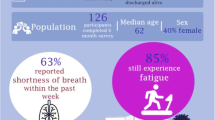
Abstract
Background
The pathophysiology, evolution, and associated outcomes of post-COVID dyspnea remain unknown. The aim of this study was to determine the prevalence, severity, and predictors of dyspnea 12 months following hospitalization for COVID-19, and to describe the respiratory, cardiac, and patient-reported outcomes in patients with post-COVID dyspnea.
Methods
We enrolled a prospective cohort of all adult patients admitted to 2 academic hospitals in Vancouver, Canada with PCR-confirmed SARS-CoV-2 during the first wave of COVID between March and June 2020. Dyspnea was measured 3, 6, and 12 months after initial symptom onset using the University of California San Diego Shortness of Breath Questionnaire.
Results
A total of 76 patients were included. Clinically meaningful dyspnea (baseline score > 10 points) was present in 49% of patients at 3 months and 46% at 12 months following COVID-19. Between 3 and 12 months post-COVID-19, 24% patients had a clinically meaningful worsening in their dyspnea, 49% had no meaningful change, and 28% had a clinically meaningful improvement in their dyspnea. There was worse sleep, mood, quality of life, and frailty in patients with clinically meaningful dyspnea at 12 months post-COVID infection compared to patients without dyspnea. There was no difference in PFT findings, troponin, or BNP comparing patients with and without clinically meaningful dyspnea at 12 months. Severity of dyspnea and depressive symptoms at 3 months predicted severity of dyspnea at 12 months.
Conclusions
Post-COVID dyspnea is common, persistent, and negatively impacts quality of life. Mood abnormalities may play a causative role in post-COVID dyspnea in addition to potential cardiorespiratory abnormalities. Dyspnea and depression at initial follow-up predict longer-term post-COVID dyspnea, emphasizing that standardized dyspnea and mood assessment following COVID-19 may identify patients at high risk of post-COVID dyspnea and facilitating early and effective management.
Similar content being viewed by others
Background
Dyspnea is a common symptom following COVID-19 and has a significant impact on quality of life [1,2,3]; however, our understanding of the mechanisms of dyspnea in this specific context also remain limited. Although some patients have cardiopulmonary abnormalities post-COVID, dyspnea can persist in others despite improvements in and normalization of cardiopulmonary function [4,5,6,7].
The underlying pathophysiology behind this ‘unexplained’ dyspnea remains unknown, with previous studies showing conflicting results. While some studies showed no difference between healthy controls and patients with post-COVID dyspnea [8], others have found exercise intolerance with evidence of circulatory and breathing pattern abnormalities [9, 10]. There is similarly only limited understanding of the evolution of post-COVID dyspnea and associated outcomes over time, and it is difficult to predict the severity of long-term respiratory symptoms following recovery from the acute phase of COVID-19.
The aim of this study was to determine the prevalence, severity, and predictors of dyspnea at 12 months following hospitalization for COVID-19, and to describe the respiratory, cardiac, and patient-reported outcomes in patients with post-COVID dyspnea. These findings would help patients and clinicians better understand the heterogeneous causes of persistent post-COVID dyspnea such that more appropriate management approaches can be considered depending on the specific clinical scenario.
Methods
Study population
We enrolled a prospective cohort of patients from the Post-COVID-19 Respiratory Clinic (PCRC) located at two academic hospitals in Vancouver, Canada [3, 5, 6]. All adult patients admitted to hospital with PCR-confirmed SARS-CoV-2 during the first wave of COVID between March and June 2020 within the Vancouver Coastal Health Authority were automatically referred to the PCRC for follow-up care after hospital discharge. Eligibility criteria included hospitalization for COVID-19, ability to complete study questionnaires in English, and provision of informed consent. There were no exclusion criteria. Research ethics board approval was obtained from the University of British Columbia (#H20-01,239).
Measurements
Patients were initially assessed 3 months post-symptom onset, with subsequent follow-up occurring at the 6- and 12-month mark. Patients completed a standardized set of questionnaires and investigations at each visit. Questionnaires included the 5-level EuroQoL 5-Dimensions (EQ-5D-5L) [11], Frailty Index [12], University of California San Diego Shortness of Breath (UCSD) Questionnaire [13], Patient Health Questionnaire-9 (PHQ-9) [14], and Pittsburgh Sleep Quality Index (PSQI) [15]. The EQ-5D-5L measures health-related quality of life based on 5 dimensions that include mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The Frailty Index quantifies frailty based on accumulated health deficits, which can predict mortality and other health outcomes. The UCSD questionnaire is a validated tool for grading the severity of dyspnea in respiratory diseases. Clinically meaningful dyspnea at baseline was defined as a UCSD score > 10, equating to a value that is double the previously established minimal important difference (MID) [16, 17]. The PHQ-9 is a self-administered tool for grading the severity of depression. A mood abnormality was defined as a PHQ-9 score ≥ 5, which has been previously validated as the threshold for mild depression [14]. The PSQI rates sleep quality over a 1-month interval. Higher values on the questionnaires represent worse outcomes except for the EQ-5D-5L.
Standard investigations performed at each clinic visit included standardized bloodwork, detailed pulmonary function tests (PFTs), transthoracic echocardiogram (TTE; only performed at the 3-month visit), and 6-min walk test (6MWT) [5, 6]. PFTs were conducted in accordance with international guidelines, with values < 80% predicted considered abnormal for forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), total lung capacity (TLC), residual volume (RV), and diffusion capacity of carbon monoxide (DLCO) [18,19,20,21]. TTEs were conducted in accordance with international guidelines and were interpreted by cardiologists with advanced echocardiography training [22, 23]. A normal left ventricular ejection fraction (LVEF) was defined as ≥ 50% and a normal pulmonary artery systolic pressure (PASP) was defined as less than < 30 mmHg based on international guidelines [22].
Statistical analysis
Data are shown as mean ± standard deviation, median (interquartile range), or number (percent). Student’s t-tests, Wilcoxon signed rank tests, or Spearman’s rank correlations were used to compare measurements between 3 and 12 months, and dyspneic and non-dyspneic patients, with the choice of test depending upon variable type (dichotomous vs continuous) and variable distribution (for continuous variables). Associations between pre-defined predictor variables and UCSD scores were determined using multivariable linear regression models. Prespecified covariates included age, sex, and body mass index (BMI). Statistical significance was defined as a two-tailed p-value < 0.05, with STATA 16.1 used for data analysis (StataCorp, Texas).
Results
Patient characteristics
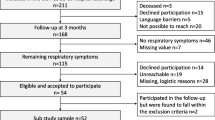
A total of 76 patients were included (Fig. 1). Pre-existing respiratory disease was present in 29% of patients with clinically meaningful dyspnea at 12 months post-COVID and 15% of patients without dyspnea (Table1). The median length of admission to hospital was 11 (5–17) days in dyspneic patients and 6.5 (5–11) days in non-dyspneic patients. The frequency of critical care admission and mechanical ventilation was similar among patients with and without dyspnea at 12 months post-COVID. Median length of supplemental oxygen requirement was 11 (7–19) days in dyspneic patients, compared to 5 (3–11) in non-dyspneic patients.
Dyspnea at 12 months post-COVID-19 infection
Evolution of dyspnea scores over time is shown in Fig. 2. Clinically meaningful dyspnea was present in 37 (49%) of patients at 3 months and 35 (46%) of patients at 12 months following COVID-19, with median UCSD scores of 10 (3–25) and 9 (2–23) respectively (p = 0.54). Of patients who were dyspneic at 12 months, 28 (80%) had dyspnea that had persisted from their 3-month visit, and 7 (20%) had new-onset of clinically meaningful dyspnea. Between 3 and 12 months post-COVID-19, 18 (24%) patients had a clinically meaningful worsening in their dyspnea, 37 (49%) had no meaningful change, and 21 (28%) of patients had a clinically meaningful improvement in their dyspnea. There was no significant difference in the severity of dyspnea comparing patients who did and did not require mechanical ventilation or comparing those requiring and not requiring intensive care unit (ICU) admission.
Dyspnea over time following COVID-19. A clinically meaningful change in dyspnea was defined as a change in UCSD score equal to or greater than 5, based on the previously established MCID for the UCSD questionnaire. Change in dyspnea, as represented by the different colours of dots, is relative to the dyspnea score at 3 months post-COVID-19
Association of dyspnea with other patient-reported, respiratory, and cardiac outcomes
There was worse sleep, mood, quality of life, and frailty in patients with clinically meaningful dyspnea at 12 months post-COVID infection compared to patients without dyspnea (Fig. 3). There was no statistically significant difference in PFT findings comparing patients with and without clinically meaningful dyspnea at 12 months post-COVID-19 infection (Fig. 3). However, patients with dyspnea had a lower percent-predicted 6-min walk distance (91 ± 15% vs 102 ± 16%; p = 0.01) compared to patients without dyspnea. There was no significant difference in the troponin or B-type natriuretic peptide (BNP) levels of patients with and without dyspnea at 12 months post-COVID-19 infection.
Patient-reported, respiratory, and cardiac outcomes 12 months post-COVID-19 stratified by presence and absence of dyspnea. Abbreviations: FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; TLC, total lung capacity; RV, residual volume; DLCO, diffusing capacity for carbon monoxide; BNP, B-type natriuretic peptide
Of the 35 patients with clinically meaningful dyspnea at 12 months, 22 (63%) had PFT abnormalities, 7 (20%) had abnormal troponin or BNP levels, 13 (37%) had an abnormal depression score, and 5 (14%) had none of these findings. The mean dyspnea score of dyspneic patients with only a PFT abnormality was 25, compared to a mean score of 32 for dyspneic patients with only a mood abnormality (Fig. 4). Patients with multiple abnormalities had a mean dyspnea score of 36. The most common PFT abnormality in dyspneic patients was a decreased DLCO, which was present in 16 patients (46% of those with dyspnea).
Predictors of dyspnea at 12 months post-COVID-19 infection
The severity of dyspnea and depressive symptoms at 3 months post-COVID-19 infection predicted severity of dyspnea at 12 months post-COVID (Table 2). Neither PFT nor cardiac findings at 3 months predicted dyspnea severity at 12 months. Sensitivity analysis excluding patients with pre-existing respiratory and cardiac comorbidities showed similar findings.
Discussion
This prospective cohort shows that dyspnea is a frequent symptom following COVID-19, and that most patients with dyspnea do not experience a meaningful improvement in the severity of their symptoms in the first year following infection. We also found that dyspnea was associated with worse sleep, mood, quality of life, and frailty in patients at 12 months post-COVID; however, there was no statistically significant difference in pulmonary function comparing dyspneic and non-dyspneic patients post-COVID. The severity of dyspnea and depressive symptoms at 3 months post-COVID were the only predictors of the severity of dyspnea at 12 months post-COVID. Together, these findings highlight the multifaceted nature of post-COVID dyspnea, with many patients having ongoing dyspnea for reasons other than overt pulmonary or cardiac consequences of COVID-19.
Mood may play a role in post-COVID dyspnea and can predict which patients are at risk for significant persistent dyspnea at 12 months. However, it is unclear whether dyspnea itself is driving these mood abnormalities, or whether the mood abnormality is instead contributing to the development of dyspnea. PTSD as a result of being hospitalized with COVID during a global pandemic may also be playing a role, as it is highly prevalent and associated with more persistent physical symptoms post-COVID [24]. There is a trend towards DLCO being reduced in patients with significant dyspnea at 12 months post-COVID, suggesting a possible underlying persistent cardiopulmonary abnormality that may be contributing to the development of dyspnea in some patients. There is also heterogeneity in the dyspneic patients as some have solely reduced DLCO, while others have primarily mood abnormalities or combinations of PFT, cardiac, and mood abnormalities. Our findings emphasize that dyspnea in the post-COVID context is a complex sensory experience that may be influenced by mood in addition to possible underlying cardiopulmonary pathology, and there may be additional factors that are contributing. Previous studies have established decreased peripheral oxygen delivery and abnormal ventilatory response to aerobic activity as mechanisms of post-COVID dyspnea in the absence of cardiopulmonary limitations on invasive cardiopulmonary exercise testing [9, 10]. Furthermore, based on our findings, the degree of contribution from these factors and the resulting severity of dyspnea appears to differ from patient to patient.
Post-COVID dyspnea was a persistent problem in our patient cohort, with 49% of patients hospitalized for acute COVID reporting no change in their dyspnea, 24% reporting an increase in their dyspnea, and 20% developing new-onset of clinically meaningful dyspnea at the 12-month mark when compared to 3 months post-COVID. The reason for the increase and new onset of dyspnea at the 12-month mark in a subset of patients is unclear but given the similar PFT findings in dyspneic and non-dyspneic patients, other suggested mechanisms may be implicated including changes in mood, peripheral oxygen delivery, and ventilatory response to aerobic activity over time [9, 10, 24]. Furthermore, the morbidity associated with the dyspnea is also persistent, as evidenced by poorer outcomes in all patient-reported variables in those who had persistent dyspnea at 12 months. Interestingly, the severity of the acute infection does not appear to influence the degree of post-COVID dyspnea, as neither the need for ICU admission or for mechanical ventilation were associated with higher dyspnea scores. Previous studies on long term outcomes in patients with acute respiratory distress syndrome (ARDS) from various etiologies have also demonstrated similar prevalence of dyspnea and mood abnormalities as well as reduced DLCO at 12 months post-ARDS, but the study populations entirely consisted of patients who required mechanical ventilation [25, 26]. We demonstrated similar long-term findings despite 82% of our patients not requiring mechanical ventilation and 51% not requiring ICU admission. This emphasizes the need to use standardized dyspnea and depression assessment tools, rather than traditional indicators like severity of illness or level of oxygen requirements, to identify patients who are at higher risk of post-COVID dyspnea given the multiple determinants of dyspnea beyond the degree of lung injury and resultant pulmonary pathology.
The persistent dyspnea and associated morbidity suggests potential benefit of using a validated dyspnea questionnaire at post-COVID follow-up visits, with similar rationale supporting utility of a standardized mood questionnaire. These can be applied in a variety of settings, including primary care clinics to both identify abnormalities and follow change over time, including potential response to intervention. Dyspnea and mood questionnaires could also be used at the time of discharge from hospital to determine who would benefit from outpatient follow-up and resources. Patients identified to have post-COVID dyspnea may benefit from early referral to supportive counselling and other psychiatric resources given the association of mood abnormalities with dyspnea. As well, dyspneic patients may also benefit from referral to pulmonary rehab, as previous literature demonstrates improvement in dyspnea and mood in the post-COVID patient population [27].
Limitations of our study include the lack of baseline characteristics for the patients prior to the initial COVID-19 diagnosis. We attempted to account for any baseline cardiopulmonary abnormalities by performing a sensitivity analysis that excluded patients with known pre-existing cardiac or pulmonary disease, which yielded consistent findings to the analysis based on all included patients. Another limitation of our study is that newer variants of SARS‑CoV‑2 may result in varying prevalence and severity of post-COVID dyspnea compared to our study cohort compared to the α variant that was responsible for wave 1 infections. Vaccination may also influence the prevalence and severity of post-COVID dyspnea [28, 29], which was not available at the time of initial infection. Thus, our results may not be generalizable to all post-COVID patient populations. Repeating this study in vaccinated and non-hospitalized post-COVID patients would help characterize post-COVID dyspnea in these populations.
In summary, post-COVID dyspnea is common, persistent, and has a significant impact on quality of life. Mood abnormalities may play a role in post-COVID dyspnea in addition to potential cardiorespiratory abnormalities. Dyspnea and depression at initial follow-up predict longer-term post-COVID dyspnea, emphasizing the need for standardized dyspnea and mood assessment following COVID-19 to identify patients at higher risk of post-COVID dyspnea and facilitate early and effective management.
Availability of data and materials
The datasets used and/or analysed during the current study are not openly available as they contain information that could compromise research participant privacy and/or consent. Data may be available from the corresponding author on reasonable request.
Abbreviations
- EQ-5D-5L:
-
5-Level EuroQoL 5-Dimensions
- UCSD:
-
University of California San Diego Shortness of Breath Questionnaire
- PHQ-9:
-
Patient Health Questionnaire-9
- PSQI:
-
Pittsburgh Sleep Quality Index
- MID:
-
Minimal important difference
- PFTs:
-
Pulmonary function tests
- TTE:
-
Transthoracic echocardiogram
- 6MWT:
-
6-Minute walk test
- FVC:
-
Forced vital capacity
- FEV1:
-
Forced expiratory volume in the first second
- TLC:
-
Total lung capacity
- RV:
-
Residual volume
- DLCO:
-
Diffusion capacity of carbon monoxide
- LVEF:
-
Left ventricular ejection fraction
- PASP:
-
Pulmonary artery systolic pressure
- ICU:
-
Intensive care unit
- BNP:
-
B-type natriuretic peptide
- ARDS:
-
Acute respiratory distress syndrome
References
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32.
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X, Qu Y, Fan Y, Li X, Li C, Yu T, Xia J, Wei M, Chen L, Li Y, Xiao F, Liu D, Wang J, Wang X, Cao B. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–58.
Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J. 2020;56(5):2003276.
Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):2003448.
Shah AS, Wong AW, Hague CJ, Murphy DT, Johnston JC, Ryerson CJ, Carlsten C. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76(4):402–4.
Shah AS, Ryu MH, Hague CJ, Murphy DT, Johnston JC, Ryerson CJ, Carlsten C, Wong AW. Changes in pulmonary function and patient-reported outcomes during COVID-19 recovery: a longitudinal, prospective cohort study. ERJ Open Res. 2021;7(3):00243–2021.
Lam GY, Befus AD, Damant RW, Ferrara G, Fuhr DP, Stickland MK, Varughese RA, Wong EY, Smith MP. Exertional intolerance and dyspnea with preserved lung function: an emerging long COVID phenotype? Respir Res. 2021;22(1):222.
Beaudry RI, Brotto AR, Varughese RA, de Waal S, Fuhr DP, Damant RW, Ferrara G, Lam GY, Smith MP, Stickland MK. Persistent dyspnea after COVID-19 is not related to cardiopulmonary impairment; a cross-sectional study of persistently dyspneic COVID-19, non-dyspneic COVID-19 and controls. Front Physiol. 2022;6(13): 917886.
Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, Possick JD, Systrom DM, Waxman AB. Persistent Exertional Intolerance After COVID-19: Insights From Invasive Cardiopulmonary Exercise Testing. Chest. 2022;161(1):54–63.
Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of Cardiopulmonary Stress Testing for Patients With Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. 2021;9(12):927–37.
Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht (NL): Springer; 2014.
Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–94.
Eakin EG, Resnikoff PM, Prewitt LM, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–24.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil. 2005;25(6):370–7.
Chen T, Tsai APY, Hur SA, Wong AW, Sadatsafavi M, Fisher JH, Johannson KA, Assayag D, Morisset J, Shapera S, Khalil N, Fell CD, Manganas H, Cox G, To T, Gershon AS, Hambly N, Halayko AJ, Wilcox PG, Kolb M, Ryerson CJ. Validation and minimum important difference of the UCSD Shortness of Breath Questionnaire in fibrotic interstitial lung disease. Respir Res. 2021;22(1):202.
Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–61.
Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–88.
Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22.
Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, MacIntyre NR, Thompson BR, Wanger J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016.
Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–84.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14.
Janiri D, Carfì A, Kotzalidis GD, Bernabei R, Landi F, Sani G. Gemelli Against COVID-19 Post-Acute Care Study Group. Posttraumatic Stress Disorder in Patients After Severe COVID-19 Infection. JAMA Psychiatry. 2021;78(5):567–9.
Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1120–8.
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93.
Gloeckl R, Leitl D, Jarosch I, Schneeberger T, Nell C, Stenzel N, Vogelmeier CF, Kenn K, Koczulla AR. Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ Open Res. 2021;7(2):00108–2021.
Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, Rescigno M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA. 2022;328(7):676–8.
Office of National Statistics.. Self-reported long COVID after infection with the Omicron variant in the UK: 18 July 2022. ONS, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidafterinfectionwiththeomicronvariant/18july2022.
Acknowledgements
Not applicable.
Funding
This work was funded by the Michael Smith Foundation for Health Research, the TB Vets charitable foundation, the Vancouver Coastal Health Research Institute, and the University of British Columbia’s Strategic Investment Fund. The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
J.S.G. and C.J.R. were responsible for conception and design of the study. All authors contributed to data collection, analysis, and interpretation. J.S.G. and C.J.R. drafted the manuscript, and all authors critically reviewed the manuscript and have approved this submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research ethics board approval was obtained from the University of British Columbia (#H20-01239). Informed consent was obtained from all subjects and/or their legal guardian(s). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Grewal, J.S., Carlsten, C., Johnston, J.C. et al. Post-COVID dyspnea: prevalence, predictors, and outcomes in a longitudinal, prospective cohort. BMC Pulm Med 23, 84 (2023). https://doi.org/10.1186/s12890-023-02376-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02376-w