Abstract
Background
Transbronchial lung cryobiopsy is useful when diagnosing lung lesions. However, prevention of associated bleeding complications is essential. This study aimed to evaluate the safety and efficacy of our novel bronchoscopic cryobiopsy technique, which uses a long nasobronchial tube to prevent blood flooding the central airway.
Methods
Patients with localized or diffuse lung lesions were prospectively enrolled and underwent cryobiopsy using a 1.9 mm diameter cryoprobe and a 4.0 mm diameter thin bronchoscope under conscious sedation. For cryobiopsy, a long silicone tube (inner diameter, 5.0 mm) was advanced through the nose to the target bronchus, then wedged to drain blood under thin-tube bronchoscopic control. The primary endpoint was the frequency of bleeding complications.
Results
Of the 80 patients initially enrolled, 73 that underwent at least one cryobiopsy were ultimately included. Mild bleeding during cryobiopsy occurred in 58 patients (79.5%), but there was no moderate or severe bleeding. Other complications occurred in four patients (two pneumothorax, one pneumomediastinum, and one pneumonia). Tube dislocation was noted in eight patients (11%). Cryobiopsy specimens were significantly larger than forceps biopsy specimens (9.0 mm2 vs. 2.7 mm2, P < .001) and allowed specific diagnoses in 50 patients (68.5%).
Conclusions
Thin bronchoscopic cryobiopsy using a nasobronchial tube in consciously sedated patients is safe and effective.
Trial registration Date of registration: 24/06/2019. UMIN-Clinical Trials Registry; Identifier: UMIN000037156 https://www.umin.ac.jp/ctr/index.htm
Similar content being viewed by others
Background
Transbronchial cryobiopsy has become popular for the diagnosis of lung lesions, as it provides larger and higher-quality specimens than obtained with conventional forceps biopsy [1,2,3]. This relative ease of sampling has changed the diagnostic approach to localized lesions as well as diffuse lung diseases [3,4,5,6,7,8,9,10,11]. The advantage of transbronchial cryobiopsy over forceps biopsy is that larger, higher-quality specimens can be obtained for diagnostic tests. However, the potential disadvantage is a higher incidence of complications [2, 3, 12], especially bleeding. The reported rate of moderate to severe bleeding in cryobiopsy is 4.9% to 39% [2, 7, 13, 14], such that routine prophylaxis for bleeding is recommended [6, 15, 16]. Several prophylactic techniques for bleeding control, including balloon occlusion, the use of a rigid tube, and a 2-scope method, have been proposed [15,16,17], but their limitations include technical complexity and the need for additional instruments. We developed a novel, wedged-tube cryobiopsy technique, based on the use of a long nasobronchial tube that prevents central airway blood flooding. The technique was described in a previously published case report [18]. In the current study, we prospectively evaluated the safety and efficacy of the technique.
Methods
Patients
This prospective study evaluated the safety and efficacy of our novel cryobiopsy (wedged-tube) technique. From September 2019 to March 2021, patients with lung lesions were recruited and underwent cryobiopsy using the wedged-tube method [18]. The principal inclusion criterion was patients with chest-computed-tomography-confirmed lung lesions in which tissue sampling by cryobiopsy was considered adequate for a pathological diagnosis. The principal exclusion criteria were lesions inappropriate for cryobiopsy (e.g., those located within the inner second ellipse from the hilum, near a large vessel, and near large lung cysts), lesions considered to be inaccessible with a cryoprobe (left upper lobe and apical segment of the right upper lobe), and the need for bronchoscopic procedures for non-target lesions in the same setting. The study was approved by the Institutional Review Board of the National Hospital Organization Nagoya Medical Center (identifier:2019–002) and registered with the University Hospital Medical Information Network-Clinical Trials Registry (identifier: UMIN000037156, registration date: 24/06/2019). Written informed consent was obtained from all participants.
Procedures
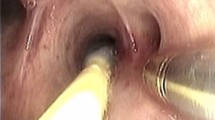
Bronchoscopic procedures were performed under local anesthesia with lidocaine and conscious sedation using IV midazolam and fentanyl. A sterilized, customized 5-mm inner diameter/7-mm outer diameter silicone tube (Phycon tube, SH No. 5; Fuji Systems, Tokyo, Japan; Fig. 1) was used for this procedure [18]. The silicone tube was cut to a length of 40 cm (for lesions in female patients and right upper lobe lesions) or 48 cm (for all lesions except right upper lobe lesions in male patients), and the distal tip was cut diagonally. A standard connector from a 5-mm endotracheal tube was attached to the proximal end of the tube to facilitate grasping (Fig. 1). The silicone tube was advanced through the nose to the target bronchus under bronchoscopic control using a thin bronchoscope (BF-P260F [4.0 mm outer diameter, 2.0 mm working channel, Fig. 1b] or BF-P290 [4.2 mm outer diameter, 2.0 mm working channel]; Olympus, Tokyo, Japan). We used radial endobronchial ultrasound (EBUS) and fluoroscopy in all patients to determine the biopsy site and target bronchus. After the silicone tube had been wedged firmly into the target bronchus, the thin bronchoscope was advanced beyond the tip of the tube toward the target lesion. After the target lesion had been localized with fluoroscopy and EBUS, a 1.9 mm reusable cryoprobe (ERBE, Tübingen, Germany) was advanced to the target site. Cryobiopsy was then performed with a freezing time of 5–7 s. During the procedure, an assistant held the bronchial tube firmly to prevent its migration from the wedged bronchus. The number of biopsies was left to the discretion of the operator. Before the cryobiopsy, a forceps biopsy was performed in some patients. These included patients with localized lesion into which the radial EBUS probe could not be inserted; in these cases, a forceps biopsy was done using an ultrathin bronchoscope (BF-MP290F; Olympus). Each histologic specimen was placed into a formalin-filled container, and submitted to the pathology department for interpretation. During the procedures, oxygen saturation was monitored using a pulse oximeter and supplemental oxygen (through the nasal cannula) was initiated or increased when the oxygen saturation was < 90% for 20 s. All patients rested in bed for 2 h after the procedures, and a chest radiograph was obtained routinely to identify complications. The cryobiopsy procedure using the wedged-tube method is shown in Fig. 2 and Additional file 1: Video 1.
Outcomes
The primary outcome was the frequency of bleeding complications. Secondary endpoints were the frequency of complications other than bleeding, pathological diagnostic yield, procedural duration, frequency of oxygen desaturation, and specimen size.
Bleeding was graded as follows [15]: grade 0, no bleeding; grade 1, mild bleeding requiring bronchoscopic suctioning but not special bronchoscopic treatment; grade 2, moderate bleeding requiring bronchoscopic occlusion, bronchoscopic bronchial collapse, or cold saline instillation; grade 3, severe bleeding causing hemodynamic or respiratory instability, or requiring tamponade, surgical interventions, blood transfusion, or admission to the intensive care unit.
Specific histological findings that resulted in a definitive diagnosis, such as malignant or benign neoplasm, epithelioid cell granuloma, organizing pneumonia, and fungal infection, were considered diagnostic. Inconclusive histological findings, such as nonspecific inflammation and fibrosis, were considered non-diagnostic. Long-term follow-up and the results of multidisciplinary discussion were not used to calculate the diagnostic yield. The size of each specimen was measured using Aperio ImageScope software (Aperio Technologies Inc.), and the sizes of the largest specimens obtained by cryobiopsy and forceps biopsy in each patient were compared.
Data analyses
The success of our technique was evaluated based on the avoidance of moderate or severe bleeding in 90% of patients. We estimated that 73 patients would be required to statistically validate the method. This number was calculated as follows: alternative completion rate of 95%, null completion rate of 80%, statistical power of 90%, and a one-sided significance level of 0.05. Eighty patients were enrolled to compensate for potential dropouts. Means and percentages are presented as appropriate. Continuous variables were compared using the Mann–Whitney U test. Statistical analyses were performed using PASW Statistics (ver. 18.0; SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to indicate a statistically significant difference.
Results
Patients
A total of 80 patients were enrolled, of whom 73 patients who underwent cryobiopsy were included in the analysis (Fig. 3). The baseline characteristics of the patients and lesions are listed in Table 1. Fifty-one patients (69.9%) had localized lesions and 22 patients (30.1%) had diffuse or infiltrative lesions. Bronchoscopic findings are listed in Table 2.
Procedures
The procedural details are shown in Table 3. Of the 73 patients who underwent cryobiopsy, 59 also underwent forceps biopsies in the same setting. The silicone tube was wedged into the mean “2.5th-generation” bronchus level (range: 1st–5th; segmental bronchi were regarded as second-generation bronchi and subsegmental bronchi as third-generation bronchi). The mean number of cryobiopsy samples was 2.5 (range: 0–6). In 72 patients, at least one cryobiopsy sample was obtained. In the remaining patient, cryobiopsy was performed once and did not provide a histological specimen. This patient had a history of bronchial asthma and had a severe cough with bleeding during bronchoscopy, such that no further attempts at cryobiopsy were made. The bronchial washing revealed eosinophilic pneumonia. Cryobiopsy, forceps biopsy, and their combination provided diagnostic materials in 68.5% (50 of 73; localized, 70.6% [36 of 51]; diffuse/infiltrative, 63.6% [14 of 22]), 57.6% (34 of 59; localized, 62.0% [31 of 50]; diffuse/infiltrative, 33.3% [3 of 9]), and 75.3% (55 of 73; localized, 78.4% [40 of 51]; diffuse/infiltrative, 68.2% [15 of 22]) of the patients, respectively. The specimens obtained via cryobiopsy were significantly larger than those harvested in forceps biopsies (9.0 mm2 vs. 2.7 mm2, P < 0.001). The median bronchoscopy time was 33.8 min (17.0–65.3 min).
Complications
As shown in Table 4, bleeding after cryobiopsy occurred in 58 patients (79.5%; localized, 74.5% [38 of 51]; diffuse/infiltrative, 90.9% [20 of 22]), and all were grade 1. Five patients (localized, n = 3; diffuse/infiltrative, n = 2) had a larger amount of bleeding (> 50 mL), but in all cases it could be controlled by simple thin bronchoscopic blood suctioning within the wedged tube. No patient needed other forms of hemostatic therapy, such as cold saline/adrenaline/thrombin administration or the use of a therapeutic bronchoscope with a larger working channel. Complications other than bleeding occurred in four patients (two pneumothorax, one pneumomediastinum, one pneumonia). Pneumothoraxes and pneumomediastinum were resolved via simple observation, and pneumonia was resolved with oral antibiotics. The wedged tube was displaced in eight patients (11%), including major displacement of the tube to other lobar location in four (right middle lobe in two, lingula in two), and minor displacement to other segments in the same lobe in four (right lower lobe in two, right upper lobe in two).
Discussion
Our study demonstrated the efficacy of the wedged-tube method in preventing blood flooding in the central airway during cryobiopsy. Moreover, this approach provides larger samples than obtained with forceps biopsy and thus contributes to a larger diagnostic bronchoscopy yield in patients with lung lesions, without compromising safety in terms of bleeding. To the best of our knowledge, this is the only transnasal cryobiopsy technique that can be performed with the patient under local anesthesia and conscious sedation.
Cryobiopsy provides larger samples than forceps biopsy, but at the expense of a higher risk of bleeding in patients with localized lung lesions or diffuse lung diseases [1–3. 10, 12]. In a randomized study of patients with diffuse lung diseases, Hetzel et al. [12] reported a significantly higher incidence of moderate to severe bleeding during cryobiopsy than during forceps biopsy (16.2% vs. 4.2%, P < 0.001). In a large retrospective study of 1024 patients with localized lung lesions who were placed under general anesthesia for cryobiopsy using both a rigid and a flexible bronchoscope, the frequency of moderate to severe bleeding was 8.5% [10]. In most cases, bleeding is controllable with bronchoscopy, however, near-fatal bleeding has been reported in rare cases [19, 20]. In addition, soiling of the airways with blood may exacerbate diffuse lung disease [21]. Several prophylactic methods for the control of central airway blood flooding have been proposed, including balloon blocking, use of a rigid tube, and a 2-scope method [15,16,17], but each has its own limitations, such as the technical difficulty, technical complexity, the need for additional instruments/equipment/medical staff, and a short but certain time lag between hemorrhage and bronchoscopic hemostasis. In the current method, the wedged tube prevents blood flooding in the central airway, by draining the blood. In fact, bleeding could be controlled with simple bronchoscopic suctioning in all patients, although the amount of bleeding differed in each one.
In the absence of a consensus definition of the bleeding grade during cryobiopsy [15, 20, 22], a comparison of our results with those of previous reports is difficult. However, our study clearly demonstrated the effectiveness of the wedged-tube method in preventing central airway blood flooding. The outer diameter of the bronchial tube used in the presence series was 7.0 mm, which is similar to the outer diameter of convex EBUS bronchoscopes. Patients can breathe spontaneously during EBUS bronchoscope procedures, as can patients who undergo wedged-tube cryobiopsy, even if the bronchial tube is filled with blood. During cryobiopsy using a balloon blocking method, the balloon may dislocate when the cryoprobe carrying the adhered frozen samples is retracted [23], possibly causing severe bleeding [20]. Inomata et al. reported an incidence of major balloon displacement, minor balloon displacement, and balloon rupture of 2.2%, 7.6%, and 1.4%, respectively [23]. Similarly, there is a risk of tube displacement in our method due to the resistance between the inner wall of the bronchial tube and the thin bronchoscope. The rate of major and minor displacements of the wedged tube was 5.5% each, which is comparable to the frequency reported by Inomata et al. Fortunately, displacement of the wedged tube did not cause bleeding complications. Although minor displacement of the tube occurred, the side wall of the tube often sealed the orifice of the target bronchus and prevented bleeding; however, major displacement can cause bleeding. To reduce this type of risk, the assistant should hold the tube firmly during the procedure. In addition, both the bronchoscope and the inner wall of the bronchial tube should be sprayed with lidocaine to facilitate the smooth removal of the bronchoscope by reducing the resistance.
The greatest advantages of the current method over other prophylactic methods are its technical simplicity and reduced invasiveness. Cryobiopsy has been performed transorally through an 8.0–9.0 mm (inner diameter) tube, using standard or large therapeutic bronchoscopes in patients under conscious sedation or general anesthesia. It has also been performed with rigid bronchoscopes in patients under general anesthesia. The current transnasal method uses a smaller-diameter bronchial tube and a thinner bronchoscope in consciously sedated patients, which is less invasive and allows the procedure to be performed on an outpatient basis. In fact, none of the 60 patients who underwent wedged-tube cryobiopsy in the outpatient setting needed unplanned hospital admission due to complications. The cryobiopsy diagnostic yields for localized lesions and diffuse lung disease range from 67 to 92% [24], and 40% to 95% [2], respectively. Many factors (e.g., size, presence of the bronchus sign, and lesion location and type) affect diagnostic yield; thus, we could not compare the diagnostic yields in the current study to published data, although ours seemed acceptable. The current technique is a good alternative to the conventional cryobiopsy technique based on the safety and efficacy profile.
Nonetheless, the wedged-tube method has a few disadvantages, especially its unavailability for the left upper lobe and right apical lung lesions. Both the left upper lobe bronchus and the right apical segmental bronchus curve sharply from the trachea, hindering advancement of the bronchial tube into those bronchi in most cases. However, the upper lobe, especially the apical segment, is rarely examined by cryobiopsy, as it is difficult to access with the stiff cryoprobe alone [25]. Another disadvantage is that larger instruments, including a 2.4 mm cryoprobe, cannot be used. However, current guidelines recommend the use of a 1.9 mm cryoprobe rather than a 2.4 mm cryoprobe in cryobiopsy [6].
Our study also had the following limitations. First, it was a single-center study conducted at an expert center, such that our results may not be generalizable. Second, long-term follow-up and the results of multidisciplinary discussions were not considered, as this study focused mainly on the safety of the wedged-tube cryobiopsy technique. The rate of false-positive results for the specific bronchoscopic findings (Table 2) was low but not zero. Third, our study excluded patients with lesions considered inappropriate for cryobiopsy or inaccessible with a cryoprobe, which may have caused selection bias. Finally, this was not a comparative study. For further insights into this technique, a large comparative study is necessary.
Conclusions
Thin bronchoscopic cryobiopsy using a long nasobronchial tube is a safe procedure in terms of bleeding. The greatest advantage of this method over conventional cryobiopsy is that it can be performed using thinner tube, inserted transnasally in patients under conscious sedation. Because of the simplicity and reduced invasiveness, wedge-tube cryobiopsy may be a useful alternative cryobiopsy method.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- EBUS:
-
Endobronchial ultrasound
References
Pajares V, Puzo C, Castillo D, Lerma E, Montero MA, Ramos-Barbón D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology. 2014;19(6):900–6.
Sethi J, Ali MS, Mohananey D, Nanchal R, Maldonado F, Musani A. Are transbronchial cryobiopsies ready for prime time?: a systematic review and meta-analysis. J Bronchology Interv Pulmonol. 2019;26(1):22–32.
Troy LK, Hetzel J. Lung cryobiopsy and interstitial lung disease: What is its role in the era of multidisciplinary meetings and antifibrotics? Respirology. 2020;25(9):987–96.
Guenther A, Krauss E, Tello S, Wagner J, Paul B, Kuhn S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):141.
Troy LK, Grainge C, Corte TJ, Williamson JP, Vallely MP, Cooper WA, et al. Cryobiopsy versus open lung biopsy in the diagnosis of interstitial lung disease alliance (COLDICE) investigators. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med. 2020;8(2):171–81.
Maldonado F, Danoff SK, Wells AU, Colby TV, Ryu JH, Liberman M, et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: CHEST guideline and expert panel report. Chest. 2020;157(4):1030–42.
Ravaglia C, Bonifazi M, Wells AU, Tomassetti S, Gurioli C, Piciucchi S, et al. Diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91(3):215–27.
Tomassetti S, Wells AU, Costabel U, Cavazza A, Colby TV, Rossi G, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193(7):745–52.
Hetzel J, Wells AU, Costabel U, Colby TV, Walsh SLF, Verschakelen J, et al. Transbronchial cryobiopsy increases diagnostic confidence in interstitial lung disease: a prospective multicentre trial. Eur Respir J. 2020;56(6):1901520.
Herth FJ, Mayer M, Thiboutot J, Kapp CM, Sun J, Zhang X, et al. Safety and performance of transbronchial cryobiopsy for parenchymal lung lesions. Chest. 2021;160(4):1512–9.
Matsumoto Y, Nakai T, Tanaka M, Imabayashi T, Tsuchida T, Ohe Y. Diagnostic outcomes and safety of cryobiopsy added to conventional sampling methods: an observational study. Chest. 2021;160(5):1890–901.
Hetzel J, Eberhardt R, Petermann C, Gesierich W, Darwiche K, Hagmeyer L, et al. Bleeding risk of transbronchial cryobiopsy compared to transbronchial forceps biopsy in interstitial lung disease - a prospective, randomized, multicentre cross-over trial. Respir Res. 2019;20(1):140.
Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. A systematic review and metaanalysis. Ann Am Thorac Soc. 2016;13(10):1828–38.
Iftikhar IH, Alghothani L, Sardi A, Berkowitz D, Musani AI. Transbronchial lung cryobiopsy and video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse parenchymal lung disease. A meta-analysis of diagnostic test accuracy. Ann Am Thorac Soc. 2017;14(7):1197–211.
Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95(3):188–200.
Colella S, Haentschel M, Shah P, Poletti V, Hetzel J. Transbronchial lung cryobiopsy in interstitial lung diseases: best practice. Respiration. 2018;95(6):383–91.
Sriprasart T, Aragaki A, Baughman R, Wikenheiser-Brokamp K, Khanna G, Tanase D, et al. A single US center experience of transbronchial lung cryobiopsy for diagnosing interstitial lung disease with a 2-scope technique. J Bronchology Interv Pulmonol. 2017;24(2):131–5.
Oki M, Saka H. Novel technique to prevent central airway blood flooding during transbronchial cryobiopsy. J Thorac Dis. 2019;11(9):4085–9.
DiBardino DM, Haas AR, Lanfranco AR, Litzky LA, Sterman D, Bessich JL. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an academic medical center. Ann Am Thorac Soc. 2017;14(6):851–7.
Kronborg-White S, Sritharan SS, Madsen LB, Folkersen B, Voldby N, Poletti V, et al. Integration of cryobiopsies for interstitial lung disease diagnosis is a valid and safe diagnostic strategy-experiences based on 250 biopsy procedures. J Thorac Dis. 2021;13(3):1455–65.
Pannu J, Roller LJ, Maldonado F, Lentz RJ, Chen H, Rickman OB. Transbronchial cryobiopsy for diffuse parenchymal lung disease: 30- and 90-day mortality. Eur Respir J. 2019;54(4):1900337.
Folch EE, Mahajan AK, Oberg CL, Maldonado F, Toloza E, Krimsky WS, et al. Standardized definitions of bleeding after transbronchial lung biopsy: a Delphi consensus statement from the Nashville working group. Chest. 2020;158(1):393–400.
Inomata M, Kuse N, Awano N, Tone M, Yoshimura H, Jo T, et al. Prospective multicentre study on the safety and utility of transbronchial lung cryobiopsy with endobronchial balloon. ERJ Open Res. 2020;6(2):00008–2020.
Sryma PB, Mittal S, Madan NK, Tiwari P, Hadda V, Mohan A, et al. Efficacy of radial endobronchial ultrasound (R-EBUS) guided transbronchial cryobiopsy for peripheral pulmonary lesions (PPL’s): a systematic review and meta-analysis. Pulmonology. 2021. https://doi.org/10.1016/j.pulmoe.2020.12.006.
Imabayashi T, Uchino J, Yoshimura A, Chihara Y, Tamiya N, Kaneko Y, et al. Safety and usefulness of cryobiopsy and stamp cytology for the diagnosis of peripheral pulmonary lesions. Cancers. 2019;11(3):410.
Acknowledgements
We thank Drs. Rieko Nishimura and Akari Iwakoshi for supporting the pathologic examinations. The English in this document has been checked by at least two professional editors, both native speakers of English.
Funding
This study was supported by a scholarship grant from TAIHO Pharmaceutical Co.
Author information
Authors and Affiliations
Contributions
MO had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MO contributed to the study design, data collection, analysis, and interpretation, wrote the initial draft of the manuscript, and performed further editing. H.S. contributed to the study design, data interpretation, and editing of the manuscript. YK, HN, AI, AY, AT, and CK contributed to data interpretation and to editing of the manuscript. MO, HS, YK, HN, AI, AY, and AT performed the procedures. All authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed following the Declaration of Helsinki and approved by the Institutional Review Board of National Hospital Organization Nagoya Medical Center (identifier: 2019-002). All participants provided written informed consent before enrollment. Clinical trial registration: UMIN000037156 at UMIN Clinical Trials Registry; www.umin.ac.jp/ctr/
Consent for publication
Informed consent was obtained from all patients before publication. The physicians videotaped in the supplementary video provided informed consent before publication.
Competing interests
MO received speaker fees from Olympus Corporation as an invited guest speaker at academic medical meetings. Other authors have no conflicts of interest directly relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Video 1. Cryobiopsy procedure using the wedged-tube method.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oki, M., Saka, H., Kogure, Y. et al. Thin bronchoscopic cryobiopsy using a nasobronchial tube. BMC Pulm Med 22, 361 (2022). https://doi.org/10.1186/s12890-022-02166-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02166-w