Abstract
Background
Pulmonary arterial hypertension (PAH) is a severe complication of mixed connective tissue disease (MCTD) and contributes to increased morbidity and mortality. Still, the demographic characteristics and risk factors of PAH in MCTD remain poorly understood. This study explored risk factors for PAH development in MCTD.
Methods
Data from patients with MCTD and PAH hospitalized from May 2009 to December 2022 in a single center were collected and compared with patients with MCTD without PAH. The variables were analyzed by logistic regression to identify the factors associated with PAH in patients with MCTD. The receiver-operating characteristic (ROC) curve was used to assess the diagnostic value of the identified factors.
Results
Finally, 119 patients with MCTD were included; 46 had PAH. The mean age at PAH onset and diagnosis was 38.9 ± 13.4 and 39.9 ± 13.7 years, respectively. The median pulmonary arterial systolic pressure (PASP) was 67.0 mmHg. The median brain natriuretic peptide (BNP) level was 180.0 pg/ml at PAH diagnosis. Red cell distribution width (RDW) (OR: 2.128; 95% confidence interval: 1.497–3.026; P < 0.001) was associated with PAH in patients with MCTD. There was a positive correlation between RDW and PASP (r = 0.716, P < 0.001). At a cutoff of 15.2%, RDW had the best sensitivity (80.4%) and specificity (82.2%) for PAH.
Conclusion
RDW may serve as a sensitive index to predict PAH in patients with MCTD.
Similar content being viewed by others
Background
Mixed connective tissue disease (MCTD) is one of the most complicated autoimmune diseases and involves various overlapping symptoms [1]. It is characterized by diverse clinical manifestations of systemic sclerosis (SSc), systemic lupus erythematosus (SLE), polymyositis or dermatomyositis (PM/DM), and rheumatoid arthritis (RA), which can also affect multiple organs, including the pulmonary system [2]. Pulmonary involvements, especially pulmonary arterial hypertension (PAH), are devastating complications among patients with MCTD, resulting in increased morbidity and mortality [3,4,5]. The prevalence of PAH is 2-24% in patients with MCTD, depending on the measurement methods used [3]. So far, little knowledge is available about the clinical characteristics of PAH patients associated with the MCTD population. The present study aimed to explore the factors associated with PAH in patients with MCTD and determine the diagnostic value of the identified factors.
Methods
Patients and diagnostic criteria
Patients with MCTD hospitalized in the rheumatology and immunology department of the affiliated Drum Tower Hospital of Nanjing University Medical School between May 2009 and December 2022 were included in this study. The exclusion criteria were (a) < 18 years of age, (b) complicated with other connective tissue diseases (CTD) such as SSc, SLE, or primary Sjögren’s syndrome (pSS), (c) PAH caused by cardiac structural lesions, such as left heart disease or valvular heart disease, (d) with severe pulmonary diseases resulted from lung cancer or chronic obstructive pulmonary disease, or (e) PAH due to liver cirrhosis, pulmonary venous occlusive disease, or chronic thromboembolism. All patients met the established MCTD criteria [6].
PAH was defined according to the 2022 ESC/ERS guidelines, using tricuspid regurgitation velocity (TRV) > 2.8 m/s [7]. The initial diagnosis or screening of PAH was established based on transthoracic echocardiography (TTE)-estimated resting pulmonary arterial systolic pressure (PASP) ≥ 36 mmHg [7], while those with PASP < 36 mmHg and without any other echocardiographic indicators suggesting PAH were included in the MCTD-non-PAH group. Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Nanjing Drum Tower Hospital (No.2020-093-01).
Data collection
Demographic data, including sex, age at MCTD onset, and disease duration, height, weight, body mass index (BMI), body surface area (BSA), and disease activity were collected. In addition, age at PAH onset and diagnosis was also included for patients with PAH, in which the former was defined as the time when the initial symptoms appeared, while the latter was when TTE examination showed abnormalities. The nonspecific symptoms were recorded, such as Raynaud’s phenomenon, sclerodactyly, serositis, limb edema, fatigue, arthralgia, and chest tightness. The World Health Organization functional classification (WHO-FC) was independently evaluated by two authors (YJ and BJ) for each patient with PAH.
TTE was performed using a Philips IE Elite machine. The included patients were all examined by TTE to evaluate the likelihood of PAH. The peak PASP was calculated using the simplified Bernoulli equation (PASP = 4 tricuspid regurgitation2 + right atrial pressure) [8]. The other parameters based on TTE were included. Autoantibodies were routinely tested. Other laboratory data, including complete blood count, uric acid (UA), blood lipid analysis, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), thyroid function test, complement, immunoglobulin, blood coagulation function test, and brain natriuretic peptide (BNP) levels were also documented. The hemoglobin and mean corpuscular volume (MCV) were used to evaluate the iron deficiency. The Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2 K) was used to evaluate disease activity [9].
Statistical analysis
Analyses were done using SPSS 19.0 (IBM, Armonk, NY, USA) and MedCalc 19.1 (MedCalc, Ostend, Belgium). The variables were presented as means ± standard deviations ± or medians with interquartile ranges. Student’s t-test or Mann-Whitney U-test was used for comparisons. The categorical variables were presented as n (%) and analyzed using the chi-square test. The correlations between two continuous variables were determined using Spearman’s correlation coefficient. Factors associated with PAH in the univariable logistic regression analyses were evaluated using multivariable logistic regression analysis. The receiver operating characteristic (ROC) curve with the area under the curves (AUC) was plotted to investigate the diagnostic value of the factor. The optimal cutoff was assessed using Youden’s index in ROC analysis. Survival was analyzed using the Kaplan-Meier method and the log-rank test. P < 0.05 was considered statistically significant.
Results
Characteristics of the patients
This study included 119 patients with MCTD, among whom 46 were diagnosed with PAH (MCTD-PAH), and 73 did not have PAH (MCTD-non-PAH). The demographics of the patients are shown in Table 1. The female-to-male ratio in the MCTD-PAH and MCTD-non-PAH groups were 44:2 and 69:4, respectively (P > 0.05). The mean age at MCTD diagnosis in the MCTD-PAH and MCTD-non-PAH groups was 37.8 ± 13.4 years vs. 39.8 ± 12.2 years (P > 0.05), while the mean age at MCTD onset in MCTD-PAH and MCTD-non-PAH groups were 35.1 ± 12.8 years vs. 36.5 ± 11.8 years (P > 0.05). The mean age at PAH onset was 38.9 ± 13.4 years, while PAH diagnosis was 39.9 ± 13.7 years, respectively. Speculatively, PAH diagnosis according to TTE was found to be 1.0 ± 1.6 years’ delayed from PAH onset. The height, weight, BMI, and BSA were comparable between two groups (all P > 0.05). Six, 33, and seven patients had low-risk, middle-risk, and high-risk PAH. They were 36.7 ± 11.9, 46.4 ± 12.9, and 42.4 ± 14.5 years of age, respectively, and there were six, 31, and seven females, respectively.
As for the symptoms (Table 1), serositis (63.0% vs. 27.4%, P < 0.001) and chest tightness (76.1% vs. 32.9%, P < 0.001) in the MCTD-PAH group were significantly more common than in the MCTD-non-PAH group. Raynaud’s phenomenon, sclerodactyly, limb edema, fatigue, disease activity and arthralgia showed no differences between the two groups.
Laboratory findings
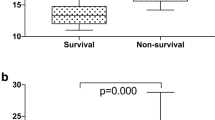
Compared with the MCTD-non-PAH group, the patients in the MCTD-PAH group displayed larger red cell distribution width (RDW) [(18.2 ± 3.4) % vs. (13.8 ± 1.7) %, P < 0.001] and higher UA levels [(320.5 ± 132.1) vs. (270.8 ± 97.9) µmol/L, P < 0.05] (Table 1). There were no significant differences in hemoglobin and MCV between the two groups (both P > 0.05). Interestingly, serum free triiodothyronine (FT3) was significantly decreased in MCTD-PAH patients [(3.0 ± 1.2) vs. (3.5 ± 1.0) pmol/L, P < 0.01], while thyroid stimulating hormone (TSH) was significantly higher in the MCTD-PAH group [2.6 (1.1–4.9) vs. 1.3 (0.8–2.7) mU/L, P < 0.05]. The autoantibody was not different between the MCTD-PAH and MCTD-non-PAH groups (Table 1). The other laboratory test values were comparable between the two groups (P > 0.05).
Echocardiographic findings
The echocardiagram findings are summarized in Table 2. Compared with the MCTD-non-PAH group, the MCTD-PAH group had significantly lower left ventricular diastolic dimension (LVDd) [(4.5 ± 0.5) vs. (4.7 ± 0.4) cm, P < 0.01], pulmonary artery velocity (PA) [(0.8 ± 0.1) vs. (0.9 ± 0.2) m/s, P < 0.05], left atrial diameter (LAD) [(3.5 ± 0.7) vs. (4.4 ± 0.6) cm, P < 0.05], and left ventricle ejection fraction (LVEF) [59.0 (58.0-60.3)% vs. 61.0 (60.0-61.5)%, P < 0.01], respectively. Besides, TTE confirmed pericardial effusion in 37.0% of the MCTD-PAH patients and 32.6% of the MCTD-non-PAH patients (P < 0.05). Other indexes, including interventricular septum thickness diastolic (IVSTd), left ventricular posterior wall thickness at end-diastole (LVPWTd), aorta diameter (AoD), left ventricular end-systolic dimension (LVDs), and mitral e peak/mitral a peak (E/A), were similar between the two groups (P > 0.05).
As shown in Table 2, many patients with PAH had severe cardiac involvement at PAH diagnosis, with a median PASP of 67.0 (interquartile range 48.5–90.0) mmHg and a median level of BNP of 180.0 (interquartile range 63.8-478.5) pg/mL. The BNP levels were higher in the MCTD-PAH group compared with the MCTD-non-PAH group (median, 180.0 vs. 57.3 pg/mL, P < 0.001). The values of PASP were not routinely tested in the MCTD-non-PAH group, and the data could not be compared. When PAH was diagnosed, nearly half (45.7%) of patients were in WHO Fc III/IV. Meanwhile, only one (1.4%) patient in the MCTD-non-PAH group reached WHO Fc III/IV.
Factors associated with PAH
Univariable and multivariable binary logistic regression analyses were performed to evaluate the clinical, laboratory, and echocardiographic factors independently associated with PAH. As shown in Table 3, only RDW (OR: 2.128; 95% confidence interval: 1.497–3.026; P < 0.001) was significant. Consistently, there was a positive correlation between RDW and PASP (r = 0.716, P < 0.001) by Spearman correlations analysis (Fig. 1).
Next, we analyzed the ROC curve of the diagnostic accuracy for RDW (Fig. 2). Using 15.2% as a cutoff level, the RDW value had 80.4% sensitivity and 82.2% specificity for PAH diagnosis. The AUC estimate for RDW was 0.9 (P < 0.001), with a Youden index of 0.6.
Survival
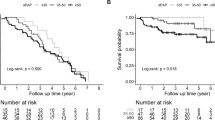
Nineteen out of 119 patients (16.0%) died during a mean follow-up period of 80 ± 38 months. Eight patients were lost to follow-up. One patient committed suicide, and one died in a car accident. The survival rate was higher in the MCTD-non-PAH group compared with the MCTD-PAH group (log-rank P < 0.001) (Supplementary Figure S1). Elevated RDW values were associated with an increased risk of all-cause death. Fourteen out of 46 patients (30.4%) died during a mean follow-up period of 68 ± 40 months. Two patients were lost to follow-up. One patient committed suicide. According to the Kaplan-Meier survival analysis, a significant difference was found between the two groups(patients with RDW > 15.2% and patients with RDW ≤ 15.2%) in terms of survival rate (log-rank P = 0.049) (Supplementary Figure S2).
Discussion
CTD-PAH constitutes the second most common subtype of PAH [10], most commonly associated with SSc, SLE, and MCTD [11]. While SSc and SLE are the leading cause of CTD-PAH [12], little is known about MCTD-PAH, despite reports showing that MCTD-PAH was more frequent than SSc and SLE. In this study, PAH diagnosis according to TTE was 1.0 ± 1.6 years’ delayed from PAH onset, accompanied by a significantly higher percentage of WHO Fc III or IV in the MCTD-PAH group compared with the controls (45.7% vs. 1.4%). RDW was the only factor significantly associated with the development of PAH in MCTD patients through multivariable binary logistic regression analysis. Consistent with the literature [13], sex, age, and disease duration were unrelated to PAH.
The first line of diagnostic testing for patients with suspected PAH involves undergoing TTE. Previous reports showed that noninvasive echocardiography had high specificity and sensitivity in detecting PAH when above 36 mmHg [14]. The median PASP by echocardiography of the MCTD-PAH patients was 67.0 mmHg, greatly higher than the diagnostic critical value.
On the other hand, significantly higher pericardial effusion values and lower indexes of LVDd, PA, LAD, and LVEF were also observed in MCTD-PAH patients. Thus, it is likely that the pathophysiology of PAH involves remodeling not only the right heart but also the left heart. In addition, in the present study, 17 of 46 patients (37.0%) had pericardial effusion by echocardiography. The literature suggests that pericardial effusion relates to RV failure and immunologically mediated inflammatory conditions [15]. Pericardial effusion is an increasingly recognized risk factor of PAH related to CTD [16].
Several biomarkers have been recommended to assess the presence and prognosis of PAH [17]. Nagaya et al. reported that PAH patients with BNP > 150 pg/mL at baseline and > 180 pg/mL after therapy had a poor prognosis [18]. The median plasma levels of BNP in the MCTD-PAH group was 180.0 pg/mL, suggesting these patients were in poor condition. UA [19], thyroid dysfunction [20], and the presence of specific autoantibodies [21] were also proposed to be related to PAH. However, the risk of both UA and thyroid dysfunction has not been confirmed in this study, and autoantibody profiles were not significantly different between the two groups.
RDW is an index of blood routine [22], which has been suggested as a biomarker of PAH in patients with other CTD [23, 24]. To our knowledge, this is the first study to identify the importance of RDW in PAH related to MCTD. The sensitivity and specificity of RDW value > 15.2% were 80.4% and 82.2%, respectively, for the prediction of MCTD-PAH. Given its positive correlation with the PASP, RDW may significantly affect the progression of PAH associated with MCTD. Recently, raised RDW values are related to the mortality of PAH and proposed as a prognostic biomarker of poor prognosis in PAH with various etiologies [23, 25], which was even superior to pro-BNP [26]. Xi et al. [27] suggested that RDW independently predicted the responsiveness of acute pulmonary vasodilator testing in idiopathic PAH patients.
The exact pathologic mechanism linking RDW to PAH is still unclear but may be related to vascular injury [28]. Routinely, the most common cause of an increased RDW is anemia related to ineffective erythropoiesis [29]. In the present study, all patients had normal hemoglobin levels with a normal range of MCV in the whole group. There was no iron deficiency; as such, the influence of iron was excluded. It was hypothesized that RDW was involved in inflammation [30]. The elevation of RDW level could reflect the circulating levels of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)- 1, and IL-6, thus leading to the occurrence of PAH [23, 31, 32]. Further studies focusing on mechanisms correlated with RDW and MCTD-PAH are necessary.
FT3 was significantly decreased in MCTD-PAH patients, while TSH was significantly higher in the MCTD-PAH group. The literature suggests that thyroid dysfunction is associated with PAH [33]. Indeed, thyroid hormone impacts cardiac contractility, cardiac output, and pulmonary and systemic vascular resistance [20, 34], and up to 20% of patients with PAH also have thyroid disease [35]. The present study report reinforces the important observation that TSH is associated with PAH, and a future study of thyroid dysfunction as a potential remediable contributor to mortality in PAH is warranted.
Limitation of the study
A major limitation of this study is not routinely applying the gold standard test for PAH-right-sided heart catheterization (RHC). As an invasive and expensive operation, RHC was not common consent by most patients. Some patients in the present study were too serious to lie flat. The retrospective nature of the study limited the data to those available in the patient charts.
Conclusion
MCTD patients with elevated RDW had a high risk for PAH. RDW can be used as a simple index for monitoring PAH in clinical practice.
Data Availability
The datasets used and/or analysed during the current study available from the corresponding author Bo Jiang on reasonable request.
Abbreviations
- PAH:
-
pulmonary arterial hypertension
- MCTD:
-
mixed connective tissue disease
- ROC:
-
receiver operating characteristic
- PASP:
-
pulmonary arterial systolic pressure
- BNP:
-
brain natriuretic peptide
- RDW:
-
red cell distribution width
- SSc:
-
systemic sclerosis
- SLE:
-
systemic lupus erythematosus
- PM/DM:
-
polymyositis or dermatomyositis
- RA:
-
rheumatoid arthritis
- CTD:
-
connective tissue disease
- TRV:
-
tricuspid regurgitation velocity
- pSS:
-
primary Sjögren’s syndrome
- TTE:
-
transthoracic echocardiography
- BMI:
-
body mass index
- BSA:
-
body surface area
- WHO-FC:
-
World Health Organization functional classification
- UA:
-
uric acid
- CRP:
-
C-reactive protein
- ESR:
-
erythrocyte sedimentation rate
- MCV:
-
mean corpuscular volume SLEDAI-2 K:Systemic Lupus Erythematosus Disease Activity Index 2000
- AUC:
-
area under the curves
- FT3:
-
serum free triiodothyronine
- TSH:
-
thyroid stimulating hormone
- LVDd:
-
left ventricular diastolic dimension
- PA:
-
pulmonary artery
- LAD:
-
left atrial diameter
- LVEF:
-
left ventricle ejection fraction
- IVSTd:
-
interventricular septum thickness diastolic
- LVPWTd:
-
left ventricular posterior wall thickness at end-diastole
- AoD:
-
aorta diameter
- LVDs:
-
left ventricular end-systolic dimension
- E/A:
-
mitral e peak/mitral a peak
- TNF:
-
tumor necrosis factor
- IL:
-
interleukin
- RHC:
-
right-sided heart catheterization
References
Ciang NC, Pereira N, Isenberg DA. Mixed connective tissue disease-enigma variations? Rheumatology (Oxford). 2017;56:326–33.
Perelas A, Arrossi AV, Highland KB. Pulmonary manifestations of systemic sclerosis and mixed connective tissue disease. Clin Chest Med. 2019;40:501–18.
Niklas K, Niklas A, Mularek-Kubzdela T, Puszczewicz M. Prevalence of pulmonary hypertension in patients with systemic sclerosis and mixed connective tissue disease. Med (Baltim). 2018;97:e11437.
Skride A, Sablinskis K, Avidan Y, Rudzitis A, Lejnieks A. Pulmonary arterial hypertension associated with connective tissue disease: insights from latvian PAH registry. Eur J Intern Med. 2017;40:e13–4.
Liu J, Wang W, Wang L, Qi XM, Sha YH, Yang T. 3-Bromopyruvate alleviates the development of monocrotaline-induced rat pulmonary arterial hypertension by decreasing aerobic glycolysis, inducing apoptosis, and suppressing inflammation. Chin Med J (Engl). 2020;133:49–60.
Kasukawa R, Kanno T, Takeda I. [Mixed connective tissue disease]. Ryoikibetsu Shokogun Shirizu 2000:396–9.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–731.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of Pulmonary Hypertension. Rev Esp Cardiol (Engl Ed). 2016;69:177.
Reiseter S, Gunnarsson R, Corander J, Haydon J, Lund MB, Aalokken TM, Taraldsrud E, Hetlevik SO, Molberg O. Disease evolution in mixed connective tissue disease: results from a long-term nationwide prospective cohort study. Arthritis Res Ther. 2017;19:284.
Yang X, Mardekian J, Sanders KN, Mychaskiw MA, Thomas J. 3rd: prevalence of pulmonary arterial hypertension in patients with connective tissue diseases: a systematic review of the literature. Clin Rheumatol. 2013;32:1519–31.
Gaine S, Chin K, Coghlan G, Channick R, Di Scala L, Galiè N, Ghofrani HA, Lang IM, McLaughlin V, Preiss R, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. 2017;50:1602493.
Liu Z, Yang X, Tian Z, Qian J, Wang Q, Zhao J, Huang C, Liu Y, Guo X, Wang H, et al. The prognosis of pulmonary arterial hypertension associated with primary Sjögren’s syndrome: a cohort study. Lupus. 2018;27:1072–80.
Iudici M, Codullo V, Giuggioli D, Riccieri V, Cuomo G, Breda S, Manfredi A, Iannace N, D’Alto M, Ghio S, et al. Pulmonary hypertension in systemic sclerosis: prevalence, incidence and predictive factors in a large multicentric italian cohort. Clin Exp Rheumatol. 2013;31:31–6.
Greiner S, Jud A, Aurich M, Hess A, Hilbel T, Hardt S, Katus HA, Mereles D. Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc. 2014;3:e001103.
Zhang N, Zhao Y, Wang H, Sun W, Chen M, Fan Q, Yang Z, Wei W. Characteristics and risk factors for pulmonary arterial hypertension associated with primary Sjögren’s syndrome: 15 new cases from a single center. Int J Rheum Dis. 2019;22:1775–81.
Huang C, Li M, Liu Y, Wang Q, Guo X, Zhao J, Lai J, Tian Z, Zhao Y, Zeng X. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic Lupus Erythematosus Patients. Med (Baltim). 2016;95:e2761.
Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: what do we know? Chest. 2013;144:274–83.
Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–70.
Voelkel MA, Wynne KM, Badesch DB, Groves BM, Voelkel NF. Hyperuricemia in severe pulmonary hypertension. Chest. 2000;117:19–24.
Marvisi M, Balzarini L, Mancini C, Mouzakiti P. Thyroid gland and pulmonary hypertension. What’s the link? Panminerva Med. 2013;55:93–7.
Sobanski V, Giovannelli J, Lynch BM, Schreiber BE, Nihtyanova SI, Harvey J, Handler CE, Denton CP, Coghlan JG. Characteristics and survival of Anti-U1 RNP antibody-positive patients with connective tissue Disease-Associated Pulmonary arterial hypertension. Arthritis Rheumatol. 2016;68:484–93.
Ju XF, Wang F, Wang L, Wu X, Jiang TT, You DL, Yang BH, Xia JJ, Hu SY. Dynamic change of red cell distribution width levels in prediction of Hospital Mortality in Chinese Elderly patients with septic shock. Chin Med J (Engl). 2017;130:1189–95.
Bellan M, Giubertoni A, Piccinino C, Dimagli A, Grimoldi F, Sguazzotti M, Burlone ME, Smirne C, Sola D, Marino P, et al. Red cell distribution width and platelet count as biomarkers of pulmonary arterial hypertension in patients with connective tissue Disorders. Dis Markers. 2019;2019:4981982.
Hui M, Zhao J, Tian Z, Wang J, Qian J, Yang X, Wang Q, Li M, Zhao Y, Zeng X. Red blood cell distribution width as a potential predictor of survival of pulmonary arterial hypertension associated with primary Sjogren’s syndrome: a retrospective cohort study. Clin Rheumatol. 2019;38:477–85.
Smukowska-Gorynia A, Tomaszewska I, Malaczynska-Rajpold K, Marcinkowska J, Komosa A, Janus M, Olasinska-Wisniewska A, Slawek S, Araszkiewicz A, Jankiewicz S, Mularek-Kubzdela T. Red blood cells distribution width as a potential prognostic biomarker in patients with Pulmonary arterial hypertension and chronic Thromboembolic Pulmonary Hypertension. Heart Lung Circ. 2018;27:842–8.
Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868–72.
Xi Q, Liu Z, Zhao Z, Luo Q. Red blood cell distribution width predicts responsiveness of acute pulmonary vasodilator testing in patients with idiopathic pulmonary arterial hypertension. Clin Chim Acta. 2015;446:272–6.
Xia YK, Tu SH, Hu YH, Wang Y, Chen Z, Day HT, Ross K. Pulmonary hypertension in systemic lupus erythematosus: a systematic review and analysis of 642 cases in chinese population. Rheumatol Int. 2013;33:1211–7.
Zuk M, Migdal A, Dominczak J, Brzezinska-Rajszys G. Usefulness of Red Cell Width distribution (RDW) in the Assessment of Children with Pulmonary arterial hypertension (PAH). Pediatr Cardiol. 2019;40:820–6.
Ozsu S, Abul Y, Gulsoy A, Bulbul Y, Yaman S, Ozlu T. Red cell distribution width in patients with obstructive sleep apnea syndrome. Lung. 2012;190:319–26.
Inuzuka R, Abe J. Red blood cell distribution width as a link between ineffective erythropoiesis and chronic inflammation in heart failure. Circ J. 2015;79:974–5.
Yan S, Li M, Wang H, Yang X, Zhao J, Wang Q, Liu Y, Lai J, Tian Z, Song H, et al. Characteristics and risk factors of pulmonary arterial hypertension in patients with primary Sjögren’s syndrome. Int J Rheum Dis. 2018;21:1068–75.
Pi H, Rayner SG, Ralph DD, Nolley S, Barros LM, Steinberg ZL, Leary PJ. Thyroid-stimulating hormone and mortality in pulmonary arterial hypertension. BMJ Open Respir Res. 2022;9:e001348.
Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–9.
Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart failure. Circulation. 2020;141:678–93.
Acknowledgements
Not applicable.
Funding
This work was supported by Clinical Research Special Fund of Nanjing Drum Tower Hospital (2022-LCYJ-MS-39 to B.J.) and Gusu Talent Project (GSWS 2022113 to Y.J.).
Author information
Authors and Affiliations
Contributions
YS J and GJ G performed a literature search, analyzed data, and wrote the manuscript. C W performed a literature search and analyzed data. B J designed the study, analyzed data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Nanjing Drum Tower Hospital (No.2020-093-01). Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jin, Y., Guo, G., Wang, C. et al. Association of red cell distribution width with pulmonary arterial hypertension in patients with mixed connective tissue disease. BMC Pulm Med 23, 299 (2023). https://doi.org/10.1186/s12890-023-02597-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02597-z