N, Zanardi G, Catino C, Savoia F, et al. Pneumothorax and/or Pneumomediastinum worsens the prognosis of COVID-19 patients with severe Acute Respiratory Failure: a Multicenter Retrospective Case-Control Study in the North-East of Italy. J Clin Med. 2021;10(21):4835.
Tonelli R, Bruzzi G, Manicardi L, Tabbi L, Fantini R, Castaniere I, et al. Risk factors for Pulmonary Air Leak and clinical prognosis in patients with COVID-19 related Acute Respiratory Failure: a retrospective Matched Control Study. Front Med (Lausanne). 2022;9:848639.
Marza AM, Petrica A, Lungeanu D, Sutoi D, Mocanu A, Petrache I, et al. Risk factors, characteristics, and Outcome in Non-ventilated patients with spontaneous pneumothorax or Pneumomediastinum Associated with SARS-CoV-2 Infection. Int J Gen Med. 2022;15:489–500.
Shaikh N, Ameri GA, Shaheen M, Abdaljawad WI, Wraidat MA, Alawi AASA, et al. Spontaneous pneumomediastinum and pneumothorax in COVID-19 patients: a tertiary care experience. Health Sci Rep. 2021;4(3):e339.
Lemmers DHL, Hilal MA, Bna C, Prezioso C, Cavallo E, Nencini N, et al. Pneumomediastinum and subcutaneous Emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6(4):00385–2020.
Steinberger S, Finkelstein M, Pagano A, Manna S, Toussie D, Chung M, et al. Barotrauma in COVID 19: incidence, pathophysiology, and effect on prognosis. Clin Imaging. 2022;90:71–7.
Nespoli S, Babini G, Piacentino E, Tognacci E, Umbrello M, Muttini S. Incidence of barotrauma in patients with severe COVID-19 acute Respiratory Failure: a descriptive study. Intensive Care Medicine Experimental. 2020;8(2):001098.
Hamouri S, Samrah SM, Albawaih O, Saleh Z, Smadi MM, Alhazymeh A, et al. Pulmonary Barotrauma in COVID-19 patients: invasive versus noninvasive positive pressure ventilation. Int J Gen Med. 2021;14:2017–32.
Guven BB, Erturk T, Kompe O, Ersoy A. Serious Complications in COVID-19 ARDS cases: pneumothorax, pneumomediastinum, subcutaneous Emphysema and haemothorax. Epidemiol Infect. 2021;149:e137.
Jones E, Gould A, Pillay TD, Khorasanee R, Sykes R, Bazo-Alvarez JC, et al. Subcutaneous Emphysema, Pneumomediastinum, and Pneumothorax in critically Ill patients with Coronavirus Disease 2019: a retrospective cohort study. Crit Care Explor. 2020;2(9):e0210.
Venkateswaran V, Soni K, Chaturvedi AC, Aggarwal R, Ganesh V, Patel N, et al. Barotrauma in critically ill COVID-19 patients: a retrospective case-control study. Anaesthesiol Intensive Ther. 2022;54(1):18–22.
Sami R, Sereshti N. Case Report: Barotrauma in COVID-19 Case Series. Am J Trop Med Hyg. 2021;105(1):54–8.
Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV Infection. Front Med. 2020;14:185–92.
Conti P, Ronconi G, Caraffa A, Gallenga C, Ross R, Frydas I, et al. Induction of proinflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2):anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–31.
Chassagnon G, Favelle O, Derogis V, Cottier JP. Spontaneous pneumomediastinum due to the Macklin effect: less is more. Intern Emerg Med. 2015;10(6):759–61.
Palumbo D, Zangrillo A, Belletti A, Guazzarotti G, Calvi MR, Guzzo F, et al. A radiological predictor for pneumomediastinum/pneumothorax in COVID-19 ARDS patients. J Crit Care. 2021;66:14–9.
Caceres M, Ali SZ, Braud R, Weiman D Jr. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg. 2008;86(3):962–6.
Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev. 2010;19(117):217–9.
Kouritas VK, Papagiannopoulos K, Lazaridis G, Baka S, Mpoukovinas I, Karavasilis V, et al. Pneumomediastinum J Thorac Dis. 2015;7:44–9.
Bwire GM. Coronavirus: why men are more vulnerable to COVID-19 than women? SN Compr Clin Med. 2020;2(7):874–6.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus Pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Ghisalberti M, Guerrera F, Vico AD, Bertolaccini L, Palma AD, Fiorelli A, et al. Age and clinical presentation for primary spontaneous pneumothorax. Heart Lung Circ. 2020;29(11):1648–55.
Pal R, Bhansali A. COVID-19, Diabetes Mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132.
Akboga SA, Gokce A, Hatipoglu M, Beyoglu MA, Inan K, Sezen AI, et al. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 Disease. Ir J Med Sci. 2022;191(4):1931–6.
Ioannidis G, Lazaridis G, Baka S, Mpoukovinas I, Karavasilis V, Lampaki S, et al. Barotrauma and pneumothorax. J Thorac Dis. 2015;7:38–43.
Gidaro A, Samartin F, Brambilla AM, Cogliati C, Ingrassia S, Banfi F, et al. Correlation between continuous positive end-expiratory pressure (PEEP) values and occurrence of Pneumothorax and Pneumomediastinum in SARS-CoV2 patients during non-invasive ventilation with helmet. Sarcoidosis Vasc Diffuse Lung Dis. 2021;38(2):e2021017.
Dondorp AM, Hayat M, Aryal D, Beane A, Schultz MJ. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102(6):1191–7.
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–36.
Aghemo A, Masarone M, Montagnese S, Petta S, Ponziani FR, Russo FP, et al. Assessing the impact of COVID- 19 on the management of patients with Liver Diseases: a national survey by the Italian association for the study of the liver. Dig Liver Dis. 2020;52:937–41.
Bardi T, Pintado V, Gomez-Rojo M, Escudero-Sanchez R, Lopez AA, Diez-Remesal Y, et al. Nosocomial Infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40(3):495–502.
Onuki T, Ueda S, Yamaoka M, Sekiya Y, Yamada H, Kawakami N, et al. Primary and secondary spontaneous pneumothorax: prevalence, clinical features, and In-Hospital mortality. Can Respir J. 2017;2017:6014967.
Chen J, Wang R, Gilby NB, Wei GW. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412–22.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Zhuan Zhong: Writing-review and editing; Data curation; Writing-original draft.Jia Guo: Conceptualization; Writing-review and editing.Xingzhao Li: Data curation; Methodology.Yingying Han: Data curation; Methodology; Writing-original draft.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, Z., Guo, J., Li, X. et al. Effects of pulmonary air leak on patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. BMC Pulm Med 23, 398 (2023). https://doi.org/10.1186/s12890-023-02710-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02710-2