Abstract
Background
CT Severity Score (CT-SS) can be used to assess the extent of severe coronavirus disease 19 (COVID-19) pneumonia. Follow-up CT-SS in patients surviving COVID-19-associated hyperinflammation and its correlation with respiratory parameters remains unknown. This study aims to assess the association between CT-SS and respiratory outcomes, both in hospital and at three months after hospitalization.
Methods
Patients from the COVID-19 High-intensity Immunosuppression in Cytokine storm Syndrome (CHIC) study surviving hospitalization due to COVID-19 associated hyperinflammation were invited for follow-up assessment at three months after hospitalization. Results of CT-SS three months after hospitalization were compared with CT-SS at hospital admission. CT-SS at admission and at 3-months were correlated with respiratory status during hospitalization and with patient reported outcomes as well as pulmonary- and exercise function tests at 3-months after hospitalization.
Results
A total of 113 patients were included. Mean CT-SS decreased by 40.4% (SD 27.6) in three months (P < 0.001). CT-SS during hospitalization was higher in patients requiring more oxygen (P < 0.001). CT-SS at 3-months was higher in patients with more dyspnoea (CT-SS 8.31 (3.98) in patients with modified Medical Council Dyspnoea scale (mMRC) 0–2 vs. 11.03 (4.47) in those with mMRC 3–4). CT-SS at 3-months was also higher in patients with a more impaired pulmonary function (7.4 (3.6) in patients with diffusing capacity for carbon monoxide (DLCO) > 80%pred vs. 14.3 (3.2) in those with DLCO < 40%pred, P = 0.002).
Conclusion
Patients surviving hospitalization for COVID-19-associated hyperinflammation with higher CT-SS have worse respiratory outcome, both in-hospital and at 3-months after hospitalization. Strict monitoring of patients with high CT-SS is therefore warranted.
Similar content being viewed by others
Background
Coronavirus disease 19 (COVID-19) is a heterogeneous [1]. Chest computed tomography (CT) scan can be helpful to diagnose a COVID-19 pneumonia. Chest CT can be of added value to differentiate between high or low probability of the infection, as reported in the CO-RADS [2]. Furthermore, chest CT can be used to visualize the extent of the disease, as reported in the CT Severity Score (CT-SS)[3, 4]. Previous research has shown that CT-SS in the acute phase of the disease correlates with disease burden and mortality risk [3, 5,6,7,8,9]. Few studies examined the medium- to long-term radiological abnormalities after COVID-19 and the possible correlation with clinical symptoms during admission. Patients surviving severe COVID-19 have higher prevalence of residual lesions on the chest CT scan in comparison with patients surviving mild COVID-19 [10, 11]. Furthermore, one-third of patients had pulmonary fibrotic-like changes six months after severe COVID-19, and these changes were associated with a higher CT-SS at hospital [12]. Nevertheless, none of the studies assessed the relationship between the severity of pulmonary involvement and respiratory outcomes, i.e. the correlation of CT-SS with pulmonary- and exercise functions tests.
A proportion of COVID-19 patients develop a hyperinflammatory state, which leads to an increased risk of respiratory insufficiency and thromboembolic events, and therefore to a higher mortality [13].
The use of immunosuppressive therapy in this state of severe COVID-19 accelerates respiratory recovery and reduces mortality [14,15,16], therefore glucocorticoids and tocilizumab are recommended in this phase [17]. None of the studies published so far focused on patients with COVID-19-associated hyperinflammation or assessed the influence of glucocorticoids and tocilizumab on the resolution of radiological findings.
The primary objective of this study was to investigate the association between CT-SS and respiratory outcomes at both the moment of hospitalization and at three months after hospitalization.
The secondary objective was to investigate whether intensive short-term immunosuppressive therapy for COVID-19 was associated with a lower CT-SS at three months after hospitalization compared with patients who did not receive this immunosuppressive therapy.
Methods
Study design and subjects
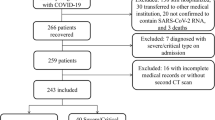
This prospective cohort study included patients who survived hospitalization for COVID-19-associated hyperinflammation. Between March and May 2020, patients hospitalized for COVID-19-associated hyperinflammation in the Zuyderland Medical Centre (ZMC), a large teaching hospital in the Netherlands, were included in the COVID High-intensity Immunosuppression in Cytokine storm syndrome (CHIC) study [14]. COVID-19-associated hyperinflammation was defined according to a set of criteria: oxygen saturation at rest ≤ 94% or tachypnoea (> 30/min); at least two out of three biomarker criteria: CRP > 100 mg/L, serum ferritin > 900 µg/L at one occasion or a twofold increase of the level at admission within 48 h and D-dimer level > 1500 µg/ [14]. From March 1st to April 1st 2020, patients were treated with standard of care (control group), consisting of oxygen support, antibiotics, chloroquine and anticoagulation. After April 1st, patients were treated according to the CHIC protocol, which was added to standard of care (treated group). This protocol included two steps: (1) intravenous methylprednisolone 250 mg on day 1, followed by methylprednisolone 80 mg intravenously on days 2–5, and an option for a two-day extension; (2) addition of tocilizumab (single dose, 8 mg/kg body weight intravenous, max 800 mg) in case of lack of improvement or worsening in respiratory status 48 h after starting with methylprednisolone. Results confirming the benefits of this therapeutical strategy during the acute setting of COVID-19-associated hyperinflammation have been published [14]. All survivors of this study were invited for an ambulatory follow-up visit at the Pulmonology department of ZMC. Patients were excluded if they were unable to visit the outpatient clinic.
Approval was obtained by the Medical Ethical Committee (METC) and the Board of ZMC, the Netherlands (number METCZ20200126). All patients provided written informed consent for the use of their data for this study.
Procedures and data collection
Patients were invited to the outpatient clinic at three months after hospital discharge. Patients were assessed by a pulmonologist and pulmonary- and exercise function tests and a chest CT were performed. Furthermore, patients completed questionnaires.
Chest CT protocol and evaluation
Chest CT scans were performed at the same time in each patient, namely during hospitalization at the moment of fulfillment of the criteria used for defining hyperinflammation and at three months after hospitalization. Chest CT was performed on either a 64-slice CT scanner (Philips Incisive) or on a 64-slice dual source scanner (Siemens Somatom Definition Flash). Scanning parameters were: collimation 64 × 0.625 or 0.6 mm,120 kVp, 667 max mA or 404 max mA, pitch 1.0 or 1.2, and matrix size 512 × 512. CT images were reconstructed with a lung kernel in the transverse plane with 1.0-mm slice thickness and 1.0-mm increment. Images were also reconstructed in axial, coronal, and sagittal planes with 3.0-mm slice thickness. All images were stored in the local picture archiving and communication system (Sectra IDS7 PACS). Severity of pulmonary involvement was assessed using CT-SS [3]. Per each of the five pulmonary lobes, the anatomic parenchymal involvement was calculated: 0, no involvement; 1, < 5% involvement; 2, 2–25% involvement; 3, 26–50% involvement; 4, 51–75% involvement; 5, > 75% involvement. CT-SS was the result of the sum of each individual lobar score (total score 0–25) [3]. For the purpose of this study, CT-SS was retrospectively scored by one experienced thoracic radiologist and one experienced pulmonologist, they scored the CT scans independently from each other and with known chronological order. They were blinded for the patients’ clinical information, including the therapy received during hospitalization. The scores of both readers were assessed separately and subsequently averaged. Reliability of the CT-SS between both readers was assessed using the intraclass correlation coefficient (ICC).
Patient reported outcomes
Patients completed the modified Medical Research Council dyspnoea scale (mMRC) to assess perceived functional limitations of breathlessness, the Hospital Anxiety and Depression Scale (HADS) questionnaire to detect states of anxiety and depression and the EuroQol 5-dimensions 5-levels (EQ-5D-5 L) questionnaire for measuring quality of [18,19,20,21].
Pulmonary- and exercise function tests
Forced vital capacity (FVC), total lung capacity (TLC) and diffusing capacity for carbon monoxide (DLCO) were tested with a pneumotachograph (Vyaire Medical, Jaeger, Würzburg, Germany). DLCO was measured using the single-breath carbon monoxide uptake. Values were calculated using Global Lung Function Initiative network references. A six-minute walk test (6MWT) with pulse oximetry was performed to objectively evaluate functional exercise. Pulmonary function tests and 6MWT were performed according to the European Respiratory Society and American Thoracic Society guidelines and values were expressed as a percentage of predicted values [22,23,24,25].
Statistical analysis
Reliability of the CT-SS, for both status scores at baseline and 3 months as well as change score (baseline minus 3-month score) was assessed using the intraclass correlation coefficient (ICC) with two-way mixed model and absolute agreement.
Descriptive statistics, namely mean and range values, were used for all outcomes. Differences in baseline characteristics between treatment groups were computed with a Mann-Whitney U test or a χ2/Fisher’s exact test. CT-SS between hospital CT scans and follow-up CT scans were compared using a paired samples t-test.
An independent samples t-test or Mann-Whitney U test was used for detecting differences in CT-SS between two different groups and one-way ANOVA or Kruskal Wallis was used for detecting differences in CT-SS across more than two groups. ANCOVA was used to correct for baseline values.
A p-value ≤ 0.05 was considered statistically significant. Data were analysed with the statistical package SPSS Statistics version 26.
Results
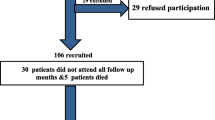
One hundred thirteen patients were included in this follow-up, having their CT scan at three months after hospitalization. Of all 113 patients, 107 performed a chest CT during hospitalization and after three months. Patient inclusion is shown in Fig. 1 and baseline characteristics are presented in Table 1. The mean age was 63 (SD 11) years and 82% of the patients were male. Hypertension was the most common comorbidity. At the moment of fulfillment of the criteria for hyperinflammation, 46% of the patients in the control group needed an oxymask/non-rebreathing mask, high flow nasal canula (HFNC) or mechanical ventilation, versus 45% in the treated group, though more patients in the control group were mechanically ventilated (16% vs. 1.6%).
The ICC between both readers was 0.87 (95% CI 0.50–0.94) and 0.89 (95% CI 0.79–0.93) for the chest CT performed during hospitalization and at the three-month follow-up visit, respectively. The ICC for the change in CT-SS between hospitalization and three-month follow-up was 0.91 (95% CI 0.87–0.94).
At baseline, i.e. during hospitalization, the mean CT-SS was 15.2 (SD 3.8). At the three-month follow-up visit, the mean CT-SS was 8.8 (SD 4.2). The mean CT-SS decreased significantly by 6.4 (SD 4.6), which corresponds to a 40.4% (SD 27.6) decrease in three months (P < 0.001). Figure 2 shows demonstrative CT scan images of two patients with pulmonary abnormalities at hospitalization and at the three-month follow-up visit.
Demonstrative CT scan images. 68-year old man with CT-SS of 19 at the acute phase of COVID-19 (A and B) and CT-SS of 0 at the three-month follow-up visit (C and D). Pulmonary function tests and six minute walk test at the three month follow-up visit were unremarkable. 86-year old patient with CT-SS of 16 at the acute phase of COVID-19 (E and F) and CT-SS of 13 at the three month follow-up visit (G and H). Pulmonary function test at the three month follow-up visit showed a TLC (Total Lung Capacity) of 58% predicted and a DLCO (Diffusing Capacity for Carbon Monoxide) of 29% predicted. The six minute walk test was not feasible due to the clinical condition
CT-SS during hospitalization and relationship with respiratory outcomes
Mean CT-SS was significantly higher in patients requiring more oxygen and in patients requiring more oxygen suppletion anytime during hospitalization (18.3 (3.7) for patients requiring mechanical ventilation vs. 13.6 (3.1) for patients requiring nasal oxygen, with a decreasing gradient between both groups, P < 0.001) (Table 2).
Patients with a higher CT-SS during hospitalization had a more impaired pulmonary function three months after hospitalization (16.6 (4.0) for patients with a TLC < 80%pred vs. 14.8 (3.7) for patients with a TLC ≥ 80%pred, P = 0.041, and 17.4 (4.2) for patients with a DLCO < 40%pred vs. 14.0 (3.9) for patients with a DLCO > 80%pred, P = 0.026). For DLCO, higher CT-SS was associated with a lower DLCO. Patients with a more pronounced desaturation during 6MWT did not have a significantly higher CT-SS during hospitalization, however the minimum CT-SS was higher in the group with a more pronounced desaturation (Table 3).
CT-SS at three-month follow-up and relationship with respiratory outcomes
Mean CT-SS at three months was significantly higher in patients who required more oxygen during hospitalization, with an increasing gradient with increasing oxygen suppletion (p = 0.007) (Table 4).
Patients with a more impaired pulmonary function had a significantly higher mean CT-SS, with an increasing gradient of CT-SS with a decreasing DLCO (P < 0.001 and P = 0.002 for TLC and DLCO, respectively). Patients with a more pronounced desaturation during 6MWT did not have a significantly higher CT-SS at that time (P = 0.098) (Table 3).
Higher mMRC score at the three-month follow-up visit was associated with higher CT-SS at three months. Mean CT-SS was 8.31 (SD 3.98) in patients with a reported mMRC of 0–2 and 11.03 (SD 4.47) in patients with a reported mMRC of 3–4 (P = 0.020).
HADS and EQ-5D questionnaire results did not correlate with CT-SS (HADS anxiety Spearman’s rho
-0.104, HADS depression Spearman’s -0.120, EQ-5D VAS Spearman’s rho 0.141).
Differences in CT-SS between treated and control groups
During hospitalization, CT-SS of the treated group (methylprednisolone with or without tocilizumab) was 14.60 (SD 3.46) and CT-SS of the control group (i.e. standard care / no immunomodulatory therapy) was 16.10 (SD 4.09), P = 0.042. At three-month follow-up, CT-SS was 9.47 (SD 4.25) and 7.91 (SD 3.86) for the treated and control group, respectively (P = 0.055). Corrected for baseline differences in CT-SS, gender, age and important comorbidities (hypertension, diabetes mellitus, COPD, cardiovascular disease, cerebrovascular disease, auto-immune disease), the difference between the groups at the three-month follow-up visit was statistically significant (p = 0.029).
The decrease in CT-SS between hospitalization and the three month follow-up visit was lower for the treatment group in comparison with the control group (5.13 (SD 4.56) versus 8.19 (SD 4.16), P = 0.001). Corrected for the same variables as previously mentioned, the difference between the treatment and control groups remained statistically significant (p = 0.004).
Discussion
This prospective cohort study shows that chest CT abnormalities decrease significantly in three months after surviving hospitalization for COVID-19-associated hyperinflammation. Patients with a higher CT-SS (in hospital and at three months) required higher maximum oxygen suppletion during hospitalization, had significant more impaired TLC and DLCO measurements and experienced more dyspnea at three months after hospitalization.
Based on these results, higher CT-SS may be used as an indicator for worse respiratory status during hospitalization, more dyspnoea and pulmonary function impairments after three months. CT-SS may therefore be used for evaluating individual burden of disease and physicians should be aware of a possible worse outcome in patients with higher CT-SS.
In the acute phase of the pandemic (especially first quarter 2020) it was common practice to use a chest CT for screening for COVID-19, mainly because of lack of polymerase chain reaction (PCR) tests. Nowadays, chest CT is not used for screening for COVID-19. The data from all performed CT-scans is helpful for follow-up though.
To our knowledge, this is the first study that compares systematically performed chest CT during the acute phase of the disease with medium-term follow-up chest CT in a homogeneous population of patients who survived COVID-19-associated hyperinflammation.
Previous studies found cross-sectional associations between higher CT-SS and more severe disease during hospitalization [3, 7, 26, 27], however data on follow-up CT-SS were not included. Other studies found that more radiological abnormalities at six and 12 months after hospitalization were associated with more oxygen requirement during hospitalization [11, 28], which is consistent with our findings. However, one study was performed retrospectively and included only 48 patients [11], the other study excluded patients with severe disease and comorbidities [28]. Additionally, in contrast to the current study, none of the previous studies performed systematically a chest CT at the acute phase of the disease. Furthermore, none of the studies assessed the relationship between CT-SS during and after hospitalization with patient reported-, pulmonary- and exercise test outcomes. CT-SS at the moment of COVID-19 differed between studies; however, one study including only patients with severe COVID-19 also found a mean CT-SS of 15 [12].
As expected, CT-SS was higher in patients requiring more oxygen. SARS-CoV-2 mainly affects the pulmonary alveoli and the surrounding vascular components. Interstitial inflammatory infiltrates and edema lead to hypoxemia. More infiltrates and edema will lead to more severe respiratory [1]. Over time, alveolar infiltrates and edema will diminish. Persisting radiological abnormalities, as well as an impaired TLC and DLCO may be the result of incomplete resolution of this damage. However, it may be possible that part of the patients had pre-existing (fibrotic) radiological abnormalities or pulmonary function impairments due to other diseases, as we did not have chest CT data or pulmonary function data before onset of COVID-19. Our study showed no higher CT-SS in patients with a more pronounced desaturation during 6MWT, however the minimum CT-SS was higher with increasing desaturation. Due to the small numbers it is hard to draw robust conclusions.
The improvement in CT-SS after three months was higher in the control group than in the treated group, even after correction for baseline differences. Also, CT-SS at 3 months was lower in the control group, compared to the treated group. However, caution must be taken in interpreting these results due to the differences between the groups at baseline. Importantly, in the CHIC study patients were included in a balanced way between treatment and control groups. In the current follow-up study, survivors of the CHIC study were included. The larger number of deaths in the CHIC-study control group, which are most likely the patients with the highest CT-SS, probably resulted in unbalanced baseline groups of the current study concerning this specific analysis [14]. This hampers a conclusion on the effect of treatment on CT-SS at 3 months. Moreover, results of pulmonary- and exercise function tests did not differ between these two groups at the three-month follow-up visit [29]. The beneficial effects of immunosuppression are therefore mainly limited to the first weeks after administation [14].
Strengths of our study are the prospective design, the systematically performed chest CT at the acute phase of the disease, the comprehensive and standardized assessment after three months with chest CT, patient reported outcomes, pulmonary- and exercise tests and the homogeneous population, as all patients included had COVID-19-associated hyperinflammation during hospitalization, which makes this population unique and distinguishes this study from previous ones. The focus on patients with COVID-19-associated hyperinflammation allows to generalize conclusions to this specific population. Also, chest CT during hospitalization was performed at the same time in each patient, namely at the moment of fulfillment of the criteria used for defining hyperinflammation, making them comparable. Furthermore, the excellent inter-rater agreement in CT-SS supports the reproducibility of this radiological scoring system. Limitations of this study include the absence of chest CT data before onset of COVID-19, which is due to the acute and severe nature of the disease. Another limitation is that follow-up chest CT was not available in a small minority of all potentially eligible patients (14/127 eligible patients: 4 control group patients and 10 treatment group patients). Also, the results of the analyses of treatment effect on CT-SS should be interpreted with caution: the extent to which the treatment and the control group are comparable is unknown due to the higher number of deaths in the control group during hospitalization. Furthermore, the relatively small number of patients, especially in subgroups, is a limitation. Besides, no minimal clinical important difference is established in CT-SS, making it hard to draw robust conclusions about absolute differences in CT-SS between groups. Nevertheless, our findings support the hypothesis that patients with more radiological abnormalities have worse respiratory status and outcomes.
Conclusion
Patients surviving hospitalization for COVID-19-associated hyperinflammation with higher CT-SS have worse respiratory outcome, both in-hospital and at 3-months after hospitalization. Strict monitoring of patients with high CT-SS is therefore warranted. Follow-up studies are needed to evaluate CT-SS and the correlation with clinical outcomes on long-term.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 19
- CT-SS:
-
CT Severity Score
- ZMC:
-
Zuyderland Medical Centre
- CHIC:
-
COVID High-intensity Immunosuppression in Cytokine storm syndrome
- METC:
-
Medical Ethical Committee
- mMRC:
-
Modified Medical Research Council dyspnoea scale
- HADS:
-
Hospital Anxiety and Depression Scale
- EQ-5D-5L:
-
EuroQol 5-dimensions 5-levels
- FVC:
-
Forced vital capacity
- TLC:
-
Total lung capacity
- DLCO:
-
Diffusing capacity for carbon monoxide
- 6MWT:
-
Six-minute walk test
- ICC:
-
Intraclass correlation coefficient
- HFNC:
-
High flow nasal canula oxygen therapy
- NRM:
-
Non-rebreathing mask
- ECMO:
-
Extracorporeal membrane oxygenation
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
References
Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324:782–93.
Ng MY, Lee EYP, Yang J, et al. Imaging Profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging. 2020;2:e200034.
Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–17.
Yang R, Li X, Liu H, et al. Chest CT severity score: an Imaging Tool for assessing severe COVID-19. Radiol Cardiothorac Imaging. 2020;2:e200047.
Lieveld AWE, Azijli K, Teunissen BP, et al. Chest CT in COVID-19 at the ED: validation of the COVID-19 Reporting and Data System (CO-RADS) and CT severity score: a prospective, Multicenter, Observational Study. Chest. 2021;159:1126–35.
Sayeed S, Faiz BY, Aslam S, et al. CT chest severity score for COVID 19 pneumonia: a quantitative imaging Tool for Severity Assessment of Disease. J Coll Physicians Surg Pak. 2021;30:388–92.
Bellos I, Tavernaraki K, Stefanidis K, et al. Chest CT severity score and radiological patterns as predictors of disease severity, ICU admission, and viral positivity in COVID-19 patients. Respir Investig. 2021;59:436–45.
Popadic V, Klasnja S, Milic N et al. Predictors of Mortality in Critically Ill COVID-19 Patients Demanding High Oxygen Flow: A Thin Line between Inflammation, Cytokine Storm, and Coagulopathy. Oxid Med Cell Longev 2021; 2021: 6648199.
Abbasi B, Akhavan R, Ghamari Khameneh A, et al. Evaluation of the relationship between inpatient COVID-19 mortality and chest CT severity score. Am J Emerg Med. 2021;45:458–63.
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–32.
Wu Q, Zhong L, Li H et al. A Follow-Up Study of Lung Function and Chest Computed Tomography at 6 Months after Discharge in Patients with Coronavirus Disease 2019. Can Respir J 2021; 2021: 6692409.
Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–86.
Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73.
Ramiro S, Mostard RLM, Magro-Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79:1143–51.
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–45.
Landewé RBM, Ramiro S, Mostard RLM. COVID-19-induced hyperinflammation, immunosuppression, recovery and survival: how causal inference may help draw robust conclusions. RMD Open. 2021;7:e001638. https://doi.org/10.1136/rmdopen-001638.
World Health Organisation (. Therapeutics and COVID-19. Living guideline 6 July 2021. 2021.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Spinhoven P, Ormel J, Sloekers PP, et al. A validation study of the hospital anxiety and Depression Scale (HADS) in different groups of dutch subjects. Psychol Med. 1997;27:363–70.
Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective based on EQ-5D. 2014.
Williams N. The MRC breathlessness scale. Occup Med (Lond). 2017;67:496–7.
Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1600016. https://doi.org/10.1183/13993003.00016-2016. Print 2017 Jan.
Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An official american thoracic society and european respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88.
Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–4.
Holland AE, Spruit MA, Troosters T, et al. An official european respiratory Society/American thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–46.
Saeed GA, Gaba W, Shah A et al. Correlation between Chest CT Severity Scores and the Clinical Parameters of Adult Patients with COVID-19 Pneumonia. Radiol Res Pract 2021; 2021: 6697677.
Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of Coronavirus Disease (COVID-19) pneumonia: a Multicenter Study. AJR Am J Roentgenol. 2020;214:1072–7.
Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–54.
Janssen MT, Ramiro S, Mostard RL, et al. Three-month and six-month outcomes of patients with COVID-19 associated hyperinflammation treated with short-term immunosuppressive therapy: follow-up of the CHIC study. RMD Open. 2021;7:e001906. https://doi.org/10.1136/rmdopen-001906.
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MTHFJ: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing - Original Draft, Project administration; MGHT: Formal analysis, Writing - Original Draft, Project administration; JK: Methodology, Validation, Investigation, Writing - Review & Editing; MG: Methodology, Validation, Investigation, Writing - Review & Editing; SR: Conceptualization, Methodology, Writing - Review & Editing; CM: Conceptualization, Methodology, Writing - Review & Editing; RBML: Conceptualization, Methodology, Writing - Review & Editing; RLMM: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Study approval was obtained by the Medical Ethical Committee (METC) and the Board of Zuyderland Medical Centre in Heerlen, the Netherlands. Number: METCZ20200126. All methods were carried out in accordance with relevant guidelines and regulations. All patients provided written informed consent for the use of their data for this study.
Consent for publication
Not applicable.
Competing interests
SR reports personal fees from AbbVie, personal fees from Eli Lilly, grants and personal fees from MSD, personal fees from Novartis, personalfees from Sanofi, personal fees from UCB, outside the submitted work. RLMM reports personal fees from Boehringer Ingelheim, personal fees from Galapagos, outside the submitted work. Rest all authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Janssen, M.T., Thijssen, M.G., Krdzalic, J. et al. Three-month follow-up after severe COVID-19 infection: are chest CT results associated with respiratory outcomes and respiratory recovery in COVID-19 patients?. BMC Pulm Med 23, 74 (2023). https://doi.org/10.1186/s12890-023-02370-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02370-2