Abstract
Background
Extra-adrenal myelolipoma is an unusual entity, and endobronchial myelolipoma is rarer, which is often ignored by clinicians, delaying the disease and affecting the prognosis.
Case presentation
A 71-year-old man with a history of chronic obstructive pulmonary disease (COPD) and type 2 diabetes mellitus, with recurrent fever, cough, and expectoration for more than 2 weeks experienced relief in cough, phlegm reduction, and glycemic control with anti-inflammatory treatment. Further examination revealed that new growths obstructing all lobar bronchi impaired flexible bronchoscope entry. In order to relieve the patient’s symptoms, under general anesthesia, we performed liquid nitrogen cryobiopsy at multiple bronchial openings, and then used argon plasma coagulation (APC) to achieve hemostasis. The pathological diagnosis was bronchial myelolipoma. The largest volume of the resected tissue was a mass measuring 0.6 cm × 0.4 cm × 0.3 cm at the bronchial opening of the upper lobe of the left lung. The patient’s condition was stable and the symptoms were partially relieved after surgery. No recurrence was observed during the 12-month follow-up, although the long-term treatment efficacy is unknown.
Conclusion
Pathological biopsy is key to the diagnosis of endobronchial myelolipoma, and the development of the endobronchial myelolipomas may have been associated with long-term poor control of steroid levels in this patient.
Similar content being viewed by others
Background
Myelolipoma is a rare benign tumor, composed of mature adipose tissue and bone marrow hematopoietic tissue, and is commonly found in the adrenal glands [1]. The majority of adrenal myelolipomas are detected inadvertently on autopsy or surgically removed specimens [2]. Although the exact pathogenesis of adrenal myelolipoma is unknown, elevated adrenocorticotropic hormone (ACTH) levels and sustained ACTH stimulation (e.g., Congenital Adrenal Hyperplasia and Cushing’s disease) likely play an important role [3,4,5].
Extra-adrenal myelolipomas are rarer, and account for only 15% of myelolipomas, of which 3% occur in the chest, mostly in the posterior mediastinum [6]. Myelolipomas can occur in the liver, spleen, lung, and other extra-adrenal sites, as reported recently [7, 8]. However, only 18 cases of pulmonary myelolipomas have been reported, both at home and abroad [9,10,11,12,13,14,15]; among these, two cases involved only the bronchus and not lung parenchyma [12].
We hereby present the case of treatment of multiple bronchial myelolipomas, along with a literature review of similar cases.
Case presentation
A 71-year-old Chinese man presented with recurrent fever and persistent cough, sputum, and asthma 2 weeks. Antibiotic treatment in the community hospital initially led to symptom alleviation, but was followed by a relapse, and the patient came to our hospital for treatment. The patient had type 2 diabetes and chronic cholecystitis with gallstones and was receiving subcutaneously injected recombinant insulin glargine (7 units QN) and insulin aspart (7 units TID), and oral acarbose and sitagliptin, but without adequate glycemic control. In the past 20 years, the patients had experienced recurrent cough, expectoration, and asthma, usually in autumn and winter. In the past 3 years, the patient was diagnosed with exertional dyspnea, and the symptoms improved after each antibiotic treatment. The patient has a long history of smoking for nearly 40 years.
The patient had a barrel chest and slight dyspnea. Percussion of both lung fields elicited hyperresonance. Auscultatory breath sounds were muffled, and widespread wheezing and rales were heard without pleural rubbings.
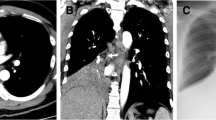
Laboratory examinations that were conducted at admission included routine blood investigation that revealed WBC 14.7 × 109/L (neutrophile granulocyte: 85.8%). Sputum smear examination did not reveal fungal, bacterial, mycobacterial, or other infection. The T-spoT test showed a negative result. Despite a normal glycated hemoglobin level, the fasting plasma glucose remained approximately 234 mg/dL. CT of the chest revealed multiple high-density pulmonary masses bilaterally in the bronchial openings of the upper, middle and lower lobes, accompanied by bronchiectasis (Fig. 1). Access for fiberoptic bronchoscopy was limited as new growth obstructed the bronchoscope lumen at all lobar bronchi. We performed forceps biopsy, brushing, and alveolar lavage at multiple bronchial openings in batches. Examination of the bronchoscopic brushing and lavage fluid revealed ciliated columnar and bronchial mucosal epithelium, respectively, but no signs of carcinoma. Pathological examination of the specimen revealed chronic inflammation of the mucosa, trabecular bone structure with calcification, fat, and neovascularization. Due to the limited amount of tissue obtained by forceps biopsy, the pathologic diagnosis only demonstrated a non-specific airway tumor. The specific diagnosis required further bronchoscopic pathological biopsy.
CT findings of multiple endobronchial masses. (A and B) Lung window and mediastinal chest CT scanning images showed an endobronchial mass (arrow) that obstructed the bronchi in the right upper lobe of the lung. (C) Mediastinal window showed a mass (arrow) that blocked the bronchi in the lower lobe of the right lung. (D) Mediastinal window showed obstruction (arrow) in the lower lobe of the left lung
To obtain definitive diagnosis and effective treatment, we scheduled bronchoscopic tumor resection and cryotherapy under general anesthesia again. The multiple bronchial openings were reluctantly selected for a liquid nitrogen cryobiopsy (Fig. 2). Then APC was used to ensure hemostasis.
Bronchoscopic images of some lesions. (A and B) Neoplasms in the bronchi of the left upper and lower lobes, with smooth surface and occluded lumen that obstructed the bronchoscope entry (black arrow indicates the site of the operation). (C and D) New growths in the openings of the right upper, middle, and lower lobes, that had a smooth surface and partially blocked the lumen
A relatively large piece of tissue taken from the bronchial opening of the upper lobe of the left lung can be seen roughly 0.6 cm × 0.4 cm × 0.3 cm grayish red with slightly hard cut texture. Microscopically, ciliated columnar epithelium covered the surface, and multifocal nodular tumor areas were seen in the fibrous tissue. The nodules were surrounded by semicircular and c-shaped trabecular bone. The tumor cells were mainly aggregated granulocytes, erythrocytes, and scattered multinucleated giant cells that were distributed among mature adipocytes (Fig. 3). Immunohistochemistry revealed a mix of megakaryocytic (CD61, FVIII, CD68, LCA, CD3, CD20, CD43) and granulocytic (MPO) origin. (Fig. 4). These results support the definite diagnosis of myelolipoma. Postoperatively, the patients experienced slight alleviation of the airway stenosis and the symptoms of infection were effectively controlled. Postoperative contrast-enhanced CT showed that the state of inflammation and atelectasis had subsided although they had not completely disappeared. Subsequently, the patient actively fought infection and controlled blood glucose, and the symptoms improved further. Although the patient’s condition was slightly reduced in terms of symptoms and examination results, multiple bronchial masses still existed, so the prognosis of the patient remains guarded.
Hematoxylin-eosin staining (HE staining) results. (A) Trabecular bone, semicircular and c-type enclosing tumor cells; magnification ×400. (B) Hematopoietic cells were seen in mature adipose tissue; magnification ×200. (C) Hematopoietic cells of erythrocyte, granulocyte, and megakaryocyte lineage; magnification ×400. (D) Trabeculae are seen in some areas; magnification ×400
Discussion
Myelolipomas were first reported in the early 20th century and were mainly related to the adrenal gland. Extra-adrenal myelolipomas are rare, and pulmonary myelolipomas are rarer. Thus far, only 18 cases of pulmonary myelolipoma have been reported at home and abroad, and we report the 19th case. Table 1 summarizes the clinicopathological features of all 19 cases: 14 (73.7%) patients were male and only 5 (26.3%) were female. The vast majority of patients were middle-aged or older adults, and the youngest patient was 29 years old. Most of the patients presented with pneumonia or other systemic diseases, and only one patient had a history of lung cancer. Pulmonary myelolipoma was often misdiagnosed because it can only be detected by surgical excision, pathological examination, and autopsy. Only two cases involved solely the bronchus, without invading the lung parenchyma, but unlike in our patient, both involved a single mass.
The etiology of myelolipoma is not fully understood, although several theories have been propounded, of which the most widely accepted one is that the myelolipoma may originate in embryonal mesenchymal cells of the adrenal gland. Under certain conditions (e.g., tissue injury, chronic infection, abnormal endocrine hormone levels, etc.), these interstitial cells can not only differentiate into bone marrow hematopoietic tissue but also undergo metaplasia into adipose tissue [16]. Patients with adrenal myelolipoma often have abnormal corticosteroid levels (with attendant obesity, hypertension, diabetes, etc.) or some syndromes that result in endocrine abnormalities (e.g., Conn syndrome, Cushing syndrome, etc.) [3,4,5], [17, 18]. Our patient had a history of type 2 diabetes with chronically poor glycemic control, which is aligned with the theory of hormonal disorders. However, the relationship between pulmonary myelolipoma and neuroendocrine dysregulation has not been reported previously. Therefore, we hypothesize that, similar to adrenal myelolipoma, endobronchial myelolipoma production may be influenced by steroids.
Histologically, myelolipoma consists of mature adipose tissue and hematopoietic tissues, including myeloid cells, erythrocytes, megakaryocytes, and, occasionally, lymphocytes. The presence of bone tissue in myelolipoma remains controversial. Our literature review revealed that trabecular or segmental bone was found in 8 of the 19 cases [10, 11, 13, 14]. A study found that a bony component of pulmonary myeloma may be related to the site of tumor occurrence, especially near the bronchial cartilage, as in our case [6].
Pathological diagnosis is the gold standard for the diagnosis of endobronchial myelolipoma, which is often similar to the pathological features of the following diseases and needs to be differentiated. (1) Extramedullary hematopoiesis: extramedullary hematopoiesis often occurs in the spleen, rarely in the lung, and often accompanied by hematological diseases [19, 20]. It is usually a diffuse and scattered small focus of bone marrow hematopoietic tissue with unclear boundary. Under the microscope, it is usually dominated by red blood cells without adipose tissue. (2) Lipoma: endobronchial lipoma is a rare benign lung tumor that can cause bronchial obstruction and parenchymal injury. A large amount of adipose tissue without hematopoietic tissue can be seen under the microscope. (3) Hamartoma: mainly occurs in the peripheral lung, < 10% occurs in the bronchus. Unlike myelolipoma, it is composed of a mixture of cartilage, fiber, fat, smooth muscle, and blood vessels without hematopoietic components [21]. (4) Ectopic bone marrow: the tissue often contains calcification and more medullary trabeculae, which is helpful to distinguish it from myelolipoma.
Benign endobronchial neoplasms are particularly rare clinical entities. Bronchoscopy is often used for palliative treatment of intrabronchial malignancies [22,23,24]. However, for several years, the use of bronchoscopy to remove benign lesions has been reported [25,26,27]. Cryotherapy or thermal ablation in the airway to treat airway diseases, including benign or malignant central airway obstruction (CAO), has become increasingly popular and is replacing traditional surgery. Common techniques include electrocautery, balloon expansion (BD), neodymium doped: yttrium aluminum garnet (Nd: YAG) laser, APC, and cryotherapy, which may usually be used in combination to confer greater effect [28].
We chose APC in combination with cryotherapy based on the location of the patient’s mass. APC is a non-contact technology that uses ionized argon (plasma) to apply a monopole current to the nearest tissue with a penetration depth of less than 3 mm. APC is very safe and is mainly used to treat superficial flat lesions [29, 30]. As argon can flow over angles and into corners, APC is suitable for treating acute-angled bronchial segments (e.g., the apical and posterior segments of the upper lobe). However, the disadvantages of APC are obvious: eschar forms easily and needs to be removed with biopsy forceps. APC, in combination with cryotherapy, enables rapid removal of intrabronchial masses, shortens the operative time, and decreases the risk of major bleeding [31].
Conclusion
We diagnosed and treated a patient multiple bronchial myelolipoma that did not involve the lung parenchyma. Tissue sections showed bony tissue, bone trabeculae, and ossification. The patient had slight symptom improvement with bronchial interventional therapy, APC, and cryotherapy. However, the relationship between pulmonary myelolipoma and neuroendocrinology has not been clearly explained, and deserves further study.
Data Availability
All data and materials are provided in the manuscript.
Abbreviations
- QN:
-
Once a night
- TID:
-
three times a day
- WBC:
-
White blood cell
- T-spoT test:
-
T cell spot test of tuberculosis infection
- CT:
-
Computerized tomography
- LCA:
-
leukocyte common antigen
- MPO:
-
myeloperoxidase
- HE staining:
-
hematoxylin-eosin staining
- FVIII:
-
Factor VIII-related antigen
References
Beiko D, Roldan H, SenGupta SK, George RL. Laparoscopic excision of a large extra-adrenal perirenal myelolipoma. Can Urol Association J. 2010;4(2):E39.
DANESHMAND S. MARCUS L. Adrenal myelolipoma: diagnosis and management. Urol J 2006.
Shi Q, Pan S, Bao Y, Fan H, Diao Y. Primary mediastinal myelolipoma: a case report and literature review. J Thorac Disease. 2017;9(3):E219.
Lin F, Pu Q, Ma L, Liu C, Mei J, Liao H, et al. Surgical treatment of primary mediastinal myelolipoma. Interact Cardiovasc Thorac Surg. 2015;21(2):206–10.
Roger BR, Dhali A, Ramesh RS, Dsouza C. Giant bilateral adrenal myelolipoma: case report. Indian J Endocrinol Metabol. 2020;24(6):551.
Chuang S-S, Liu H, Ye H, Martín-Subero JI, Siebert R, Huang W-T. Pulmonary mucosa-associated lymphoid tissue lymphoma with strong nuclear B-cell CLL/lymphoma 10 (BCL10) expression and novel translocation t (1; 2)(p22; p12)/immunoglobulin κ chain-BCL10. J Clin Pathol. 2007;60(6):727–8.
Wood WG, Restivo TE, Axelsson KL, Svahn JD. Myelolipoma in the spleen: a rare discovery of extra-adrenal hematopoietic tissue. J Surg Case Rep. 2013;2013(3):rjt007.
Li K-Y, Wei A-L, Li A. Primary hepatic myelolipoma: a case report and review of the literature. World J Clin Cases. 2020;8(19):4615.
Huang W, Zhao Y, Yin X, Qi Q. Primary myelolipoma of the lung: a case of report and review of literature. Pol J Pathol. 2012;63(3):204–6.
Huang W-t, Zhao S-j, Lin D-M. Pulmonary-bronchus myelolipoma and review on extra-adrenal myelolipomas in chinese literature. Chin Med J. 2012;125(17):3188–90.
Mašić S, Vučić M, Seiwerth S. Pulmonary myelolipoma containing osseous tissue: an unexpected finding at autopsy. Respiratory Med Case Rep. 2017;22:254–6.
Chung HS, Lee KM, Eom JS, Kim I, Park S, Ahn J, et al. Bronchoscopic management of solitary bronchial myelolipoma: a case report. BMC Pulm Med. 2019;19(1):1–5.
Lu X, Xiao L. Myelolipoma of the lung: a case report. Chin Med J. 2003;116(06):951–3.
Sabate CJ, Shahian DM. Pulmonary myelolipoma. Ann Thorac Surg. 2002;74(2):573–5.
Xu Q, Yin X, Huang W, Sun J, Wu X, Lu L. Intrapulmonary myelolipoma and its computed tomography features: a case report and literature review. Oncol Lett. 2015;9(4):1677–80.
Shen C, Han Z, Che G. A bilateral neoplasm in chest: a case report and literature review. BMC Surg. 2014;14(1):1–6.
Surrey LF, Thaker AA, Zhang PJ, Karakousis G, Feldman MD. Ectopic functioning adrenocortical oncocytic adenoma (oncocytoma) with myelolipoma causing virilization. Case Reports in Pathology 2012;2012.
Arshad MF, Kaluarachchi VT, Ross R, Balasubramanian SP. Development of a large adrenal myelolipoma in the context of long-term elevated ACTH. BMJ Case Reports CP. 2021;14(1):e240493.
Hosseinzadeh M, Omidifar N, Kumar PV, Rasekhi A. Pulmonary extramedullary hematopoiesis in a patient with chronic asthma resembling Lung Cancer: a Case Report. Case Rep Med. 2012;2012:1–3.
Mubarak V, Fanning S, Windsor M, Duhig E, Bowler S. Pulmonary extramedullary haematopoiesis. Case Reports 2011;2011(dec02 1):bcr0620114392–2.
Mertoglu A, Tellioglu E, Yucel N. Multiple endobronchial hamartoma. Clin Respir J. 2017;11(2):263–6.
Xie X, Chen Y, Ding C, Yu X, Zou L, Xu B, et al. Primary pulmonary leiomyosarcoma: a case report. Oncol Lett. 2016;11(3):1807–10.
Huang Y-K, Liu Y-H, Ko P-J, Wu Y-C, Liu H-P. Successful treatment of tracheal mucoepidermoid carcinoma using rigid bronchoscopy combined with conventional surgical resection. Chang Gung Med J. 2003;26(7):530–4.
Park CW, Kim W, Oh IJ, Kim KS, Choi YD, Kwon YS. Solitary extramedullary plasmacytoma presenting as an endobronchial mass. Intern Med. 2013;52(18):2113–6.
Ozgul MA, Toru U, Acat M, Ozgul G, Cetinkaya E, Dincer HE et al. A RARE TUMOUR OF TRACHEA: INFLAMMATORY MYOFIBROBLASTIC TUMOUR DIAGNOSIS AND ENDOSCOPIC TREATMENT. 2014.
Wu Z, Wan H, Shi M, Li M, Wang Z, Yang C, et al. Bronchoscopic resection of bronchial angiolipoma: a rare case report. Mol Clin Oncol. 2016;5(6):850–2.
Kim J-H, Cho S-H, Kim E-K, Lee J-H, Jeong H-C. Endobronchial malignant fibrous histiocytoma: case report of an unusual presentation and palliative flexible bronchoscopic resection. Respir Care. 2013;58(8):e92–4.
Sachdeva A, Pickering EM, Lee HJ. From electrocautery, balloon dilatation, neodymium-doped: yttrium-aluminum-garnet (nd: YAG) laser to argon plasma coagulation and cryotherapy. J Thorac Disease. 2015;7(Suppl 4):363.
Rosell A, Stratakos G. Therapeutic bronchoscopy for central airway diseases. Eur Respiratory Rev 2020;29(158).
Tremblay A, Marquette C-H. Endobronchial electrocautery and argon plasma coagulation: a practical approach. Can Respir J. 2004;11(4):305–10.
Gao H, Ding X, Wei D, Cheng P, Su X, Liu H, et al. Endoscopic management of benign tracheobronchial tumors. J Thorac Disease. 2011;3(4):255.
Acknowledgements
The authors thank the patient’s family who agreed to publish this case report. The authors thank all anonymous reviewers and editors for their constructive suggestions to improve the quality of this manuscript.
Funding
This work was supported by the Wuxi Municipal Health Commission [MS201937, T201937].
Author information
Authors and Affiliations
Contributions
Jiali Ji and Hongqin Zhong drafted the manuscript and assisted with the clinical data collection and interpretation. Xian Ren and Ting He contributed to the physical examination and data analysis. Guijuan Xie contributed to pathological examination and diagnoses. Xun Wang performed surgery and participated in revising the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethical approval to report this case was not required due to its retrospective nature.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, J., Zhong, H., Ren, X. et al. Bronchoscopic treatment of multiple bronchial myelolipomas: a case report and literature review. BMC Pulm Med 23, 317 (2023). https://doi.org/10.1186/s12890-023-02608-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02608-z