Abstract
Background
Pulmonary fibrosis (PF) is caused by a heterogeneous group of diseases, with a high inter-individual variability in disease trajectory. Identifying disease progression in patients with PF has impact on clinical management decisions. However, strategies to early identify and predict disease progression for these patients are currently lacking. In this study, we aim to assess long-term FVC change in patients with PF measured with home spirometry, and evaluate the feasibility of a multinational patient-led registry in PF. In addition, we will assess validity of patient-reported outcomes (PROMs) for the different subgroups of patients with PF.
Methods
In this international, prospective, multicenter, observational study, we aim to include 700 patients across seven European countries. Patients will monitor their disease course for a period of two years using an online home monitoring program (I-FILE), which includes home spirometry, pulse oximetry, and PROMs. Results will be directly sent to the hospital via the online application. Patients will be asked to perform daily home spirometry and pulse oximetry in the first three months, followed by once weekly measurements for a period of two years. PROMs will be completed in the online I-FILE application every six months, including the King’s brief Interstitial Lung Disease Health Status, The EuroQol five dimensions five-level, Visual Analogue Scales on cough, dyspnea, fatigue and general complaints, Leicester Cough Questionnaire, Fatigue Assessment Scale, Work Productivity and Activity Impairment Questionnaire, Global Rating of Change Scale, and Living with Pulmonary Fibrosis questionnaire.
Discussion
This study will provide much needed insights in disease trajectories of the different subgroups of patients with PF. Simultaneously, the I-FILE study will yield valuable information on the use and feasibility of home-based data collection. This international patient-led registry will facilitate trans-border collaboration to further optimize care and research for patients with PF.
Trial registration: The study was registered on the 12th of March 2020 in the International Clinical Trial Registry, www.clinicaltrials.gov; Identifier: NCT04304898.
Similar content being viewed by others
Background
Pulmonary fibrosis (PF) is a manifestation of different interstitial lung diseases, characterized by fibrotic remodeling of the lung, leading to pulmonary function loss [1, 2]. Disease course and prognosis of PF widely vary between individuals and due to different underlying etiologies [3,4,5,6]. Symptoms such as cough, dyspnea, impaired exercise tolerance, fatigue, anxiety and depression, significantly impair health-related quality of life (HRQOL) of patients with PF [7,8,9]. With appropriate and timely management, some forms of PF have the potential for stabilization or, in a subgroup with more inflammatory disease, even improvement [1]. However, a subset of patients have a progressive disease course, characterized by increasing symptoms, decline in pulmonary function and HRQOL, and ultimately early mortality [2,3,4,5,6, 10, 11]. With a median survival of two to three years without treatment, idiopathic pulmonary fibrosis (IPF) is the most progressive form of PF [12]. Nevertheless a group of patients with other forms of pulmonary fibrosis have a similar rapidly progressive disease course. New guidelines have introduced the term progressive pulmonary fibrosis (PPF) for this progressive phenotype of PF in patients with a diagnosis other than IPF. Due to the heterogeneity of PF, predicting the disease course for individual patients remains challenging. Especially for the more rare and ultra-rare forms of PF, detailed data about disease behavior and trajectory is currently lacking. Therefore, more comprehensive data for the different forms of PF is needed.
Nowadays, identifying disease progression has direct treatment implications. Anti-fibrotic medication is the mainstay of treatment for IPF, as it reduces pulmonary function decline and prolongs survival [13,14,15]. More recently, studies demonstrated beneficial effects of the anti-fibrotic medications Nintedanib and Pirfenidone for PPF [16,17,18,19]. This has led to the approval of anti-fibrotic treatment (Nintedanib) for this patient group [16, 17]. However, data on timing of treatment initiation, and combination with immunomodulatory treatment in patients with PPF are still scarce. Moreover, real-life data about adverse events and effectiveness in patients with comorbidities or severely impaired pulmonary function, and not eligible for clinical trials, are currently also not available.
In current guidelines, disease progression is defined by a combination of worsening symptoms, radiological and/or physiological progression [20]. In-hospital forced vital capacity (FVC) trends are accepted as current standard to monitor physiological disease progression. Nevertheless, FVC measurements have an inherent variability and are usually measured at low frequency during visits to the pulmonologist, possibly delaying detection of disease progression, and consequently, management decisions. Online home monitoring, using eHealth technology, enables more frequent measurements of pulmonary function and symptoms by patients at home, which will help to get a more detailed overview of disease course in individual patients. Home monitoring in PF has gained increasing interest in recent years, with studies demonstrating its feasibility and reliability in this patient population, despite technical and analytical issues [18, 21,22,23,24,25]. Results of an international survey revealed that ILD healthcare providers are positive towards the use of online home monitoring for daily care, but also for research and registry purposes [26]. A large online multinational patient registry could be a major step towards the better understanding of PF heterogeneity, and thus improve its management. eHealth technology can help lift the burden of frequent data collection, and provide a more practical way of data collection. It could facilitate trans-border collaboration and pooling of data in a patient registry, as distances are bridged online.
In the I-FILE study, we aim to gain more insights in disease behavior and response to treatment in patients with PF using online home monitoring. In addition, we will evaluate the feasibility of a patient-led registry in PF.
Methods
Objectives
The main aim of this study is to assess long-term FVC change in patients with PF measured with home spirometry. Our secondary aims are to evaluate feasibility, adherence, patient and healthcare provider experiences and satisfaction, and to assess validity existing PROMs in patients with PF.
Study design and participants
The I-FILE study is an ongoing prospective multicenter, multinational, observational pilot study (NCT04304898). Patients with ILD, newly diagnosed in a multidisciplinary discussion (MDD), and PF on high-resolution computed tomography (HRCT), will be included in both community and tertiary centers in Belgium, France, Germany, Greece, the Netherlands, Norway and The United Kingdom. The trial is currently underway, and patients are being recruited. We aim to include approximately 700 patients in total. Patients will be considered newly diagnosed, when the diagnosis has been established within six months before inclusion in an MDD. In addition, patients should be either treatment naïve or have received treatment for their PF for a maximum of one month before inclusion. For more detail, inclusion—and exclusion criteria are shown in Table 1.
Study procedures
In the I-FILE study, patients will use an online home monitoring program, consisting of the online I-FILE application integrated with home spirometry, for two years. If eligible for participation, patients will receive information on the I-FILE study by their treating physician. Written informed consent will be obtained before start of the study. At time of inclusion, patients will be instructed on the use of the I-FILE application and spirometer. Patients will perform once daily home spirometry and oximetry measurements for the first three months, followed by once weekly measurements (three consecutive home spirometry and one oximetry measurement). In addition, patients are asked to complete patient reported outcomes (PROMs) in the online application every six months. Permission will be asked separately to reassess medical records and online data for another five years after the end of the study. After two years, patients will be asked to fill in a twelve-item survey on their experiences with the home monitoring program. After two years, patients are offered the possibility to continue using the system if they wish.
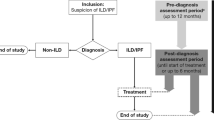
Baseline characteristics, such as patient demographics, clinical characteristics, medication, data on HRCT, histology, comorbidities, hospital-based pulmonary function tests, and laboratory results will be collected at time of inclusion. An overview of the data collection is shown in Additional file 1: Table S1. No adjustments will be made in regular care of participating patients. Every six months, during outpatient clinic visits, patients will be asked about hospitalizations, changes in medication use and side-effects of medication for PF. Hospital-based spirometry, consisting of FVC, Forced Expiratory Volume in 1 s (FEV1), and diffusion capacity of the lung for carbon monoxide (DLCO), will be performed according to international guidelines. An overview of the study flow is shown in Fig. 1. After completion of the study, healthcare providers and research staff are asked to fill in a survey on their opinions and experiences with the home monitoring program in an online twelve item survey.
I-FILE application
I-FILE is an online eHealth application (Curavista, Gezondheidsmeter, Geertruidenberg, the Netherlands) (www.i-file.app). This application has been successfully used in earlier (multi-center) home monitoring studies [23,22,27,24]. At time of inclusion, all patients will receive a password-protected personal account, and the I-FILE application will be installed on their smartphone or tablet in their native language. If patients do not own a smart device, they will be provided with a tablet computer for the duration of the study. The I-FILE application includes home spirometry, pulse oximetry, online completion of questionnaires and symptom scales, possibility for eConsultation with healthcare providers, an option for private notes for the patients, and a real-time overview of results over time (Fig. 2).
At first login, patients will be asked to tick a box to consent that their data can be stored in a database and used for research purposes; they can recall this consent at any time. All study results will be encrypted and directly sent to a secured and approved server (Gezondheidsmeter, the Netherlands), which has the highest European Certification for safety (NEN7510 and ISO27001), is CE-marked and complies with all safety regulations (i.e. General Data Protection Regulation).
Patients will be provided with an instruction manual on paper for the use of the I-FILE application. They will find additional information about the study on the I-FILE website, which includes animated instruction videos on the I-FILE study and the use of the spirometer. In addition, both patients and research staff will have access to a technical helpdesk in their native language.
Healthcare providers and research staff will receive a password for an online dashboard with an overview of all participating patients from their center. They will have real-time access to all patient data, except for the part with private notes. Some hospitals will have direct access to the I-FILE platform via the electronic patient file in their hospital. The data will be made available to all collaborating investigators and patient associations for different research questions and post-hoc data analyses.
Patients will receive automated reminders if measurements are not performed according to the study protocol. In case of repeated missing measurements, research staff will receive automated alerts with instructions to contact the patient. In addition, healthcare professionals are instructed to check the results of the included patient on a regular basis. Healthcare providers do not receive automated alerts in case of changes in pulmonary function, oxygen saturation or PROMs.
Home spirometry and pulse oximetry
Home spirometry and oximetry measurements will be performed using a Bluetooth-enabled validated, CE-marked home spirometer (Spirobank Smart or Spirometer OXI MIR, Italy). Patients will be instructed by a trained member of the research team and will perform a minimum of three test blows on the home spirometer to ensure reliability of the home spirometry results. Home spirometry measurements will be considered reliable if the highest FVC value deviates less than ten percent from the in-hospital FVC value conducted at baseline, and if the difference between two consecutive measurements is less than 150 ml [28]. Patients are instructed to perform home spirometry approximately at the same time to reduce variability [29]. Oxygen saturation is measured in the subgroup of patients who use the Spirometer OXI, by placing their finger on the integrated sensor for 30 s. All measurements will be directly sent to the research team with a personal 5-digit code. Both patient and research team will have real-time access to the flow-volume loops, quality assessment of the measurements, and a trend line of the FVC and the FEV1 over time.
Patient reported outcome measures
Patients will complete online PROMs (Table 2), consisting of HRQOL questionnaires and symptom scores, and can see a graphical overview of their main results.
Endpoints and statistical analysis
We will evaluate absolute changes in FVC (L and % predicted) measured with home spirometry, and oxygen saturation (%) measured with pulse oximetry, from baseline to 6, 12, and 24 months. These changes will be analyzed using a linear mixed model with disease and country as fixed effects. Repeated measurements within a patient will be corrected by random effects (intercepts or slopes, depending on the data). This model corrects for random missing data. Possible non-linear evolutions and correlations between subsequent observations will be accounted for through flexible modeling of the time variable and structured error terms in the variance–covariance matrix of the random effects. Absolute in-hospital FVC changes between baseline, 6, 12 and 24 months (L and %predicted), HRQOL, and symptom scores will also be assessed with linear mixed models. The correlation of home and hospital spirometry will be analyzed by calculating Pearson’s correlation coefficient, taking the individual mean FVC changes for each patient into account. Results will be used to optimize analytical methods to deal with outliers and missing data. Besides, a delta (Δ) change will be computed for HRQOL and symptom changes from baseline to 6, 12 and 24 months. Furthermore, we will use group based trajectory modeling and joint models to predict outcomes in individual patients (e.g. respiratory related and all cause hospitalizations, disease progression, and mortality) To measure time to decline in HRQOL, we will use the minimal clinically important difference (MCID) for all individual patient reported outcomes. Joint models will be used to assess time to reach the MCID. Adherence to the home monitoring program will be assessed by evaluating the completeness of data. The total number of measurements from each participant will be divided by the number of expected measurements, for pulmonary function as well as PROMs. The incidence rate of new oxygen prescription, non-elective hospitalization (respiratory and all cause), mortality (all cause and respiratory-related) will be summarized descriptively by diagnosis cohort. Disease progression will be defined according to the guideline criteria and its individual components, and will be compared with criteria used in different trials [16,17,18, 20]. We will also perform analyses with different cut-off values for FVC and DLCO. Multivariate logistic regression, ROC curve analysis and a multivariate Cox proportional hazards model will be used to identify possible predictive factors for disease progression and/or mortality. Differences between countries will be analyzed with ANOVA. If possible, HRQOL questionnaires will be validated for the different forms op PF by assessing internal consistency, discriminative ability, concurrent validity, and responsiveness. Patient and healthcare providers satisfaction with the home monitoring program will be summarized descriptively. An overview of the pre-specified endpoints is shown in Table 3.
Discussion
The I-FILE study will be the first international patient registry for PF using online home monitoring. This study will help us gain better insights in disease course of different forms of PF through frequent collection of physiological parameters and PROMs and will yield important information about the feasibility of a patient registry using eHealth technology.
During the last decade, many patient registries have been established for PF, with most registries focusing on IPF. Registry data are especially of added value for rare diseases with a heterogeneous disease trajectory, to gain more real-life insights in disease behavior and response to treatment. Compared to randomized controlled trials, registries have less strict inclusion criteria, as they operate more aligned with daily clinical practice. Therefore, registries include patients with a broader range of disease severity, who are often older, have more pulmonary function impairment, and more comorbidities. Moreover, larger patient groups can be included and monitored for a longer time period. However, registry data also have drawbacks. Combining data from different registries is hampered by factors such as data ownership, diverse patient populations, and differences in study parameters and outcomes. Due to the study design and less strict in- and exclusion criteria, registries often encounter more missing and heterogeneous data compared to clinical trials. In addition, patients with more severe disease can possibly have a higher dropout rate, whereas patients with a relatively mild disease course may be lost to follow up if they are referred to local centers for follow up. Until now, most PF registries are retrospective and often included patients from a single country. The I-FILE study provides solutions to overcome these hurdles. The eHealth technology used in this study facilitates uniform and frequent data collection from different countries across Europe. Since patients are tracking their disease course at home, they can provide data for a long period of time independent of their treating center. Data entry in traditional registries is mostly conducted by healthcare providers or research staff and is often very time consuming. In the I-FILE study, we chose to place patients in the lead by giving them ownership of their own study data. Patients collect data and give authorization for using their data for scientific research. We hypothesize that, compared to existing registry processes, patient-led data registration is less laborious for healthcare providers and research staff, and provides a more practicable way of frequent data logging at minimal burden for patients. The experience and satisfaction of all stake holders will be evaluated.
Results of this study will provide insights into disease behavior across different forms of PF. Because of the sample size and duration, we expect to be able to evaluate relatively large subgroups of individual diseases and rare events, such as acute exacerbations. Frequent home spirometry measurements may facilitate early detection of acute exacerbations and disease progression. This is particularly relevant in this patient population, since there is a paucity of prognostic and predictive biomarkers. Data from this study can shed light on whether the use of home spirometry can optimize timing of treatment initiation for individual patients. The I-FILE study will also reveal potential differences in clinical management and outcomes of patients with PF across different European countries.
Earlier studies have elucidated the potential benefits of online home monitoring for patients with PF, both for regular care and research purposes. A randomized controlled trial in IPF found that an online home monitoring program, including home spirometry and online completion of patient-reported outcome measures, tended to improve psychological wellbeing compared to standard care, and was highly appreciated by patients. In addition, online home monitoring allowed for early treatment adjustments in individual patients in case of pulmonary function decline or side-effects of treatment [22]. Results of the I-FILE will build on these insights and show whether home monitoring of patients with potentially progressive disease remains viable and beneficial on the long-term. We postulate that real-time access to pulmonary function, symptoms, and HRQOL data can enhance patient engagement and self-management, as patients gain more insights in their own disease course. The I-FILE study will also reveal whether disease course has influence on the adherence to home monitoring. For research purposes, frequent online data collection using home monitoring can facilitate early identification of patients with a progressive phenotype for inclusion in clinical trials, as well as possibilities for real-time safety monitoring, and broader access to trials [21].
Technical issues are frequently mentioned as expected hurdles for further implementation of eHealth technology in clinical practice. We aim to overcome this hurdle with a technical helpdesk in the native language, accessible for patients, healthcare providers, and research staff. Furthermore, the home monitoring program used in the I-FILE study has been co-developed together with patients and extensively evaluated in earlier studies [23,22,27,24, 25]. We will host an annual patient meeting to actively incorporate patient’s experience with the platform, their perspectives on study and will also encourage input on research questions from all stakeholders. We hope that in this way the traditional model of registries where patients “deliver” data and researcher analyze, will gradually shift to a more collaborative effort. Insights gained in this study can be used to further optimize eHealth applications and collaborative registries for patients with PF in the future.
In this study, we will also collect longitudinal data about HRQOL, symptoms, and disease burden. Although PROMs are increasingly used in clinical trials, many have not been validated in different forms of PF or in real-life settings, which we aim to do in the current study. Besides clinical trials, PROMs can also be used in daily practice, although it can be time consuming or difficult for patients to complete lengthy PROMs on a regular basis. Previous research indicated that the use of online visual analogue scales in patients with IPF is feasible and reliable, and the visual analogue scale is an easy tool to assess symptoms over time [41]. Here, we aim to validate and extend these results in a larger and more diverse patient population over a prolonged period of time.
Although lessons were learned from previous eHealth studies conducted in patients with PF, we still anticipate challenges during the setup and throughout the execution of the study. Since the I-FILE will be conducted in different countries, we anticipate the initiation of the study to be complicated by differences in legislation between countries with regard to home monitoring. During the conduct of the study, one of the anticipated issues could be a lack of internet access or technological skills in a subgroup of patients. Therefore, one of our aims is to evaluate feasibility of this international patient-led registry and gain more insights in differences between countries.
In conclusion, the I-FILE registry study is the ultimate collaboration between researchers, healthcare providers and patients. This study will yield valuable information about the clinical course of patients with PF and potential differences in disease management across Europe. Simultaneously, the I-FILE study will provide broad insights on the use and feasibility of home-based data collection. This international patient-led registry can facilitate trans-border collaboration to further optimize care and research for patients with PF.
Availability of data and materials
The full protocol is available from the corresponding author on reasonable request.
Abbreviations
- PF:
-
Pulmonary fibrosis
- PROMs:
-
Patient reported outcome measures
- K-BILD:
-
The King’s brief Interstitial Lung Disease Health Status
- EQ5D-5L:
-
The EuroQol five dimensions five-level
- VAS:
-
Visual analogue scale
- LCQ:
-
Leicester cough questionnaire
- FAS:
-
Fatigue assessment scale
- WPAI:
-
Work productivity and activity impairment questionnaire
- GRoC:
-
Global rating of change scale
- L-PF:
-
Living with pulmonary fibrosis
- HRQOL:
-
Health-related quality of life
- IPF:
-
Idiopathic pulmonary fibrosis
- PPF:
-
Non-IPF progressive pulmonary fibrosis
- FVC:
-
Forced vital capacity
- MDD:
-
Multidisciplinary-discussion
- FEV1:
-
Forced expiratory volume in 1s
- DLCO:
-
Diffusion capacity of the lung for carbon monoxide
References
Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022.
Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. 2020;383(10):958–68.
Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
Yount SE, Beaumont JL, Chen SY, Kaiser K, Wortman K, Van Brunt DL, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. Lung. 2016;194(2):227–34.
Glaspole IN, Chapman SA, Cooper WA, Ellis SJ, Goh NS, Hopkins PM, et al. Health-related quality of life in idiopathic pulmonary fibrosis: data from the Australian IPF registry. Respirology. 2017;22(5):950–6.
Lubin M, Chen H, Elicker B, Jones KD, Collard HR, Lee JS. A comparison of health-related quality of life in idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Chest. 2014;145(6):1333–8.
Cox IA, Borchers Arriagada N, de Graaff B, Corte TJ, Glaspole I, Lartey S, et al. Health-related quality of life of patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Eur Respir Rev. 2020;29(158):200154.
Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. Marked deterioration in the quality of life of patients with idiopathic pulmonary fibrosis during the last two years of life. BMC Pulm Med. 2018;18(1):172.
Kreuter M, Swigris J, Pittrow D, Geier S, Klotsche J, Prasse A, et al. Health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: Insights-IPF registry. Respir Res. 2017. https://doi.org/10.1186/s12931-017-0621-y.
Kolb M, Vašáková M. The natural history of progressive fibrosing interstitial lung diseases. Respir Res. 2019;20(1):57.
Wells AU, Brown KK, Flaherty KR, Kolb M, Thannickal VJ, Group IPFCW. What’s in a name? That which we call IPF, by any other name would act the same. Eur Respir J. 2018. https://doi.org/10.1183/13993003.00692-2018.
Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40.
Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82.
Crestani B, Huggins JT, Kaye M, Costabel U, Glaspole I, Ogura T, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. 2019;7(1):60–8.
Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27.
Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–28.
Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–57.
Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, Bonella F, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9(5):476–86.
Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an Update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–47.
Johannson KA, Vittinghoff E, Morisset J, Lee JS, Balmes JR, Collard HR. Home monitoring improves endpoint efficiency in idiopathic pulmonary fibrosis. Eur Respir J. 2017. https://doi.org/10.1183/13993003.02406-2016.
Moor CC, Mostard RLM, Grutters JC, Bresser P, Aerts JGJV, Chavannes NH, et al. Home monitoring in patients with idiopathic pulmonary fibrosis: a randomized controlled trial. Am J Respir Crit Care Med. 2020;202(3):393–401.
Moor CC, Gür-Demirel Y, Wijsenbeek MS. Feasibility of a comprehensive home monitoring program for sarcoidosis. J Pers Med. 2019;9(2):476.
Moor CC, Van Leuven SI, Wijsenbeek MS, Vonk MC. Feasibility of online home spirometry in systemic sclerosis-associated interstitial lung disease: a pilot study. Rheumatology. 2021;60(5):2467–71.
Moor CC, Wapenaar M, Miedema JR, Geelhoed JJM, Chandoesing PP, Wijsenbeek MS. A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: a pilot study on experiences and barriers. Respir Res. 2018;19(1):105.
Nakshbandi G, Moor CC, Johannson KA, Maher TM, Kreuter M, Wijsenbeek MS. Worldwide experiences and opinions of healthcare providers on eHealth for patients with interstitial lung diseases in the COVID-19 era. ERJ Open Res. 2021;7(3):00405-2021. https://doi.org/10.1183/23120541.00405-2021.
Moor CC, Van Manen MJG, Tak NC, Van Noort E, Wijsenbeek MS. Development and feasibility of an eHealth tool for idiopathic pulmonary fibrosis. Eur Respir J. 2018;51:3.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–88.
Moor CC, Van Den Berg CAL, Visser LS, Aerts JGJV, Cottin V, Wijsenbeek MS. Diurnal variation in forced vital capacity in patients with fibrotic interstitial lung disease using home spirometry. ERJ Open Res. 2020;6(1).
Nolan CM, Birring SS, Maddocks M, Maher TM, Patel S, Barker RE, et al. King’s Brief Interstitial Lung Disease questionnaire: responsiveness and minimum clinically important difference. Eur Respir J. 2019. https://doi.org/10.1183/13993003.00281-2019.
Patel AS, Siegert RJ, Brignall K, Gordon P, Steer S, Desai SR, et al. The development and validation of the King’s Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax. 2012;67(9):804–10.
Swigris J, Cutts K, Male N, Baldwin M, Rohr KB, Bushnell DM. The Living with Pulmonary Fibrosis questionnaire in progressive fibrosing interstitial lung disease. ERJ Open Res. 2021. https://doi.org/10.1183/23120541.00145-2020.
Swigris JJ, Bushnell DM, Rohr K, Mueller H, Baldwin M, Inoue Y. Responsiveness and meaningful change thresholds of the living with pulmonary fibrosis (L-PF) questionnaire dyspnoea and cough scores in patients with progressive fibrosing interstitial lung diseases. BMJ Open Respir Res. 2022;9(1):e001167.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Tsai APY, Hur SA, Wong A, Safavi M, Assayag D, Johannson KA, et al. Minimum important difference of the EQ-5D-5L and EQ-VAS in fibrotic interstitial lung disease. Thorax. 2021;76(1):37.
Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–43.
De Vries J, Michielsen H, Van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS). Br J Health Psychol. 2004;9(Pt 3):279–91.
de Kleijn WP, De Vries J, Wijnen PA, Drent M. Minimal (clinically) important differences for the Fatigue assessment scale in sarcoidosis. Respir Med. 2011;105(9):1388–95.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–70.
Moor CC, Mostard RLM, Grutters JC, Bresser P, Aerts JGJV, Wijsenbeek MS. Patient-reported outcomes in idiopathic pulmonary fibrosis; validity and reliability of visual analogue scales. Eur Respir J. 2021;58(suppl 65):PA3749. https://doi.org/10.1183/13993003.congress-2021.PA3749.
Acknowledgements
Not applicable.
Funding
The I-FILE study is sponsored by the Erasmus Medical Center and funded by Boehringer Ingelheim. Boehringer Ingelheim has no influence on the study design, procedures and has no access to the study data.
Author information
Authors and Affiliations
Contributions
MW, CM contributed to the study concept and design. GN, CM and MW drafted the manuscript. MW, EK, MK, AV, CV, KA, PM, WW read and revised the manuscript for important intellectual content. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study will be performed in accordance with the Declaration of Helsinki. The medical ethical committee of the Erasmus Medical Center has approved the trial with the protocol reference number MEC-2020-0029. Approval from the Medical Ethics Committee of all participating sites will also be obtained. Written informed consent will be obtained from the participants.
Consent for publication
Not applicable.
Competing interests
GN: Financial grant from Boehringer Ingelheim paid to institution. CM: Financial grant from Boehringer Ingelheim, Astra—Zeneca, Daiichi-Sankyo paid to institution. Payments for presentations/lectures from Boehringer-Ingelheim and Hoffman—la Roche paid to institution. KA: Consulting fees from Boehringer Ingelheim, Hoffman—la Roche and GSK. Payments for presentations/lectures from Boehringer Ingelheim, Hoffman—la Roche, Chiesi, Menarini, GSK, AstraZeneca. VC: Financial grant from Boehringer Ingelheim paid to institution. Consulting fees from Astra Zeneca, Boehringer Ingelheim, Celgene/BMS, CSL Behring, GSK, Pliant, Pure Tech, RedX, Hoffman—la Roche, Sanofi, Shionogi, United Therapeutics/Ferrer. Payments for presentations/lectures from Boehringer Ingelheim and Hoffman—La Roche. Support for attending meetings from Boehringer Ingelheim and Hoffman—La Roche. Participation on data and safety monitoring board of Galapagos and Galecto. Leadership role in Fibrogen adjudication committee. AV: Grants from ARXX therapeutics, Boehringer Ingelheim, Hoffman—la Roche, Bayer, Merck Sharp&Dohme, Lilly and Medscape. Consulting fees from ARXX therapeutics, Boehringer Ingelheim, Roche, Merck Sharp&Dohme, Lilly, Medscape, Genentech. Payments for presentations/lectures from ARXX therapeutics, Boehringer Ingelheim, Roche, Bayer, Merck Sharp&Dohme, Lilly and Medscape. Support for attending meetings from Boehringer Ingelheim and Janssen. Leadership role in EULAR convenor for ERS/EULAR CTD-ILD guideline, EULAR quality of care committee, PH Nordic group. MK: Financial grant from Boehringer Ingelheim and Hoffman—la Roche. Consulting fees from Boehringer Ingelheim and Hoffman—la Roche. Payments for presentations/lectures from Boehringer Ingelheim and Hoffman—la Roche. Leadership role in ERS and DGP. PM: Financial grant from Astra Zeneca and Action for Pulmonary Fibrosis payed to institution. Advisory board fees from Hoffman—la Roche, Boehringer Ingelheim, Qureight, Trevi, AstraZeneca. Speakers fees from Astra Zeneca, Boehringer Ingelheim, Hoffman—La Roche. WW: Financial grant from Hoffman—la Roche, Boehringer Ingelheim, Galapagos paid to institution. Consulting fees from Hoffman—la Roche, Boehringer Ingelheim, Galpagos paid to institution. Payments for presentations/lectures from Boehringer Ingelheim paid to institution. Participation on Advisory board by Boehringer Ingelheim, Galapagos, Hoffman—La–Roche paid to institution. MW: Financial grant from Boehringer Ingelheim, Hoffman—La Roche, The Netherlands Organization for Health Research and Development; The Dutch Lung Foundation; The Dutch Pulmonary Fibrosis, all paid to institution. Consulting fees from Bristol Myers Squibb, Boehringer Ingelheim, Galapagos, Galecto, Hoffman la Roche, Horizon therapeutics, Kinevant Sciences, Molecure, Nerre Therapeutics, Novartis, PureTech Health, and Respivant, all paid to institution. Support for attending meeting from Boehringer Ingelheim, Hoffman la Roche, Galapagos. Participation on advisory board from Savara, Galapagos, all paid to institution. Leadership role as Chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, Member of the board of the Netherlands Respiratory Society, Member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and related disorders federation, Chair of the educational committee of the European Reference Network for rare Lung Diseases, Advisory board of the Dutch Lung fibrosis and Sarcoidosis patient associations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Data collection I-FILE study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakshbandi, G., Moor, C., Antoniou, K. et al. Study protocol of an international patient-led registry in patients with pulmonary fibrosis using online home monitoring: I-FILE. BMC Pulm Med 23, 51 (2023). https://doi.org/10.1186/s12890-023-02336-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02336-4