Abstract
Purpose
Autoimmune rheumatic diseases (ARD) are groups of diseases that are commonly associated with cardiac and pulmonary manifestations and may affect the morbidity and mortality of the patients. The study aimed to the assessment of cardiopulmonary manifestations and their correlation with the semi-quantitative scoring of high-resolution computed tomography (HRCT) in ARD patients.
Methods and patients
30 patients with ARD were included in the study (mean age 42.2 ± 9.76 years) [10 patients were scleroderma (SSc), 10 patients were rheumatoid arthritis (RA), and 10 patients were systemic lupus erythematosus (SLE)]. They all met the diagnostic criteria of the American College of Rheumatology and underwent spirometry, echocardiography, and chest HRCT. The HRCT was assessed by a semi-quantitative score for parenchymal abnormalities. Correlation between HRCT lung scores and: inflammatory markers, lung volumes in spirometry, and echocardiographic indices has been performed.
Results
The total lung score (TLS) by HRCT was 14.8 ± 8.78 (mean ± SD), ground glass opacity score (GGO) was 7.20 ± 5.79 (mean ± SD) and fibrosis lung score (F) was 7.63 ± 6.05 (mean ± SD). TLS correlated significantly with ESR (r 0.528, p 0.003), CRP (r 0.439, p 0.015), PaO2 (r -0.395, P 0.031) FVC% (r -0.687, p 0.001), and echocardiographic Tricuspid E (r -0.370, p 0.044), Tricuspid E/è (r -0.397,p 0.03), ESPAP (r 0.459,p 0.011), TAPSE (r -0.405, p 0.027), MPI-TDI (r -0.428, p 0.018) and RV Global strain(r -0.567, p 0.001). GGO score correlated significantly with ESR (r 0.597, p 0.001), CRP (r 0.473, p 0.008), FVC% (r -0.558, p 0.001), and RV Global strain(r -0.496, p 0.005). F score correlated significantly with FVC% (r -0.397, p 0.030), Tricuspid E/è (r -0.445, p 0.014), ESPAP (r 0.402, p 0.028), and MPI-TDI (r -0.448, p 0.013).
Conclusion
The total lung score and GGO score in ARD were found to be consistently significantly correlated with FVC% predicted, PaO2, inflammatory markers, and RV functions. Fibrotic score correlated with ESPAP. Therefore, in a clinical setting, most clinicians who monitor patients suffering from ARD should concern with the applicability of semiquantitative HRCT scoring in clinical practice.
Similar content being viewed by others
Introduction
Autoimmune rheumatic diseases (ARD) are disorders with autoimmune characteristics that can influence any of the body systems causing organ damage. Examples include scleroderma or progressive systemic sclerosis (SSc), rheumatoid arthritis (RA), autoimmune myopathies, and systemic lupus erythematosus (SLE) [1]. They can involve any part of the cardiac or pulmonary system and are commonly accompanied by interstitial lung diseases (ILDs) which might be the primary cause of death in most patients, especially with usual interstitial pneumonia [2, 3].
Rather than being dependent on definite validation, the utility of the HRCT in the identification of ILDs indicates universal practical knowledge of discontent with alternative methodologies. In other words, during the last 20 years, HRCTs is practically beneficial in the identification of ILDs; as a result, they acquired a crucial role [4].
HRCT has been considered the gold standard in the detection of ARD-related ILD, particularly in the early stages of the disease. [5, 6]. The most prevalent radiologic feature in ARD is diffuse parenchymal interstitial lung disease (ILD), which can manifest by ground glass opacity (GGO), reticulation, tractional bronchiectasis, or honeycombings (HC) [7].
A lot of CT scoring system was described in the ILD associated with ARD, some automated or computer-aided, and others depend on reader visual assessment and semiquantitative (semi-QA) methods [8, 9].
Although many studies looked at the degree and severity of ILD detected by HRCT and whether they were correlated with the level of respiratory insufficiency and lung functions, the majority of studies focused on SSc patients rather than all ARD patients, and only a small number of studies linked CT scoring to right ventricular function [10,11,12,13]. More knowledge concerning the link between distinct CT abnormalities and underlying disease pathophysiology and illness progression may be gained by assessing the relation of any abnormalities with the clinical data [14]. So, in this study, the spirometry, inflammatory markers including ESR and CRP, and right ventricular (RV) function by echocardiography were assessed, together with their correlation with semi-quantitative scoring of HRCT in the patients with different ARD.
Patients and methods
Study population
This prospective study was conducted on 30 patients (mean age 42.2 ± 9.76 years), diagnosed with ARD, attending Menoufia University Hospitals from the period of January 2021 to March 2022. All of the patients met the diagnostic criteria of the American College of Rheumatology that including RA [15], SSc [16], and SLE [17], and their age was > 18 years old. Exclusion criteria were any patient with chronic pulmonary, or cardiac disease, lung cancer history, a known cause of interstitial lung fibrosis e.g., occupational, age less than 18 years old, patients, who couldn’t perform spirometry, or patient refusal. The Menoufia University Hospital Research Ethical Committee gave its approval to the study (IRB: 6/2022CHES4-2).
Study design
The patients underwent comprehensive history taking, clinical evaluations, and basic laboratory tests like C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) and PaO2(mmHg), spirometry, HRCT, and echocardiography.
Spirometric function evaluation
Spirometry was done using a computerized spirometer (THOR Laboratories Kft Spirometer, Hungary) in the Unit of pulmonary function test (PFT) in Menoufia University Hospital, taking the data with a particular emphasis on the ratio of forced expiratory volume in 1st second (FEV1) / forced vital capacity (FVC) and FVC% of the predicted values following the guidelines of the American Thoracic Society/European Respiratory Society (ATS/ERS) [18]. The test was repeated at least 2 times to report the best values. Restrictive abnormality was determined if the FVC% < 80% together with a normal ratio of FEV1/FVC.
Transthoracic echocardiography (ECHO)
The ECHO was performed with an S4-2 probe (Vivid9, General Electric health care Vingmed, Norway) by an experienced echocardiographer and all patients underwent:
-
1.
Conventional 2-dimensional (2D) transthoracic ECHO with functional evaluation of the RV which includes:[19] measuring EPASP (using the tricuspid regurgitation velocity and the Bernoulli equation), Tricuspid annular plane systolic excursion (TAPSE), RV-fractional area change (RV-FAC), TDI derived RV myocardial performance index (TDI-MPI), pulsed wave derived tricuspid E wave and A wave and early tricuspid diastolic wave velocity (è) and E/ è ratio that measured by TDI to assess the diastolic function of the RV.
-
2.
The 2 D-speckle tracking echocardiography (2D STE): to assess and analyze the RV global strain as the conventional 2-D ECHO isn’t sensitive enough for detection of subclinical RV changes in autoimmune disease.
HRCT assessment and HRCT score
All patients underwent a multi-slice CT study without contrast for the chest. The CT was performed using multi-slice 16 detectors (CT Toshiba Aquilion 1) and the CT acquisition parameters were as follows: Slice thickness was set at 1.0 mm, reconstruction interval was between 1.0 and 3.0 mm, and a sharp reconstruction technique was employed. The tube voltage ranged from 120 to 160 kVp.
Semiquantitative (SemiQA) scoring of HRCT
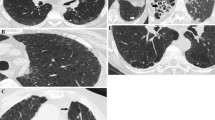
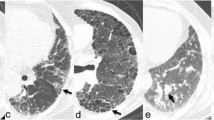
Semi-quantitative scoring is determined by evaluating the extent of the illness and assigning a score; higher scores coincide with more progression of the disease. All CTs were examined by an expert chest radiologist blinded to any of the clinical presentations or the diagnosis of the patient. We used the scoring methods modified from that described by Goldin et al. [20] used in the Scleroderma Lung Study (Table 1). In this study, The upper, middle, and lower zones of each lung were divided, and each zone was independently scored. From the lung apex to the arch of the aorta is considered the upper zone, the middle zone is from the arch of the aorta to the inferior pulmonary veins, while from the inferior pulmonary veins to the diaphragm is considered the lower zone. The abnormalities were divided into ground-glass opacity (GGO) and total fibrosis (F) (Fig. 1). GGO is defined radiologically as a hazy opacity in the lung parenchyma without any reticulations or architectural distortion and F is defined as any reticulations (inter- or intra-lobular), traction bronchiectasis, or honeycombing (HC) which is defined as multiple layers of dense walled air-filled cysts [21]. The grading of abnormality extension is expressed in a scale in which (0 = absent; 1 = 1–25%; 2 = 26–50%; 3 = 51–75% and 4 = 76–100%). Finally, The total CT score was defined as the summation of the grades in the Six zones. This scoring was derived from the scoring of Kazerooni et al. [22] in which the lung zones were changed into lobes.
Statistical analysis
An IBM personal computer running version 20 of the Statistical Package of Social Science was used to analyze the data (SPSS, Inc, Chicago, Illinois, USA). The qualitative data were presented as % and numbers, while the quantitative data were presented as mean, standard deviation (SD), and range. Quantitative data were correlated using Spearman’s correlation. P values lower than 0.05 were deemed statistically significant.
Results
This study was conducted on 30 patients (mean age 42.2 ± 9.76 years, 27 females and 3 males) diagnosed with ARD; 10 patients with SSc, 10 patients with RA, and 10 patients with SLE. The duration of the disease ranged between 2 and 15 years. The Socio-demographic together with the clinical data of the studied participants shown in (Table 2).
Table 3 showed mean values of HRCT scoring, laboratory parameters, spirometry values, and ECHO finding among the studied patients.
The total lung score was 14.8 ± 8.78 (mean ± SD) where GGO was 7.20 ± 5.79, and the total fibrosis (F) was 7.63 ± 6.05.
Correlations between total lung score, GGO score, and lung fibrosis score by HRCT and: inflammatory markers, echocardiographic indices as well as lung volumes by spirometry are presented in Table 4.
Total lung score showed statistically significant positive correlations with inflammatory markers (ESR and CRP (r 0.528, p 0.003 and r 0.439, p 0.015 respectively) together with ESPAP (r 0.459, p 0.011). Furthermore, it was statistically significant and inversely correlated with PaO2(r -0.395, P 0.031), FVC% (r -0.687, p 0.001), and RV functions including Tricuspid E(r= -0.370, p = 0.044), Tricuspid E/è (r -0.397,p 0.03), TAPSE (r -0.405, p 0.027), MPI-TDI(r -0.428, p 0.018) and RV Global strain (r -0.567, p 0.001).
GGO score correlated significantly with ESR (r 0.597, p 0.001), CRP (r 0.473, p 0.008), FVC% (r -0.558, p 0.001), and RV Global strain(r -0.496, p 0.005).
F score correlated significantly with FVC% (r -0.397, p 0.030), Tricuspid E/è (r -0.445,p 0.014), ESPAP (r 0.402,p 0.028), and MPI-TDI (r -0.448, p 0.013).
Discussion
HRCT is considered a noninvasive reference approach for the diagnosis of ILD because it gives good parenchymal detail [23, 24].
Semi-quantitative scoring is determined by evaluating the extent of the illness and assigning a score; higher scores coincide with more progression of the disease, while the exact percent of lung involvement is employed in quantitative approaches [11]. In this current study, we use the semi-QA scoring method described by Goldin et al. [20], the method described in the Scleroderma Lung Study. The main results of this study were that spirometric FVC%, PaO2, inflammatory markers, and RV functions by ECHO were correlated with the total lung score by the Semi-QS method in ARD patients.
Semi-QA scoring methods, in multiple studies, were compared to QA using computer-aid approaches. Computer-aid methods were well correlated with the visual scoring methods in the identification of lung fibrosis together with the assessment of the disease extent and there was no intrareader variation encountered in visual scoring methods [8, 25,26,27,28]. So, we tried to use the Semi-QA scoring in HRCT in patients with ARD and its correlation with the inflammatory markers, spirometry, and echocardiographic RV functions.
In this current study, the total lung score was 14.8 ± 8.78 (mean ± SD) where GGO was 7.20 ± 5.79 and the total fibrosis (F) was 7.63 ± 6.05. The spirometry showed restrictive type with the FVC % − 64.4 ± 9.20 (mean ± SD) and FEV1/FVC% − 82.1 ± 6.03 A significant inverse correlation between the total lung score, GGO score, and F score with the FVC % was found (r -0.687, p 0.001; r -0.558, p 0.001 and r -0.397, p0.030 respectively).
The spirometry in our study coincided with the study of Mena-Vázquez et al. that showed the character and progress of ILD in ARD patients between the period of 2015 and 2020. The FVC of all patients (n = 204) was 72 ± 16.6 (mean ± SD) and at the end of the study was 68.2 ± 16.2(mean ± SD) [29].
In the study of Marten et al., all patients with collagen vascular disease (CVD) (n = 52) had findings of ILD in thin-section CT. The extent of ILD was 36.3 ± 27.2% determined by the readers (the extent of reticulation was 27.0 ± 23.3%, the GGO extent was 9.2 ± 17.0%, the extent of the coarseness of the reticulation was 1.1 ± 0.6%). There were significant correlations between the ILD extent in CT and FVC and FEV1 (r − 0.559, P 0.0002 and r = − 0.379, P = 0.014 respectively). The reticulation extent was correlated moderately with FVC (r = − 0.436, P = 0.005), while no significant correlations were found between FEV1 and reticulation extent. There were no significant correlations between the GGO, and coarseness extent with either FVC or FEV1 [30].
Most studies used the semi-quantitative scoring systems to assess the PFT in Systemic sclerosis patients and found that the total score of HRCT was significantly and negatively correlated with TLC, FVC%, and DLCO [10, 31,32,33].
Our results were near that of Zexuan et al. who involved the scleroderma patients using the scoring system used by Goldin et al., they found that the total score was 14.35 ± 6.18 where GGO was 7.15 ± 3.94, PF 5.58 ± 2.91, and HC 1.36 ± 2.66. But they put also emphysema in the scoring which was 0.28 ± 1.05 [34].
Goldin et al. studied the HRCT scan on 162 scleroderma patients. They found that the F and the GGO scores were the commonest abnormalities in symptomatic scleroderma patients, and the extent of the F score had a statistically significant negative correlation with DLCO (r − 0.44), FVC (r − 0.22), and TLC (r − 0.36). They suggested that pure GGO on CT might be reversible as it represents inflammation because its extent was correlated with acute inflammatory cells in bronchoalveolar fluid and not correlated with PFT (r = 0.28) [20].
The study of Wangkaew et al. involved 31 participants with SSc and used the HRCT score that classified the parenchymal abnormalities into 4 items: GGOs, lung fibrosis, bronchiectasis, and HCs. In concordance with our study, they found a significant and inverse correlation between FVC % and total GGO (r − 0.43; P 0.05), total Fibrosis score (r − 0.56; P 0.01), total bronchiectasis score (r − 0.43; P 0.05) and total scores of HRCT (r − 0.52; P 0.01) together with significant correlations between HRCT scores and O2 saturation (r − 0.47; P 0.01) and ESR (r 0.38, P 0.05) [35].
We tried to assess the correlation of total lung score with right ventricular echocardiographic function in different ARD. We found that the total lung score has statistically significant correlations with Tricuspid E (r -0.370, p 0.044), Tricuspid E/è (r -0.397, p 0.03), TAPSE (r -0.405, p 0.027), MPI-TDI (r -0.428, p 0.018), RV Global strain (r -0.567, p 0.001), and ESPAP (r 0.459, p 0.011). The total F score was significantly correlated with EPASP (r 0.402, p 0.028), Tricuspid E/è (r -0.445, p 0.014), and MPI-TDI (r -0.448, p 0.013).
There were limited studies that correlate the CT score in ARD with RV function by ECHO. For example, Pandey et al. found that the total HRCT score correlated with elevated pulmonary arterial pressures (PAP). Their study showed a significant relationship between the peak PAP and the total CT score (p < 0.0001). They stated that the fibrotic score was the most determinant factor of pulmonary hypertension (PH) on ECHO, which can help in the screening of SSc patients for PH by the extent of pulmonary fibrosis [13].
Some patients had significant elevations in the PAP although they had small or no lung fibrosis. This could explain why multiple factors contribute to the occurrence of PH in people with scleroderma, including pulmonary vasculopathy and capillary bed obliteration, and lung fibrosis may be the main factor [13].
The study by Mukerjee et al. found that the EPASP on echo (mmHg) was 39 ± 15 in scleroderma patients without lung fibrosis and 46 ± 18 in scleroderma patients with lung fibrosis detected by HRCT [36]. In the study of Mohammed et al., the mean EPASP in the studied SLE patients was 31 ± 5.1 mmHg with no significant variation between the diseased and control group [37].
There were some limitations in this study e.g., the total number of participants was small in each group of diseases, not all diseases of ARD are involved, the scoring system used didn’t include other CT abnormalities e.g., emphysematous changes in ARD, and more PFT parameters need to be evaluated e.g., TLCO. We recommend further studies on a large number of participants with ARD with the comparison between different types of CT scoring systems that include more abnormalities recording.
Conclusion
Semi-quantitative approaches of HRCT are distinguished by the precise estimation of interstitial lung disease extent and character. The higher grade equates to more advanced lung disease. The total lung score and GGO score in ARD were found to be consistently significantly correlated with FVC% predicted, PaO2, inflammatory markers, and RV functions. Fibrotic score correlated with ESPAP. Therefore, in a clinical setting, most clinicians who monitor patients suffering from ARD should concern with semi-quantitative HRCT scoring in clinical practice.
Data availability
On reasonable request, the corresponding author will provide the datasets used and/or analyzed during the current work.
Change history
25 June 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12890-024-03111-9
Abbreviations
- ARD :
-
Autoimmune rheumatic diseases
- CRP :
-
C-reactive protein
- ECHO :
-
Echocardiography
- EPASP :
-
Estimated pulmonary artery systolic pressure
- ESR :
-
Erythrocyte sedimentation rate
- F :
-
Fibrosis
- FEV1/ FVC :
-
forced expiratory volume in 1st second / forced vital capacity
- GGO :
-
Ground glass opacity
- HC :
-
Honeycombing
- HRCT :
-
high-resolution computed tomography
- ILD :
-
interstitial lung disease
- MPI :
-
myocardial performance index
- PFT :
-
Pulmonary function test
- RA :
-
rheumatoid arthritis
- RV-FAC :
-
right ventricle- fractional area change
- semi-QA :
-
semiquantitative
- SLE :
-
systemic lupus erythematosus
- SSc :
-
scleroderma
- STE :
-
speckle tracking echocardiography
- TAPSE :
-
Tricuspid annular plane systolic excursion
References
Jawad H, McWilliams SR, Bhalla S. Cardiopulmonary manifestations of collagen vascular diseases. Curr Rheumatol Rep. 2017;19(11):1–10.
Frankel SK, Brown KK. Collagen vascular diseases of the lung. Clin Pulmonary Med. 2006;13(1):25–36.
Nurmi HM, et al. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med. 2016;16(1):107.
Ruaro B et al. High-Resolution Computed Tomography: Lights and Shadows in Improving Care for SSc-ILD Patients. Diagnostics (Basel), 2021. 11(11).
Pignone A, et al. High resolution computed tomography in systemic sclerosis. Real diagnostic utilities in the assessment of pulmonary involvement and comparison with other modalities of lung investigation. Clin Rheumatol. 1992;11(4):465–72.
Sergiacomi G, et al. Non-invasive diagnostic and functional evaluation of cardiac and pulmonary involvement in systemic sclerosis. in vivo. 2004;18(2):229–36.
Launay D, et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J Rhuematol. 2006;33(9):1789–801.
van Royen FS, et al. Automated CT quantification methods for the assessment of interstitial lung disease in collagen vascular diseases: a systematic review. Eur J Radiol. 2019;112:200–6.
Warrick JH, et al. High resolution computed tomography in early scleroderma lung disease. J Rhuematol. 1991;18(10):1520–8.
Ooi G, et al. Interstitial lung disease in systemic sclerosis: an HRCT-clinical correlative study. Acta Radiol. 2003;44(3):258–64.
Assayag D et al. High Resolution Computed Tomography Scoring Systems for Evaluating Interstitial Lung Disease in Systemic Sclerosis Patients. Rheumatology, 2012. 2012: p. 1–6.
Emilsson ÖI, et al. Different chest HRCT scan protocols change the extent of ground glass opacities. BMC Pulm Med. 2022;22(1):430.
Pandey AK, et al. Predictors of pulmonary hypertension on high-resolution computed tomography of the chest in systemic sclerosis: a retrospective analysis. Can Assoc Radiol J. 2010;61(5):291–6.
Shah RM, Jimenez S, Wechsler R. Significance of ground-glass opacity on HRCT in long-term follow-up of patients with systemic sclerosis. J Thorac Imaging. 2007;22(2):120–4.
Arnett FC, et al. The american Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1988;31(3):315–24.
Van Den Hoogen F, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthr Rhuem. 2013;65(11):2737–47.
Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1982;25(11):1271–7.
Miller MR, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–61.
Rudski LG, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the canadian society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713.
Goldin JG, et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 2008;134(2):358–67.
Zisman DA, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132(3):773–9.
Kazerooni EA, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. Am J Roentgenol. 1997;169:977–84.
Muller NL, Miller RR. Computed tomography of chronic diffuse infiltrative lung disease. Am Rev Respir Dis. 1990;142:1206–15.
Remy-Jardin M, et al. Pulmonary involvement in progressive systemic sclerosis: sequential evaluation with CT, pulmonary function tests, and bronchoalveolar lavage. Radiology. 1993;188(2):499–506.
Shin KE, et al. Quantitative computed tomographic indexes in diffuse interstitial lung disease: correlation with physiologic tests and computed tomography visual scores. J Comput Assist Tomogr. 2011;35(2):266–71.
Kim H, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol. 2010;28(5 Suppl 62):S26.
Kazantzi A, et al. Automated 3D ιnterstitial lung disease εxtent quantification: performance evaluation and correlation to PFTs. J Digit Imaging. 2014;27(3):380–91.
Goh NS, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–54.
Mena-Vázquez N, et al. Characteristics and predictors of Progression interstitial lung Disease in Rheumatoid Arthritis compared with other Autoimmune Disease: a retrospective cohort study. Diagnostics. 2021;11(10):1794.
Marten K, et al. Interstitial lung disease associated with collagen vascular disorders: disease quantification using a computer-aided diagnosis tool. Eur Radiol. 2009;19(2):324–32.
Diot E, et al. Relationship between abnormalities on high-resolution CT and pulmonary function in systemic sclerosis. Chest. 1998;114(6):1623–9.
Camiciottoli G, et al. Lung CT densitometry in systemic sclerosis: correlation with lung function, exercise testing, and quality of life. Chest. 2007;131(3):672–81.
Bellia M, et al. HRCT and scleroderma: semiquantitative evaluation of lung damage and functional abnormalities. Radiol Med. 2009;114(2):190–203.
Zexuan Z, et al. HRCT imaging features of systemic sclerosis-associated interstitial lung disease. J Radiol Oncol. 2021;5:035–41.
Wangkaew S, et al. Correlation of delta high-resolution computed tomography (HRCT) score with delta clinical variables in early systemic sclerosis (SSc) patients. Quant imaging Med Surg. 2016;6(4):381.
Mukerjee D, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology. 2004;43(4):461–6.
Mohammed MA, et al. Echocardiographic findings in systemic lupus erythematosus and its relation to disease activity and damage index. Egypt Rheumatologist. 2018;40(3):173–8.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Study design: S.E. Data collection: A.E, E.Z, M.S, and M.A. Data analysis: S.A and M.E. Interpretation of results: A.E, M.E, S.E. Initial draft: A.E and M.E. final review of the manuscript content: all authors.
Corresponding author
Ethics declarations
The ethics approval and consent to participate
The Menoufia University Hospital Research Ethical Committee gave its approval to the study, which adhered to the Declaration of Helsinki’s guidelines (IRB: 6/2022CHES4-2). Following the guidelines of the Menoufia University Hospital’s local research ethical committee, written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they do not have any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article corrected in 2024.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Kalashy, M.M., Elbeltagy, S.A., Zahran, E.S. et al. Assessment of cardiopulmonary manifestations and its correlation with semi-quantitative scoring of high-resolution computed tomography in patients with autoimmune rheumatic diseases. BMC Pulm Med 23, 131 (2023). https://doi.org/10.1186/s12890-023-02404-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02404-9