Abstract
Background
Prolonged mechanical ventilation (PMV), mostly defined as mechanical ventilation > 72 h after lung transplantation with or without tracheostomy, is associated with increased mortality. Nevertheless, the predictive factors of PMV after lung transplant remain unclear. The present study aimed to develop a novel scoring system to identify PMV after lung transplantation.
Methods
A total of 141 patients who underwent lung transplantation were investigated in this study. The patients were divided into PMV and non-prolonged ventilation (NPMV) groups. Univariate and multivariate logistic regression analyses were performed to assess factors associated with PMV. A risk nomogram was then established based on the multivariate analysis, and model performance was further examined regarding its calibration, discrimination, and clinical usefulness.
Results
Eight factors were finally identified to be significantly associated with PMV by the multivariate analysis and therefore were included as risk factors in the nomogram as follows: the body mass index (BMI, P = 0.036); primary diagnosis as idiopathic pulmonary fibrosis (IPF, P = 0.038); pulmonary hypertension (PAH, P = 0.034); primary graft dysfunction grading (PGD, P = 0.011) at T0; cold ischemia time (CIT P = 0.012); and three ventilation parameters (peak inspiratory pressure [PIP, P < 0.001], dynamic compliance [Cdyn, P = 0.001], and P/F ratio [P = 0.015]) at T0. The nomogram exhibited superior discrimination ability with an area under the curve of 0.895. Furthermore, both calibration curve and decision-curve analysis indicated satisfactory performance.
Conclusion
A novel nomogram to predict individual risk of receiving PMV for patients after lung transplantation was established, which may guide preventative measures for tackling this adverse event.
Graphic Abstract

Similar content being viewed by others
Background
More than 4,000 lung transplantations are currently performed worldwide per year [1]. However, the mortality and morbidity of lung transplant remain at relatively high levels compared with other solid organ transplantations [2]. Prolonged mechanical ventilation (PMV) is a prognostic marker for short-term adverse outcomes in patients after lung transplantation [3, 4]. Previous reports also show that PMV is associated with impaired long-term survival [5]. Thus, the discovery of predictors for PMV may assist in developing precautionary measures to ameliorate the high morbidity and mortality.
Primary graft dysfunction (PGD) is a form of acute lung injury that occurs in about 30% of patients after lung transplantation within 72 h, which can be characterized by hypoxemia and alveolar infiltrates in the allograft(s) [6]. PGD is reported as the most frequent cause of early death after lung transplantation and is also strongly correlated with other late outcomes [7,8,9]. Although the presence of PGD is associated with an increased duration of mechanical ventilation [9], recently, Schwarz and colleagues found that the value of PGD in predicting PMV was limited [3]. Instead, a model combing three ventilation parameters better predicted PMV. However, the predictive value of this model was still moderate, with an area under the curve (AUC) of 0.727 [3]. Thus, a more precise model is urgently required.
Apart from PGD and ventilation parameters, other factors, such as cold ischemia time (CIT), may also provide valuable information for predicting PMV, and CIT is closely associated with ischemia–reperfusion injury (IRI) [9, 10]. A recent study revealed that CIT is a risk factor for developing airway complications after lung transplantation [11], even though the correlation between CIT and PMV remains unknown. According to the latest guidelines, idiopathic pulmonary fibrosis (IPF) and idiopathic pulmonary arterial hypertension (IPAH) are strongly associated with risk for PGD in primary diagnoses[9]. However, to date, no research has considered the role of different primary diagnoses in predicting the early adverse events after lung transplant besides PGD. Therefore, our research aimed to develop a nomogram combing clinical variables (including primary diagnosis), CIT, PGD grading, and ventilation parameters to improve the predictive accuracy of PMV.
Methods
Study design and participants
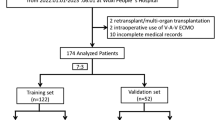
With the Research Ethics Commission of Shanghai Pulmonary Hospital (Shanghai, China) approval (No. L20-352), we conducted a single-center, retrospective observational cohort study. Data from 146 patients who underwent lung transplantation at Shanghai Pulmonary Hospital were retrospectively extracted from electronic medical records between January 1, 2018, and February 1, 2022. The exclusion criteria included: patients with missing data; re-transplantation; postoperatively extended extracorporeal membrane oxygenation (ECMO) with clear chest radiographs (PGD ungradable) [6]. The use of postoperative extended ECMO was defined as the use of ECMO or re-use of ECMO to maintain life after arriving in the intensive care unit (ICU) after surgery [12]. According to the exclusion criteria, 141 patients who underwent lung transplant were included in our study cohort (Additional file 1: Figure S1).
Data acquisition
The baseline characteristics and demographics of the patients (age, gender, BMI, smoking history, and documented pulmonary hypertension), primary diagnosis before the operation, the CIT, and length of mechanical ventilation were retrospectively collected from the medical case database of Shanghai Pulmonary Hospital. In this study, the length of mechanical ventilation was classified as PMV or non-prolonged mechanical ventilation (NPMV), with the threshold to be 72 h. The extubation criteria were as follows in our clinical practice as modified from the consensus on weaning from mechanical ventilation [13]: (1) successful spontaneous breathing trial lasting for 120 min; (2) hemodynamic stability; (3) No sedation or adequate mentation on sedation; (4) P/F ratio > 150 mm Hg with FiO2 ≤ 0.4, positive end-expiratory pressure ≤ 8 cm H2O. Patients who were extubated but needed reintubation within 72 h after lung transplantation were also included in the PMV group.
The ventilation parameters of T0, T24, T48, and T72 were also obtained. T0, T24, T48, and T72 were defined as the 2nd, 24th, 48th, and 72nd hours after the arrival at the ICU after transplantation, respectively. The ventilation parameters mainly included inhaled oxygen concentration fraction, arterial oxygen partial pressure, tidal volume (TV), peak inspiratory pressure (PIP), and positive end-expiratory pressure (PEEP). Dynamic compliance was calculated as tidal volume/(peak inspiratory pressure-positive end-expiratory pressure), while partial pressure of the oxygen fraction of inspired oxygen (P/F) ratio was calculated as arterial oxygen partial pressure (PaO2)/inhaled oxygen concentration fraction (FiO2).
The PGD diagnosis method in this study refers to the standard judgment of the International Society for Heart and Lung Transplantation (ISHLT) on PGD in 2016 [6]. Notably, patients receiving mechanical ventilation with FiO2 > 0.5 on nitric oxide > 48 h from lung transplant or using extracorporeal lung support (ECLS) with bilateral pulmonary edema on chest X-ray, which indicated ECLS is primarily hypoxemia, were classified as grade 3. In addition, using atomized prostacyclin or other drugs that may improve oxygenation did not affect PGD classification [14].
Statistical analysis
The categorical variables were summarized as the absolute frequency and percentage, while the continuous variables were presented in the median and interquartile range (IQR). Fisher’s exact test and a non-parametric Mann–Whitney U test were performed to compare the categorical and continuous data, respectively. Subsequently, univariate and multivariate binary logistic regressions were calculated to test the effect of the PGD grading, CIT, and the ventilation parameters for predicting PMV [15]. Candidate factors with a univariate significance of P < 0.1 were selected for the multivariate analysis. The final multivariate model was displayed in the nomogram format to illustrate all the selected predictors of the individual risk of PMV. The linear relationship between the nomogram score and the length of mechanical ventilation was estimated by calculating Pearson’s correlation coefficient.
Performance assessments
Bootstrapped calibration curves were used to assess the predictive probability of this model. The assessment determines whether the model is biased as a result of the overfitting of the model. The receiver operating characteristic curve (ROC) analysis was then performed to quantify the discrimination ability of the nomogram and the subjects included in it. The Bootstrap test was used to compare the area under the curve (AUC) of the different smoothed ROCs. The clinical utility was determined using decision-curve analysis (DCA), assessing the clinical net benefit associated with the use of the model [16]. The vertical axis, namely the net benefit (NB), was defined as the true positive rate minus the false positive rate over a range of threshold probability defining high risk. Each decision curve graphically illustrated the NB of the model and every indicator through a range of threshold probabilities of the outcome [17,18,19]. This study used R software (R-4.1.0) and SPSS v26.0 for the data analysis. The graphics were made with R or GraphPad Prism 9.0.0. Two-sided P values < 0.05 were used to declare statistical significance.
Organ procurement statement
Voluntary organ donation by citizens has become the only legal source of deceased donor organ transplantation in China since starting on January 1, 2015, and the origins of all organs were registered in the Chinese organ donation system and have been traceable since that date. All the donation procedures were approved by The Institutional Ethics Committees of the Organ Procurement Organization (OPO). Donated lungs were prioritized to the listed candidates following the national organ allocation principles while considering the priority based on lung allocation score (LAS), a comprehensive measure of transplantation urgency and utility. Organ procurement was performed according to the standard protocol through the China Organ Transplant Response System (COTRS) [20]. Hence, it can be guaranteed that no organ used for lung transplantation during the study period was procured from executed prisoners.
Results
Patient characteristics
The demographical characteristics of our study cohort are presented in Table 1. Of the 141 patients in the cohort, the median age [interquartile range (IQR)] was 62 (56–66) years, with 103 (73.0%) male patients. Sixty-four (45.4%) patients received bilateral lung transplant, and seventy-seven (54.6%) patients received unilateral lung transplant. The most frequent diagnosis was idiopathic pulmonary fibrosis (IPF), followed by chronic obstructive pulmonary diseases (COPD) and interstitial lung disease (ILD). The median length of mechanical ventilation was 49 h, and 45 (31.9%) patients underwent PMV in the ICU after lung transplant. Other baseline characteristics of this retrospective cohort are listed in Table 1.
Comparison between the PMV and NPMV patients
Patients in the PMV group tended to be older (65 vs. 60 years, p = 0.041) and were more likely to have a higher BMI (22.7 vs. 20.5, P = 0.011) and longer CIT (P < 0.001) compared with the NPMV group. In addition, patients with primary diagnoses as IPF were more likely to undergo PMV than those diagnosed with other diseases (60.0% vs. 30.2%, P = 0.003). A similar trend was found for the presence of pulmonary hypertension (55.5% vs. 33.3%, P = 0.010, Table1), which was considered a complication of primary diagnoses. However, no statistically significant difference was found between the two groups regarding gender, smoking history, other diagnoses, and type of transplant.
As for the mechanical ventilation parameters at T0, more patients in the PMV group had controlled ventilation status than those in the NPMV group (95.0% vs. 79.1%, P = 0.015). Nevertheless, there was no significance in the detailed ventilation modes between the PMV and NPMV groups. Furthermore, patients who underwent PMV had a significantly higher peak inspiratory pressure (PIP, 19 vs. 16 cmH2O, P = 0.039) and lower dynamic compliance (Cdyn, 27.80 vs. 32.92, P = 0.018) and PaO2/FiO2 ratio (P/F ratio, 222 vs. 306, P = 0.041, Table 2). More detailed ventilation parameters are presented in the supplementary materials (Additional file 4: Table S1). We also investigated the difference in PGD grading between the subgroups. PGD grading was significantly higher in the PMV group, whereas the difference decreased over time (all P < 0.05, table 3). Additionally, no statistically significant differences were found in donor characteristics between the PMV and NPMV groups (Additional file 6: Table S3).
Prophylactic noninvasive ventilation after extubation was applied in 32 (22.7%) transplant recipients and the percentage of patients receiving noninvasive ventilation were similar between the NPMV and PMV groups (30.2% vs 44.4%; P = 0.272). Twenty-five (17.7%) patients underwent reintubation and the majority of patients underwent reintubation were in the PMV group (37.8% vs. 8.3%, P < 0.01, Additional file 7: Table S4).
Logistic regression analyses
Possible correlations between PMV and thirteen parameters for the patients in this cohort were evaluated by univariate logistic regression. BMI, CIT, PGD grading at all times, pulmonary hypertension as a complication, primary diagnosis as IPF, and four ventilation parameters at T0 (ventilation status, PIP, P/F ratio and Cdyn) were identified as potential predictors for PMV (all P < 0.05), while age, gender and smoking history were considered not predictive. Further multivariate logistic regression identified 8 independent variables. BMI (odds ratio [OR] with 95% confidence interval [CI] 1.425[1.323–1.767]; P = 0.032), CIT (OR with 95% CI 1.777[1.065–2.889]; P = 0.012), PGD grading at T0 (OR with 95% CI 1.557[1.331–1.899]; P = 0.011), pulmonary hypertension (OR with 95% CI 1.894[1.243–3.001]; P = 0.034), primary diagnosis as IPF (OR with 95% CI 1.788[1.245–3.634]; P = 0.038), PIP (OR with 95% CI 1.961[1.211–2.747]; P < 0.001), P/F ratio (OR with 95% CI 0.991[0.980–0.996]; P = 0.015) and Cydn (OR with 95% CI 1.266[1.121–1.473]; P = 0.001) remained independent predictors of PMV (Table 4).
In contrast, ventilation status and PGD grading at other times were not appropriate for inclusion in the final nomogram (all P > 0.05). We further investigated the prediction value of the donor factors using the univariate logistic regression analysis and we found no statistically significant differences in our results (Additional file 8: Table S5).
Predictive nomogram for PMV
Based on the multivariate logistic regression, a nomogram incorporating BMI, CIT, PGD grading at T0, PIP, and Cdyn for predicting PMV after lung transplantation was established (Fig. 1). The model demonstrated excellent discrimination, with an AUC of 0.895 (95%CI, 0.852–0.955, Fig. 2) and an accuracy of 0.90 (Additional file 5: Table S2). A bootstrapped calibration curve was further established to estimate the predictive ability of the model, which demonstrated a superior ability with a preserved calibration. (Fig. 3). The Bootstrap test for the different ROC curves demonstrated significant differences between the nomogram and each variable included in it (P < 0.001). Other performance metrics are listed in the supplementary materials (Additional file 5: Table S2). As the DCA depicted in Fig. 4, the nomogram added clinical risk prediction within the range of the PMV threshold probability < 0.80, which presented satisfactory clinical usefulness. In addition, a simplified nomogram that only included four preoperative variables (pulmonary hypertension, primary diagnosis as IPF, BMI, and CIT) can be helpful in the preoperative risk assessment and early prevention of PMV (Additional file 2: Figure S2). An AUC of 0.793 also demonstrated a moderate predictive ability of this simplified nomogram (Additional file 3: Figure S3).
Risk prediction nomogram of logistic regression. Nomogram constructed to predict prolonged mechanical ventilation in lung transplant recipients after surgery. The included variables were cold ischemia time, ventilation parameters at T0 (including peak inspiratory pressure, tidal volume, dynamic compliance and oxygenation index), and PGD grade at T0. The full point density and risk density plots show their distribution. For category variables, their distribution is reflected by the size of the box. Rank the importance of each variable according to the standard deviation on the Nomogram scale. When using the Nomogram image, specific points (black spots) for each patient are located on each variable axis. Draw lines to determine the points received by each variable; The sum of these points is placed on the total point line and a line drawn down the risk line to obtain the total predicted risk of prolonged ventilation after surgery. CIT, cold ischemia time; PGDT0, primary graft dysfunction at T0; BMI, body mass index; Cydn, dynamic compliance; PIP, peak inspiratory pressure; PAH pulmonary hypertension; IPF, idiopathic pulmonary fibrosis
ROC analysis for the nomogram of the prediction model of recipients with prolonged mechanical ventilation after lung transplantation based on all indicators and all variables. ROC curve summation of various factors, including cold ischemia time, ventilation parameters, and PGD grade at T0. The final integrated model in the figure has an area under the ROC curve of 0.895. Among the indicators, the area under ROC curve of cold ischemia time was the largest, reaching 0.789
The decision curve analysis (DCA) of the prediction model of recipients with prolonged mechanical ventilation after lung transplantation based on all indicators and all variables. The prediction model or index with the largest net benefit has the best clinical guidance efficiency. Net benefit is defined as the true positive rate minus the weighted false positive rate under a given threshold probability, which defines the high risk of prolonged mechanical ventilation after lung transplantation
Discussion
Lung transplantation is the ultimate treatment option for selected patients with end-stage lung diseases. However, the risks associated with lung transplant remain considerable. One of the most important risk factors after lung transplant is PMV, which leads to an increased cost of care and a greater risk of death for the patient [21]. Predicting patients at risk of PMV helps clinicians devise personalized care plans to mitigate the risk of PMV and timely decide on tracheostomy if ventilatory support is still required. However, tools to accurately predict PMV after lung transplant are limited. In the present study, we established a nomogram incorporating patients’ BMI, pulmonary hypertension, primary diagnosis as IPF, three ventilation parameters, CIT, and PGD grading at T0 to predict PMV. Compared with ventilation parameters alone, this nomogram achieved a better predictive value. Since the variables included in this nomogram are easily obtainable, the utility of this nomogram to predict the risk of PMV and guide treatment decisions may be considered routine clinical practice shortly. In more detail, lung-protective ventilation, fluid restriction, prophylactic use of ECLS, and pulmonary vasodilators may be viable options for preventing PMV in high-risk individuals from this model.
Although a series of studies have confirmed the negative prognostic impact of PMV [22, 23], the definition of PMV is still controversial, ranging from 5 h to 21 days [24]. In 2005, a report by the National Association for Medical Direction of Respiratory Care (NAMDRC) consensus conference defined PMV as mechanical ventilation for \(\ge\) 21 consecutive days [25]. However, the definitional criteria may not fit all studies due to subject cohort variations. For lung transplantation, most patients undergo extubation within the first 72 h. Two previous studies defined PMV as mechanical ventilation > 72 h based on their finding that most patients (77.1% and 80.6%, respectively) were already extubated at T72 [3, 26]. They thus referred to > 72 h as the threshold to define PMV. A similar extubation rate (96/141, 68.1%) within the first 72 h after transplantation was observed in the present study. Therefore, we used the same criteria as in the two previously mentioned studies to define PMV.
In our study, BMI, pulmonary hypertension, primary diagnosis as IPF, PGD grading at T0, relevant ventilation parameters, and cold ischemia time were included in the nomogram. Obesity has long been considered an independent predictor of the length of mechanical ventilation in mechanically ventilated patients in the ICU setting [27]. Obesity and overweight are also risk factors for PGD and mortality after lung transplantation [28,29,30]. In the present study, although the mean BMIs in both NPMV and PMV groups do not meet the World Health Organization (WHO) criteria for overweight or obesity, however, previous reports demonstrate that Asian population develop health complications at lower BMIs than people of other races [31], and the mean BMI in the PMV group is close to the Asian-specific overweight criteria (≥ 23) [32]. Therefore, Asian patients with higher BMI should be given particular caution regarding perioperative management even though they were considered as normal weight according to international BMI chart. Thus, obese recipients may be given particular caution regarding perioperative management based on the trend for higher BMI means higher risk of PMV.
Despite previous studies that have reported that IPF and IPAH were independent predictors of increased PGD [9, 33], our findings are the first study to implicate the predictive ability of IPF as a primary diagnosis and pulmonary hypertension as a complication for early adverse events after lung transplant besides PGD. As for PGD grading at T0, we demonstrated that patients with NPMV were more likely to be PGD grade 0 than patients with PMV (60.4% vs. 24.4%). A previous report also revealed that patients with PGD grade 0 at T0 had a shorter length of mechanical ventilation than those with PGD grade 1–3 [3]. However, the AUC of PGD grading for predicting PMV was only 0.634, slightly smaller than our study (AUC = 0.747). Thus, the predictive value of PGD grading at T0 alone for PMV was limited. Although PGD grading at a later time point is reported to be more closely related to long-term outcomes after lung transplant[34], only PGD grading at T0 remained statistically significant in the multivariate logistic regression analysis (P = 0.011). A likely reason for this result is that what led to long-term outcomes did not necessarily generalize to some early outcomes, such as PMV.
The length of mechanical ventilation is closely related to the ventilation parameters [35]. Three ventilation parameters, P/F ratio, PIP, and Cdyn, were included in the nomogram in our study. Similarly, Schwarz and colleagues [3] also found these three ventilation parameters were predictors of PMV after lung transplantation. According to Ripoll et al., elevated PIP is associated with the development of acute respiratory distress syndrome (ARDS) in liver transplant recipients [36]. Moreover, Laffey et al. [37] demonstrated that higher PIP and lower P/F ratio contribute to increased hospital mortality in patients with ARDS. Cdyn was reported as a critical parameter for evaluating graft function after ex vivo lung perfusion in a previous study [38]. However, we show that in our multivariate logistic regression analysis, PIP was the strongest predictor of PMV. Only mechanical ventilation parameters at T0 were included in our study. This is because only ventilation parameters in the immediate postoperative period were thought to have predictive value while ventilation parameters at later times hold value for assessing the status of those patients after lung transplantation rather than being predictive.
Among these variables included in the nomogram, CIT outperformed other individual factors for predicting PMV. Since the pathological basis of PGD is consistent with IRI [39], CIT is closely related to early allograft function [40]. Recently, CIT was also reported to have a significant correlation with postoperative complications of lung transplantation [41]. However, whether CIT could be used to predict PMV remains unknown. In the present study, we demonstrated for the first time that longer CIT was an independent risk factor for PMV.
Our study has several limitations. First, one major limitation in this single-center study is that the absence of external validation may limit the application of the nomogram. Regrettably, despite repeated attempts to add a validation cohort, we ultimately failed to establish such a cohort because there are so few lung transplantation centers in China. However, both the lung transplantation centers and the annual number of lung transplants has markedly increased in recent years in China [20]. Hopefully, this preliminary result will be validated in multicenter studies in the future. Second, the sample size was relatively small. Third, the majority of the patients in our study cohort underwent a unilateral lung transplantation, which may influence the estimation of the PGD grading’s impact on length of mechanical ventilation. Although the Report of the ISHLT Working Group does not recommend separately grading PGD for bilateral and single lung transplant recipients routinely [6], previous publications do show that single lung transplantation may have an elevated overall incidence of PGD [33, 42]. In addition, the residual pulmonary function of the contralateral lung may influence the length of mechanical ventilation, which could not be evaluated in our study. Finally, this model can only be applied post-operatively to evaluate the risk for PMV after lung transplant. This may limit the interventions available to reduce the incidence of PMV and hence restricts potential applications.
Conclusions
As shown in Visual Abstract, we established a novel nomogram that could efficiently predict individual risk of receiving PMV for patients after lung transplantation, which facilitates early diagnosis and rational intervention. Still, additional prospective validation cohorts from more clinical centers will be needed to confirm the practical utility of the newly established nomogram before its translation to wide-accepted clinical practice.
Availability of data and materials
All data that support our research will be available with the International Society for Heart and Lung Transplantation (ISHLT) following ISHLT standardized embargo and policies. The data request should be sent to the corresponding author on chenthoracic@163.com.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- Cdyn:
-
Dynamic compliance
- CI:
-
Confidence interval
- CIT:
-
Cold ischemia time
- DCA:
-
Decision-curve analysis
- ECLS:
-
Extracorporeal life support
- ECMO:
-
Extended extracorporeal membrane oxygenation
- FiO2 :
-
The fraction of inspiration O2
- ICU:
-
Intensive care unit
- IRI:
-
Ischemia–reperfusion injury
- ISHLT:
-
The International Society for Heart and Lung Transplantation
- NPMV:
-
Non-prolonged mechanical ventilation
- PaO2 :
-
Partial pressure of oxygen
- PEEP:
-
Positive end-expiratory pressure
- PGD:
-
Primary graft dysfunction
- PIP:
-
Peak inspiratory pressure
- PMV:
-
Prolonged mechanical ventilation
- ROC:
-
Receiver operating characteristic curve
References
Hachem RR. Advancing lung transplantation. Clin Transpl. 2015;31:239–47.
Raskin J, Vanstapel A, Verbeken EK, Beeckmans H, Vanaudenaerde BM, Verleden SE, Neyrinck AP, Ceulemans LJ, Van Raemdonck DE, Verleden GM, et al. Mortality after lung transplantation: a single-centre cohort analysis. Transpl Int. 2020;33(2):130–41.
Schwarz S, Benazzo A, Dunkler D, Muckenhuber M, Sorbo LD, Di Nardo M, Sinn K, Moser B, Matilla JR, Lang G, et al. Ventilation parameters and early graft function in double lung transplantation. J Heart Lung Transplant. 2021;40(1):4–11.
Van Herck A, Frick AE, Schaevers V, Vranckx A, Verbeken EK, Vanaudenaerde BM, Sacreas A, Heigl T, Neyrinck AP, Van Raemdonck D, et al. Azithromycin and early allograft function after lung transplantation: a randomized, controlled trial. J Heart Lung Transplant. 2019;38(3):252–9.
Efrati O, Bylin I, Segal E, Vilozni D, Modan-Moses D, Vardi A, Szeinberg A, Paret G. Outcome of patients with cystic fibrosis admitted to the intensive care unit: Is invasive mechanical ventilation a risk factor for death in patients waiting lung transplantation? Heart Lung. 2010;39(2):153–9.
Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, Pelaez A, Whelan TP, Perch M, Bag R, et al. Report of the ISHLT Working Group on primary lung graft dysfunction, part I: definition and grading—a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097–103.
Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–6.
Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–13.
Diamond JM, Arcasoy S, Kennedy CC, Eberlein M, Singer JP, Patterson GM, Edelman JD, Dhillon G, Pena T, Kawut SM, et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary lung graft dysfunction, part II: epidemiology, risk factors, and outcomes—a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1104–13.
Fiser SM, Kron IL, Long SM, Kaza AK, Kern JA, Cassada DC, Jones DR, Robbins MC, Tribble CG. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transplant 2001;20(12):1291–6.
Mendogni P, Pieropan S, Rosso L, Tosi D, Carrinola R, Righi I, Damarco F, Musso V, Bonitta G, Morlacchi LC, et al. Impact of cold ischemic time on airway complications after lung transplantation: a single-center cohort study. Transplant Proc. 2019;51(9):2981–5.
Hoetzenecker K, Schwarz S, Muckenhuber M, Benazzo A, Frommlet F, Schweiger T, Bata O, Jaksch P, Ahmadi N, Murakozy G, et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg. 2018;155(5):2193-2206 e2193.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–56.
Van Raemdonck D, Hartwig MG, Hertz MI, Davis RD, Cypel M, Hayes D Jr, Ivulich S, Kukreja J, Lease ED, Loor G, et al. Report of the ISHLT Working Group on primary lung graft dysfunction part iv: prevention and treatment: a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1121–36.
Aziz A, May M, Burger M, Palisaar RJ, Trinh QD, Fritsche HM, Rink M, Chun F, Martini T, Bolenz C, et al. Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort. Eur Urol. 2014;66(1):156–63.
Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell’Oglio P, Bravi CA, Fallara G, Pellegrino F, Nocera L, Karakiewicz PI, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. 2017;72(4):632–40.
Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804.
Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313(4):409–10.
Steyerberg EW, Vickers AJ. Decision curve analysis: a discussion. Med Decis Mak. 2008;28(1):146–9.
Dong NG, Hu XJ, Wang HB, Chen JY, Wan S. Should we tolerate biased critiques in cardiothoracic surgery journals? J Thorac Cardiovasc Surg. 2022. https://doi.org/10.1016/j.jtcvs.2022.03.033.
Hadem J, Gottlieb J, Seifert D, Fegbeutel C, Sommer W, Greer M, Wiesner O, Kielstein JT, Schneider AS, Ius F, et al. Prolonged mechanical ventilation after lung transplantation—a single-center study. Am J Transplant. 2016;16(5):1579–87.
Fernandez-Zamora MD, Gordillo-Brenes A, Banderas-Bravo E, Arboleda-Sánchez JA, Hinojosa-Pérez R, Aguilar-Alonso E, Herruzo-Aviles Á, Curiel-Balsera E, Sánchez-Rodríguez Á, Rivera-Fernández R. Prolonged mechanical ventilation as a predictor of mortality after cardiac surgery. Respir Care. 2018;63(5):550–7.
Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(7):544–53.
Rose L, McGinlay M, Amin R, Burns KE, Connolly B, Hart N, Jouvet P, Katz S, Leasa D, Mawdsley C, et al. Variation in definition of prolonged mechanical ventilation. Respir Care. 2017;62(10):1324–32.
MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–54.
Pilcher DV, Scheinkestel CD, Snell GI, Davey-Quinn A, Bailey MJ, Williams TJ. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(4):912–8.
Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–8.
Madill J, Gutierrez C, Grossman J, Allard J, Chan C, Hutcheon M, Keshavjee SH, Toronto Lung Transplant P. Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90–day mortality following transplantation. J Heart Lung Transplant. 2001;20(3):288–96.
Lederer DJ, Wilt JS, D’Ovidio F, Bacchetta MD, Shah L, Ravichandran S, Lenoir J, Klein B, Sonett JR, Arcasoy SM. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180(9):887–95.
Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant. 2010;29(9):1026–33.
Mui P, Hill SE, Thorpe RJ Jr. Overweight and obesity differences across ethnically diverse subgroups of Asian American men. Am J Mens Health. 2018;12(6):1958–65.
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527–34.
Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, Dahlberg PS. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26(10):1004–11.
Figueroa-Casas JB, Dwivedi AK, Connery SM, Quansah R, Ellerbrook L, Galvis J. Predictive models of prolonged mechanical ventilation yield moderate accuracy. J Crit Care. 2015;30(3):502–5.
Ripoll JG, Wanta BT, Wetzel DR, Frank RD, Findlay JY, Vogt MNP. Association of perioperative variables and the acute respiratory distress syndrome in liver transplant recipients. Transplant Dir. 2020;6(1): e520.
Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, Brochard L, Clarkson K, Esteban A, Gattinoni L, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42(12):1865–76.
Arni S, Maeyashiki T, Citak N, Opitz I, Inci I. Subnormothermic ex vivo lung perfusion temperature improves graft preservation in lung transplantation. Cells. 2021. https://doi.org/10.3390/cells10040748.
Wang X, O’Brien ME, Yu J, Xu C, Zhang Q, Lu S, Liang L, An X, McDyer JF, Mallampalli RK. Prolonged cold ischemia induces necroptotic cell death in ischemia–reperfusion injury and contributes to primary graft dysfunction after lung transplantation. Am J Respir Cell Mol Biol. 2019;61(2):244–56.
Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochettino A, Lewis J, Christie JD. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23(6):819–30.
Necki M, Antonczyk R, Pandel A, Gaweda M, Latos M, Urlik M, Stacel T, Wajda-Pokrontka M, Zawadzki F, Przybylowski P, et al. Impact of cold ischemia time on frequency of airway complications among lung transplant recipients. Transplant Proc. 2020;52(7):2160–4.
Prekker ME, Nath DS, Walker AR, Johnson AC, Hertz MI, Herrington CS, Radosevich DM, Dahlberg PS. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006;25(4):371–8.
Acknowledgements
We would like to thank the thoracic surgery and ICU staff of the Shanghai Pulmonary Hospital for making this research possible and all patients and their family members for participating in this study.
Funding
This research was supported by the scientific and technological innovation action plans of Science and Technology Commission of Shanghai Municipality (No.20DZ2253700, 22Y2190050, and SHDC22021310-A).
Author information
Authors and Affiliations
Contributions
PG and CL analyzed the data and wrote the paper; YZ, YN and JW collected the data; XL, PZ and JD checked the integrity of the data and the accuracy of the data analysis; CC, YS and WH designed the study and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Commission of Shanghai Pulmonary Hospital (No. L20-352). The requirement for informed consent was waived by the Research Ethics Commission of Shanghai Pulmonary Hospital, Tongji University School of Medicine because of the retrospective nature of the study. All procedures were in accordance with relevant guidelines and regulations (Declaration of Helsinki). We confirm that our retrospective data collection didn’t subject the patients to any additional experimental protocols.
Consent for publication
Not applicable.
Competing interests
No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Figure S1. Study flochart.
Additional file 2
. Figure S2. A simplified nomogram.
Additional file 3
. Figure S3. ROC analysis for the simplified nomogram.
Additional file 4
. Table S1. Detailed Ventilation Parameters of the 104 Patients at T0, T24, T48, T72.
Additional file 5
. Table S2. Comparison of the performance metric for PMV.
Additional file 6
. Table S3. Donor characteristics.
Additional file 7
. Table S4. Primary outcomes after extubation.
Additional file 8
. Table S5. Univariate logistic regression analysis testing effects of donor characteristics on predicting PMV in 141 patients after LuTx.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, P., Li, C., Wu, J. et al. Establishment of a risk prediction model for prolonged mechanical ventilation after lung transplantation: a retrospective cohort study. BMC Pulm Med 23, 11 (2023). https://doi.org/10.1186/s12890-023-02307-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02307-9