Abstract
Background
Frailty has been increasingly identified as a risk factor of adverse outcomes in chronic obstructive pulmonary disease (COPD). The prevalence and impact of frailty on health outcomes in people with COPD require clarification.
Methods
PubMed, Embase, The Cochrane Library and Web of Science (January 1, 2002, to July 1, 2022) were comprehensively searched to identify studies related to frailty and COPD. Comparisons were made between people who did and did not have frailty for pulmonary function, dyspnea severity, 6-minute walking distance, activities of daily life, and mortality.
Results
Twenty studies (9 cross-sectional, 10 cohort studies,1 clinical trial) from Europe (9), Asia (6), and North and South America (4), Oceania (1) involving 11, 620 participants were included. The prevalence of frailty was 32.07% (95% confidence interval (CI) 26.64–37.49) with a range of 6.43–71.70% based on the frailty tool used. People with frailty had lower predicted forced expiratory volume in the first second (mean difference − 5.06%; 95%CI -6.70 to -3.42%), shorter 6-minute walking distance (mean difference − 90.23 m; 95%CI -124.70 to -55.76), poorer activities of daily life (standardized mean difference − 0.99; 95%CI -1.35 to -0.62), higher CAT(COPD Assessment Test) score(mean difference 6.2; 95%CI 4.43 to 7.96) and mMRC (modified Medical Research Council) grade (mean difference 0.93; 95%CI 0.85 to 1.02) compared with those who did not (P < 0.001 for all). Meta-analysis showed that frailty was associated with an increased risk of long-term all-cause mortality (HR 1.68; 95% CI 1.37–2.05; I2 = 0%, P < 0.001).
Conclusion
Frailty is prevalent in people with COPD and linked with negative clinical outcomes including pulmonary function, dyspnea severity, exercise capacity, quality of life and mortality.
Similar content being viewed by others
Introduction
Frailty is a complex geriatric syndrome characterized by a decline in physiological capacity across several organ systems, accompanied by an increased risk of adverse outcomes including falls, delirium, disability, hospitalization, and mortality in older adults [1]. The Fried frailty phenotype(FFP) [2], clinical frailty scale (CFS) [3], the Hospital Frailty Risk Score (HFRS) [4] and the frailty index are usually used to evaluate the frailty of older persons.
Frailty can predict the negative prognosis of several chronic diseases, such as chronic kidney disease [5], lower extremity peripheral artery disease [6], atrial fibrillation [7] and heart failure [8]. However, the relationship between frailty and chronic obstructive pulmonary disease (COPD) are needed to further clarify. Frailty is common in individuals with COPD. Patients with COPD appear to have an increased risk of presenting frailty. Marengoni et al. [9] found that the pooled prevalence of frailty in individuals with COPD was 19% and patients with COPD had two-fold increased risk of frailty comparing those without COPD. Previous studies indicated that frailty appears to have a negative impact upon clinical outcomes related to function and health [10,11,12,13]. Frailty was associated with longer-duration hospitalization, poorer quality of life and higher risk of readmission in patients with COPD [10, 14], but the real clinical impact has not yet been explicitly quantified. Significantly, frailty status in older adults can be improved by the targeted interventions [15]. Further understanding of the relationship between the frailty and COPD may guide the comprehensive management of patients with COPD. In this study, therefore, we aim to conduct a systematic review with meta-analysis to quantify the impacts of frailty upon health outcomes.
Methods
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines 2020 [16]. The protocol for this review was registered in PROSPERO(CRD42022369111).
Search strategy and inclusion and exclusion criteria
PubMed, Embase, The Cochrane Library and Web of Science were searched for studies using the following free-text and subject heading terms: ‘Pulmonary Disease’, ‘Chronic Obstructive Bronchitis’, ‘chronic obstructive pulmonary disease’, ‘COPD’, ‘Chronic Obstructive Airway Disease’, ‘Chronic Obstructive Lung Disease’, ‘emphysema’, ‘bronchitis’ AND ‘frail elderly’, ‘frail’, ‘frailty’ (Additional Table 1). The most used model to assess the frailty-the phenotype model was developed by Fried et al. in 2001 [2]. Recognition of frailty is becoming increasingly important in recent years. Therefore, the search period was from the January 1, 2002, to July 1, 2022.
The inclusion criteria were: (1) articles in English; (2) the design was a cross-sectional, case-control, prospective, or retrospective cohort study or clinical trial in humans; (3) studies must have been conducted on adults with COPD;(4) patients had a definite diagnosis of frailty, defined according to any criteria provided it was stated in the methodology; (5) studies that provided comparative data between people with COPD who did and did not have frailty, as follows: (a) pulmonary function measured by spirometry (e.g. FEV1% predicted); (b) dyspnea severity including CAT(COPD Assessment Test) and mMRC (modified Medical Research Council) grade; (c) physical function, derived from common clinical assessment including six minutes walking test (6MWT) and activities of daily living(ADL); (d)hospital readmission, acute exacerbation and all-cause mortality.
Articles were excluded if they: (1) did not investigate the aims of the review; (2) were not original (e.g. editorial, review, congress abstract); (3) if frailty was assessed only with a single symptom or measure (e.g. only gait speed or grip strength); and (4) was a duplicate.
Quality assessment
We used the tool from the Newcastle-Ottawa Scale (NOS) for cohort studies [17] and Agency for Healthcare Research and Quality (AHRQ) scale for cross-sectional studies [18]. For cohort studies, scores > 7 were considered a low risk of bias; 5 to 7, a moderate risk; and < 5, a high risk. Each cross-sectional study was scored as follows: 0–3, low quality; 4–7, medium quality; and 8–11, high quality. The Cochrane Collaboration’s tool for assessing risk of bias was used for randomized controlled trials [19]. Studies with high risk of bias in at least one of the six areas were assumed to have an overall high risk of bias. Two authors (L.W. and X.Z.) independently examined the sources of bias of the included studies and any disagreement was resolved through discussion. A third author (X. L.) was consulted when consensus was not achieved.
Data extraction and study outcomes
Data from the different studies was extracted in a prespecified spreadsheet in Microsoft Excel. The extracted data elements consisted of (1) name of first author, publication year; (2) design type of study; (3) the sample settings and size; (4) the characteristics of the population, including gender, age, and smoking status; (5) assessment of frailty; (6) number of frailty and non-frailty; (7) the data of hospital readmission, acute exacerbation, and mortality. (8) FEV1% predicted, 6MWT distance, ADL, CAT score, mMRC grade, means (and standard deviation) were extracted.
Statistical analysis
Where individual studies reported different measurements of frailty, if able to be determined, the most ‘conventional’ type was used. If the studies provide the continuous outcome data as median and interquartile range, we converted the median and interquartile range to mean and standard deviation [20, 21]. Clinical outcome data from studies comparing people with COPD who did and did not have frailty were meta-analyzed via Stata version 17(Stata Corporation, College Station, TX, USA). Continuous outcome data evaluated using homogenous metrics were summarized as mean differences, while data arising from heterogenous metrics were summarized as standardized mean differences (SMDs) and 95% confidence intervals (CI). The impact of frailty on mortality was summarized by pooling the fully adjusted hazard ratio (HR) with 95% CI using a random effect (DerSimonian-Laird) model. Statistical heterogeneity was quantified by using the I2 statistic (values < 25% considered low, 50–75% moderate, and > 75% high). If moderate or substantial heterogeneity was identified, we used random effects models to pool outcomes. Otherwise, a fixed effects model was used. Publication bias was assessed with the funnel plots and Egger tests. Two-tailed P values of < 0.05 were considered statistically significant.
Results
Description of included studies
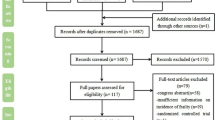
A flow diagram detailing the literature search is provided in Fig. 1. Of the 1301 abstracts identified during the search, 118 were selected for full-text reading, and 1183 were excluded because they did not relevant to the topic of the review. After reading the full text, twenty articles involving 11, 620 participants were included in the final review. Of these, 10 were observational cohort studies, 9 adopted a cross-sectional design, and one was a randomized clinical trial. Characteristics of included studies are presented in Table 1. These studies were conducted during a diverse range of populations, including nine studies from Europe, six from Asia, four from North and South America and one from Oceania.
Quality of included studies
The details of the quality assessment are shown in Additional Tables 2, 3 and 4. The quality assessment showed that of the 20 studies included, one article [22] was of low quality and 6 articles [23,24,25,26,27,28] were medium quality; the remaining articles were all rated as high quality.
Methods used to assess frailty
For frailty evaluation, 10 studies [10, 11, 13, 23,24,25, 29,30,31,32](50%) used the criteria of FFP; frailty was also measured by using other measurements, such as the Timed “Up and Go”(TUG) test [22](1, 5%) the comprehensive geriatric assessment (CGA) [26](1, 5%), frailty index [26, 30, 33](3, 15%), Frailty Staging System [34](1,5%), the Kihon Checklist [27, 28](2,10%), FRAIL Scale [35](1,5%), the Reported Edmonton Frailty Scale(REFS) [14](1,5%) and HFRS [12](1,5%). One study used six criteria including weight loss, physical activity, mobility, hearing, strength for physical frailty, and anxiety/depression to assess frailty [36].
Frailty prevalence
The prevalence of frailty ranged from 6.43 to 71.70% based on the frailty tool used. Overall frailty prevalence was 32.07% (95% CI 26.64–37.49; Fig. 2). The high statistical heterogeneity in this analysis (I2 = 98.12%) meant that individual study weighting was uniform (range 3.91–5.45%). Visual examination of asymmetrical funnel plots suggested publication bias (Additional Fig. 1), and Egger’s test indicated strong evidence of publication bias detected in the meta-analysis of prevalence of frailty(Z = 5.57,P < 0.01). Trim-and-fill analysis was performed to show the effect of the publication bias. The pooled estimate value was 26.60% (95% CI, 20.37–34.74; P < 0.001; random-effects model), which did not alter the significance of the results. The funnel plot after trimming is provided in Additional Fig. 7.
Impact of frailty on clinical outcomes
Data from 15 studies [10, 11, 13, 14, 22,23,24,25,26,27, 29, 32, 33, 35, 36] involving 4,122 participants meta-analyzed showed that those with frailty presented poorer FEV1% predicted than those without frailty [mean difference − 5.06% (95%CI -6.70 to -3.42%); I2 = 36.94%, Fig. 3A].
Data from 10 studies [11, 22,23,24, 26, 27, 29, 32, 33, 35] involving 2,392 participants were available for meta-analysis of CAT score, showing that those with frailty presented higher CAT score than those without frailty [mean difference 6.20(95%CI 4.43 to 7.96); I2 = 84.95%, Fig. 3B]. Similarly, the meta-analysis of mMRC grade from nine studies [11, 13, 23,24,25,26, 29, 33, 35] showed that having frailty was associated with higher mMRC grade [mean difference 0.93(95%CI 0.85 to 1.02; I2 = 0.00%, Fig. 3C].
Seven studies evaluated the association between frailty status and 6-minute waking test [13, 22,23,24,25,26, 33]. Frailty was associated with shorter 6MWD [mean difference − 90.23 m (95%CI -124.70 to -55.76); I2 = 83.92%, Fig. 4A]. Four studies involving 2,430 participants reported data on activities of daily living via the Katz Activities of Daily Living [11, 33], Lawton scale [11, 24, 33], Barthel index [12]. Having frailty was associated with poorer ADL [SMD − 0.99 (95%CI -1.35 to -0.62); I2 = 86.74%, Fig. 4B].
The overall pooled analysis of the 7 studies [10, 11, 13, 30, 31, 33, 34]demonstrated a 1.68-fold increase in the risk of long-term all-cause mortality for frail patients (95% CI 1.37–2.05; P < 0.0001) compared with non-frail patients (Fig. 5). No significant heterogeneity among the 7 studies was observed (P = 0.60, I2 = 0.00%). The results of the funnel plot suggested little publication bias for the above analyses of frailty upon clinical outcomes (Additional Figs. 2–6).
A summary of findings related to the rehospitalization and acute exacerbation is presented in Table 2; Quantitative meta-analysis was not possible due to lack of sufficient data. Compared with non-frail individuals, those with frailty tend to have heightened risk of rehospitalization [10, 11, 30]. Only two studies examined acute exacerbation risks for frailty COPD patients. Of them, Halon et al. [30] found that frailty increased the risk of hospitalized exacerbation and community exacerbation adjusting for FEV1. On the contrary, the other study showed that the frailty measured by FFP was not associated with COPD exacerbations [13].
Discussion
This meta-analysis evaluated the impact of frailty on health outcomes related to pulmonary function, symptom burden, physical function, and risk of mortality in patients with COPD. The quality of included most studies was grouped in terms of moderate to low risk of bias. The main finding of our meta-analysis is that frailty is associated with reduced FEV1% predicted, higher CAT score and mMRC grade, shorter six minutes walking distance (6MWD) and poorer ADL; patients with COPD and frailty had a higher risk of long-term all-cause mortality. Therefore, frailty is a prospective predictor in the risk classification of COPD.
In this review, the proportion of patients with COPD and frailty ranged from 6.43 to 71.70%. However, frailty prevalence in most studies ranged from 20 to 50%. Frailty has been estimated as occurring in up to 19% of people with stable COPD [9] in previous study and more than 50% of patients with acute exacerbations of COPD (AECOPD) [14]. The difference in the prevalence of frailty may be due to the heterogeneity of frailty assessment tools and the severity of COPD.
The pathophysiological mechanism of frailty is multidimensional including higher chronic inflammation, immune activation, dysregulation of the musculoskeletal and endocrine systems and higher level of oxidative stress [37]. A growing evidence supports the contribution of chronic inflammation and immune system dysfunction to frailty [38, 39]. Inflammation may accelerate the catabolism of skeletal muscle and adipose tissue, inducing the muscle weakness and weight loss that are symbols of frailty [40]. Patients with COPD also show signs of chronic inflammation; higher levels of systemic proinflammation biomarkers are associated with poorer outcomes [41]. Frailty patients often have decline in the ability to cough and weak cough diminishes the ability of airway clearance. In COPD patients, weak cough is associated with increased two-year mortality after a scheduled extubation [42].
Although there is no consensus on which frailty measures are most suitable for patients with COPD at present. FFP is still a widely accepted reference model [43]. This was further confirmed in 50% of the included studies where the FFP was used as the measurement [10, 11, 13, 23,24,25, 29,30,31,32]. However, the participants in the above studies focused on the community-based population and stable outpatients with COPD. For patients with advanced and critical lung disease, FFP has proven limited utility [44]. HFRS [12] and REFS [14] were used to predict the outcome of patients with AECOPD in previous studies. Frailty measured by REFS can predict the risk of early hospital readmission in patients hospitalized for AECOPD [14]. HFRS was associated with prolonged hospitalization, but had poor predictive performance of mortality after adjusting for covariates [12]. It is reasonable to consider using various tools for different health status of COPD to evaluate the effect of frailty on outcomes in clinical practice.
Frailty is an increasingly recognized and potential therapeutic risk factor in acute exacerbations of chronic airway diseases [45]. If physical frailty is present, comprehensive and multicomponent interventions except for respiratory drug therapy seem necessary. Rehabilitation serves as an important component of the management of COPD. Pulmonary rehabilitation (PR) can significantly improve a range of clinical outcomes in frail patients with COPD, including symptom burden (mMRC grade and CAT score), exercise performance, physical activity level and health status in the short term [29]. For frail COPD patients with chronic respiratory failure, these benefits were maintained more than 6 months after the end of PR [46]. Physical frailty was not a barrier for benefiting from the intervention. Indeed, physical frailty can be reversed from PR intervention at least partially. After rehabilitation, more than half of previously frail patients improved their frailty status [46]. Future research studies are needed to determine the most effective PR program and the effect in frail patients with COPD in the long term.
The results of our meta-analysis highlight that frailty evaluation may improve risk stratification in patients with COPD. Comprehensive geriatric assessment is proven beneficial to the management of frail patients, which increases possibility of being alive and returning homes after an emergency admission to hospital [47]. Frailty is common in patients with COPD and associated with poorer clinical outcomes. Clinicians should stratify patients according to their frailty status and take timely interventions, which may reverse the frailty status and improve the prognosis of patients with COPD especially in the older adults. Notably, clinicians should be aware of the importance of PR for frail patients with COPD.
There are several limitations that should be noted. First, although this review had included studies to investigate the prevalence of frailty in COPD, the funnel plot suggested the publication bias. After correction of the publication bias by the trim-and-fill method, the pooled estimate value (26.60%) was slightly lower than the original 32.07%, whereas the difference remained statistically significant. Secondly, different studies used different measures of frailty. The inadequate and inconsistent definition of frail status may affect the predictive value of frailty. Thirdly, the follow-up time ranged from 90 days to 12 years, with most of studies focused on the long-term mortality. Therefore, no further analysis was made to investigate the impact of frailty on short-term mortality. Future studies are warranted to investigate the correlation between frailty and AECOPD. Finally, this review was unable to elucidate the direct relationship between frailty and readmission and acute exacerbation due to a lack of data.
Conclusion
Frailty is prevalent in people with COPD and negatively impacts clinical outcomes. Assessment of the frailty status of patients with COPD can potentially guide clinical management of this population. Patients living with COPD and frailty may benefit from some interventions such as pulmonary rehabilitation.
Data Availability
All data generated or analyzed during this study are included in this published article. The data could be freely available for anyone interested.
Abbreviations
- ADL:
-
activities of daily living
- AECOPD:
-
acute exacerbation of chronic obstructive pulmonary disease
- AHRQ:
-
Agency for Healthcare Research and Quality
- COPD:
-
chronic obstructive pulmonary disease
- CI:
-
confidence interval
- CAT:
-
COPD Assessment Test
- CFS:
-
clinical frailty scale
- CGA:
-
the comprehensive geriatric assessment
- FFP:
-
the Fried frailty phenotype
- mMRC:
-
modified Medical Research Council
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- HR:
-
hazard ratio
- HFRS:
-
the Hospital Frailty Risk Score
- PR:
-
pulmonary rehabilitation
- REFS:
-
Reported Edmonton Frail Scale
- SMD:
-
standardized mean difference
- TUG:
-
Timed “Up and Go” test
- 6MWD:
-
six minutes walking distance
References
Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD: Management of frailty: opportunities, challenges, and future directions. Lancet (London, England) 2019, 394(10206):1376–1386.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G et al: Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001, 56(3):M146-156.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A: A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173(5):489–495.
Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW: Association of Frailty with 30-Day outcomes for Acute Myocardial Infarction, Heart failure, and Pneumonia among Elderly adults. JAMA cardiology 2019, 4(11):1084–1091.
Mei F, Gao Q, Chen F, Zhao L, Shang Y, Hu K, Zhang W, Zhao B, Ma B: Frailty as a predictor of negative health outcomes in chronic kidney disease: a systematic review and Meta-analysis. Journal of the American Medical Directors Association 2021, 22(3):535–543.e537.
Zhang H, Jie Y, Wang P, Sun Y, Wang X, Fan Y: Impact of frailty on all-cause mortality or major amputation in patients with lower extremity peripheral artery disease: a meta-analysis. Ageing Res Rev 2022, 79:101656.
Proietti M, Romiti GF, Raparelli V, Diemberger I, Boriani G, Dalla Vecchia LA, Bellelli G, Marzetti E, Lip GY, Cesari M: Frailty prevalence and impact on outcomes in patients with atrial fibrillation: a systematic review and meta-analysis of 1,187,000 patients. Ageing Res Rev 2022, 79:101652.
Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T: Frailty and Clinical Outcomes in Heart failure: a systematic review and Meta-analysis. Journal of the American Medical Directors Association 2018, 19(11):1003–1008 e1001.
Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K: The Relationship between COPD and Frailty: a systematic review and Meta-analysis of Observational Studies. Chest 2018, 154(1):21–40.
Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP, Fishman AP, Bozzarello BA, Al-Amin A, Katz M et al: Frailty and clinical outcomes in chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2019, 16(2):217–224.
Luo J, Zhang D, Tang W, Dou LY, Sun Y: Impact of Frailty on the risk of exacerbations and all-cause Mortality in Elderly patients with stable chronic obstructive Pulmonary Disease. Clin Interv Aging 2021, 16:593–601.
Ushida K, Shimizu A, Hori S, Yamamoto Y, Momosaki R: Hospital Frailty risk score predicts Outcomes in Chronic Obstructive Pulmonary Disease Exacerbations. Arch Gerontol Geriatr 2022, 100:104658.
Yee N, Locke ER, Pike KC, Chen Z, Lee J, Huang JC, Nguyen HQ, Fan VS: Frailty in Chronic Obstructive Pulmonary Disease and Risk of Exacerbations and Hospitalizations. Int J Chron Obstruct Pulmon Dis 2020, 15:1967–1976.
Bernabeu-Mora R, Garcia-Guillamon G, Valera-Novella E, Gimenez-Gimenez LM, Escolar-Reina P, Medina-Mirapeix F: Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis 2017, 11(10):383–392.
Sun X, Liu W, Gao Y, Qin L, Feng H, Tan H, Chen Q, Peng L, Wu IXY: Comparative effectiveness of non-pharmacological interventions for frailty: a systematic review and network meta-analysis. Age Ageing 2023, 52(2).
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372:n71.
Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010, 25(9):603–605.
Atkins D, Fink K, Slutsky J, Agency for Healthcare R, Quality, North american evidence-based practice C: better information for better health care: the evidence-based Practice Center program and the Agency for Healthcare Research and Quality. Ann Intern Med 2005, 142(12 Pt 2):1035–1041.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA et al: The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343:d5928.
Luo D, Wan X, Liu J, Tong T: Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018, 27(6):1785–1805.
Wan X, Wang W, Liu J, Tong T: Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014, 14:135.
Finamore P, Scarlata S, Delussu AS, Traballesi M, Incalzi RA, Laudisio A: Frailty Impact during and after Pulmonary Rehabilitation. COPD 2021, 18(5):518–524.
Kagiali S, Inal-Ince D, Cakmak A, Calik-Kutukcu E, Saglam M, Vardar-Yagli N, Tekerlek H, Sonbahar-Ulu H, Arikan H, Bozdemir-Ozel C et al: Daily living activities, exercise capacity, cognition, and balance in COPD patients with and without frailty. Ir J Med Sci 2022, 191(2):817–824.
Naval E, Cruz Gonzalez M, Giraldos S, Calatayud J, Jornet M, Lluch I, Meseguer M, Ruiz Cubillan JJ, Vina J, Jose Tarazona-Santabalbina F: Frailty Assessment in a stable COPD cohort: is there a COPD-Frail phenotype? COPD 2021, 18(5):525–532.
Gephine S, Mucci P, Grosbois JM, Maltais F, Saey D: Physical Frailty in COPD patients with chronic respiratory failure. Int J Chron Obstruct Pulmon Dis 2021, 16:1381–1392.
Gale NS, Albarrati AM, Munnery MM, Hubbard RE, Tal-Singer R, Cockcroft JR, Shale DJ: Frailty: a global measure of the multisystem impact of COPD. Chron Respir Dis 2018, 15(4):347–355.
Kusunose M, Oga T, Nakamura S, Hasegawa Y, Nishimura K: Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir Res 2017, 4(1):e000196.
Hirai K, Tanaka A, Homma T, Kaneko K, Uno T, Sato H, Manabe R, Ohta S, Kusumoto S, Yamaguchi F et al: Comparison of three frailty models and a sarcopenia model in elderly patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int 2019, 19(9):896–901.
Maddocks M, Kon SS, Canavan JL, Jones SE, Nolan CM, Labey A, Polkey MI, Man WD: Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016, 71(11):988–995.
Hanlon P, Lewsey J, Quint JK, Jani BD, Nicholl BI, McAllister DA, Mair FS: Frailty in COPD: an analysis of prevalence and clinical impact using UK Biobank. BMJ Open Respir Res 2022, 9(1).
Lahousse L, Ziere G, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, Tiemeier H, Joos GF, Hofman A, Ikram MA et al: Risk of Frailty in Elderly with COPD: a Population-Based study. J Gerontol A Biol Sci Med Sci 2016, 71(5):689–695.
Medina-Mirapeix F, Bernabeu-Mora R, Gimenez-Gimenez LM, Escolar-Reina P, Gacto-Sanchez M, de Oliveira-Sousa SL: Physical frailty characteristics have a differential impact on symptoms as measured by the CAT score: an observational study. Health Qual Life Outcomes 2018, 16(1):140.
Scarlata S, Finamore P, Laudisio A, Cardaci V, Ramaccia M, D’Alessandro F, Pedone C, Antonelli Incalzi R, Cesari M: Association between frailty index, lung function, and major clinical determinants in chronic obstructive pulmonary disease. Aging Clin Exp Res 2021, 33(8):2165–2173.
Galizia G, Cacciatore F, Testa G, Della-Morte D, Mazzella F, Langellotto A, Raucci C, Gargiulo G, Ferrara N, Rengo F et al: Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res 2011, 23(2):118–125.
Dias LS, Ferreira ACG, Junior JLRDS, Conte MB, Rabahi MF: Prevalence of frailty and evaluation of associated variables among COPD patients. International Journal of COPD 2020, 15:1349–1356.
Park SK: Frailty in Korean patients with chronic obstructive pulmonary disease, using data from the Korea National Health and Nutrition Examination Survey, 2015 and 2016. Appl Nurs Res 2021, 59:151417.
Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, Dupuis G, Baehl S, Camous X, Witkowski JM, Larbi A: Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr 2015, 41:26–40.
Pansarasa O, Mimmi MC, Davin A, Giannini M, Guaita A, Cereda C: Inflammation and cell-to-cell communication, two related aspects in frailty. Immun Ageing 2022, 19(1):49.
Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S et al: Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 2016, 31:1–8.
Schaap LA, Pluijm SM, Deeg DJ, Visser M: Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006, 119(6):526 e529-517.
Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, Nordestgaard BG: Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013, 309(22):2353–2361.
Hong Y, Deng M, Hu W, Zhang R, Jiang L, Bai L, Duan J: Weak cough is associated with increased mortality in COPD patients with scheduled extubation: a two-year follow-up study. Respir Res 2022, 23(1):166.
Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L, Ding YY, Lim WS: Frailty in hospitalized older adults: comparing different Frailty Measures in Predicting Short- and long-term patient outcomes. Journal of the American Medical Directors Association 2018, 19(5):450–457.e453.
Baldwin MR, Singer JP, Huang D, Sell J, Gonzalez WC, Pollack LR, Maurer MS, D’Ovidio FF, Bacchetta M, Sonett JR et al: Refining low physical activity measurement improves Frailty Assessment in Advanced Lung Disease and Survivors of critical illness. Ann Am Thorac Soc 2017, 14(8):1270–1279.
McDonald VM, Osadnik CR, Gibson PG: Treatable traits in acute exacerbations of chronic airway diseases. Chron Respir Dis 2019, 16:1479973119867954.
Gephine S, Saey D, Grosbois JM, Maltais F, Mucci P: Home-based Pulmonary Rehabilitation is effective in Frail COPD patients with chronic respiratory failure. Chronic Obstr Pulm Dis 2022, 9(1):15–25.
Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P: Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ 2011, 343:d6553.
Acknowledgements
Not applicable.
Funding
This work was supported by National Key R&D Program of China(2020YFC2008804).
Author information
Authors and Affiliations
Contributions
X. L. is the guarantor of the article and designed the study. L.W. and X.Z. performed the literature search; L.W. performed the statistical analyses; L.W. and X.Z. wrote the manuscript. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not include any studies with human or animal participants that were performed by any of the authors. For this type of study, formal consent was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Zhang, X. & Liu, X. Prevalence and clinical impact of frailty in COPD: a systematic review and meta-analysis. BMC Pulm Med 23, 164 (2023). https://doi.org/10.1186/s12890-023-02454-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02454-z