Abstract
Background
Regulatory T cells (Treg cells) in the peripheral blood of patients with pulmonary tuberculosis (PTB) may be closely related to the progression of PTB. In this study, the distribution characteristics and clinical importance of CD8+CD28− Treg cells in patients with tuberculosis were systematically analyzed, and the role and importance of CD8+CD28− Treg cells in influencing the immune response and progression of tuberculosis were discussed, which will provide immunological indices and reference values for the clinical diagnosis of tuberculosis.
Methods
Flow cytometry, sputum smears and computed tomography imaging were used to analyze the distribution characteristics of CD8+CD28− Treg cells in the peripheral blood of patients with PTB and the correlation between CD8+CD28−Treg cells and clinical and immune indices.
Results
The percentages of CD4+CD25high and CD8+CD28− Treg cells in the peripheral blood of patients with PTB were significantly higher than those in the healthy control (HC) group. Further analysis showed that the percentage of CD4+CD25highTreg cells in the Stage II group was significantly higher than that in the HC group. The percentages of CD4+CD25high and CD8+CD28− Treg cells increased significantly in patients in the Stage II group. The proportion of CD8+CD28− Treg cells was directly proportional to the degree of positivity in sputum smears, while CD4+CD25highTreg cells did not exhibit this trend.
The correlations between the percentage of CD4+CD25high and CD8+CD28− Treg cells and the percentage of lymphocyte subsets were examined. The percentage of CD8+CD28− Treg cells was negatively correlated with the percentage of CD4+T cells and positively correlated with the CD8+T cell percentage in the HC and PTB groups. The percentage of CD4 + CD25highTreg cells was positively correlated with the percentage of CD4+T cells only in the PTB group.
Conclusions
This study was the first to show that the proportion of CD8+CD28− Treg cells in the peripheral blood of patients with PTB was significantly increased, and the increase in CD8+CD28− Treg cells was related to the progression of PTB, which may affect the proportion of immune cell subsets by inhibiting the immune response, resulting in the progression of PTB.
Similar content being viewed by others
Background
Tuberculosis (TB) is a chronic respiratory infectious disease caused by Mycobacterium tuberculosis (MTB), which seriously threatens human health and is the 13th leading cause of death worldwide. According to the 2021 Global Tuberculosis Report, there are approximately 9.9 million new TB patients worldwide, with 1.28 million deaths and a mortality rate of 17/100,000. Furthermore, due to the effect of the COVID-19 pandemic, the number of deaths due to TB in 2021 was higher than that observed in the previous year [1].
After MTB infection, cell-mediated immunity (CMI) and delayed type hypersensitivity (DTH) play important roles in the occurrence, development and prognosis of TB. CMI refers to the presence of a large number of activated macrophages produced by amplified levels of T lymphocytes and cytokines specific to MTB antigens, including those involved in the innate immune response and acquired immune response. Multiple immune cell subsets, their cytokines and specific TB-presenting cells are involved in the immune response and immune regulation of TB infection.
In recent years, the abnormal increase in the number of regulatory T cells (Treg cells) and the occurrence and development of immune responses and related diseases have attracted increasing attention. Studies have shown that the increase in the number of CD4+CD25highTreg cells in patients with pulmonary tuberculosis (PTB) may be related to low cellular immunity and lead to prolonged and repeated attacks of PTB [2]. When Treg cells are inhibited, anti-MTB immunity can return to normal, so Treg cells may be an important factor in the immune escape of TB. Treg cells play an immunosuppressive role mainly through cytokines and cell contact. Treg cells participate in the occurrence and development of diseases by inhibiting the immune response to self or foreign antigens. Many subsets of Treg cells have been identified, including CD4+CD25highTreg cells and CD8+CD28−Treg cells [3].
CD8+CD28−Treg cells are CD8+T cells lacking the CD28 molecule. CD8+CD28−Treg cells play a negative regulatory role in the activation of T cells and play an important regulatory role in controlling autoimmune diseases and rejection after organ transplantation. Recent studies have confirmed that CD8+CD28−Treg cells are an important subset that have the same immunosuppressive effects as CD4+CD25highTreg cells. CD8+CD28−Treg cells can upregulate the expression of IL-3, IL-4 and other inhibitory factors on the surface of antigen-presenting cells (APCs), which makes these cells immunologically tolerant; downregulates the expression of CD80, CD86 and other costimulatory molecules; and inhibits the activity of Th cells [4,5,6,7]. The transcription factor Foxp3 regulates the immunosuppressive effects of CD8+ Treg cells. The higher the expression levels of Foxp3, the stronger the inhibitory activity of CD8+ Treg cells, indicating a positive correlation. The ratio of CD8+CD28−Treg cells in the peripheral blood and tumor tissues of patients with primary liver cancer and gastric cancer was found to be significantly higher than that in normal individuals [8, 9]. In tumors, it was found that the number and activity of CD8+CD28−Treg cells in peripheral blood increased, which inhibited the antitumor immune response and reduced the antigen presentation ability of dendritic cells (DCs), ultimately leading to the onset of tumors [10,11,12]. In a study of HIV, the number of CD8+CD28−Treg cells was significantly increased and negatively correlated with the number of CD4+T cells, while in a study of pneumonia pathogens [13], CD8+CD28−Treg cells could be used to specifically diagnose Mycoplasma pneumoniae infection [14].
Because CD8+CD28−Treg cells exhibit increased secretion of IL-6 [15], which can inhibit the effects of IFN-γ and reduce the sensitivity of uninfected macrophages to IFN-γ stimulation, CD8+CD28−Treg cells may participate in the immune escape of MTB. However, there has been no report on the correlation between TB and CD8+CD28−Treg cells. Therefore, this study systematically analyzed the relationship between peripheral blood CD8+CD28−Treg cells and the course of TB and the immune response and further clarified the immune mechanism of TB.
Methods
Subjects and inclusion criteria
Subjects: From September 2018, to August 2019, 79 patients with PTB were recruited from the Tuberculosis Department of the Infectious Disease Hospital Affiliated with Soochow University. Computed Tomography (CT) imaging was combined with the clinical characteristics of patients, and the patients were divided into a severe PTB group (Stage II) and a mild PTB group (Stage I). In addition, according to the acid-fast staining results of sputum smears, the amount of mycobacteria excretion in PTB patients was measured and divided into four grades: + , + + , + + + and + + + + + . Samples were collected from healthy controls (HCs) at the physical examination center of the fifth people’s hospital of Suzhou. The HCs were TB-IGRA negative, and no other respiratory tract infections or other diseases were confirmed in these participants.
Inclusion and exclusion criteria: The diagnosis of confirmed PTB met the diagnostic criteria for PTB, and patients were screened for sputum smears (+ and higher) and MTB cultures. CT imaging was combined with the clinical characteristics of patients to divide the patients into two groups: patients with lesions involving more than three lung fields; respiratory symptoms such as cough, expectoration and chest tightness; and a body temperature ≥ 39 °C were in the severe TB group (Stage II); in contrast, those with mild illness were in the mild group (Stage I). All cases of bacterial pneumonia were confirmed by clinical manifestations, etiological examinations and chest CT. Patients with other infectious diseases, including HIV, HCV, HDV and HBV infections, and other complications, such as diabetes, were excluded from the study.
Reagents and instruments
Anti-human monoclonal antibodies (CD3-APC, CD4-PerCP CD25-FITC and CD28-PE) were purchased from BD Pharmingen Company (San Diego, CA, USA). Erythrocyte lysis buffer was purchased from BD Biosciences Company (San Diego, CA, USA). MTB drug-sensitive Roche medium (Roche, USA) was used.
Centrifuges (Jouan, France), an electronic balance (Eppendorff, Germany), a TB culture apparatus (BACTEC MGIT-960) and BBL MGIL culture tubes (BD Company, USA), a flow cytometer (BD Company, USA) and Philips MX4000 Dual Spiral CT Instrument (Philips Corporation, USA) were used.
Detection of Treg cell subsets in human peripheral blood
CD25 was also considered to be a marker of CD4+Treg cells, and its expression level was correlated with Foxp3 and CD127 expression levels. (Additional file 1: Fig. S1), CD3+CD4+CD25highcells were considered to be CD4+Tregs in this study. Peripheral blood (100 μL) was collected from healthy individuals and PTB patients in each test group, and then antibodies (CD3-APC, CD4-PerCP CD25-FITC and CD28-PE) were added and incubated for 15 min. Then, red blood cells were lysed in erythrocyte lysis buffer, and the cells were centrifuged, washed with PBS, suspended in 0.5 mL of PBS, acquired by flow cytometry and analyzed by FlowJo software.
Acid-fast staining of sputum smears
Sputum samples were examined by the acid-fast staining method, and the results of the microscopic examination were reported according to the following criteria: negative acid-fast bacilli ( − ) were observed continuously in 300 different visual fields, and no acid-fast bacilli were observed. The positive rate of acid-fast bacilli ( +) was 3–9 strips/100 visual fields. The positive rate of acid-fast bacilli (+ +) was 1–9 strips/10 visual fields. The positive rate of acid-fast bacilli (+ + +) was 1–9/field; the positive rate of acid-fast bacilli (+ + + + +) was ≥ 10 strips/field.
Culture of MTB
The collected sputum samples were combined with an equal amount of 4% sodium hydroxide and mixed well. Five milliliters of the treated specimens was placed into a labeled sharp-bottomed centrifuge tube, shaken vigorously, and mixed well, after which PBS buffer was added, and the samples were centrifuged at 4000 rpm for 15 min. The supernatant was removed, and the pellet was resuspended in 12 mL of PBS. First, 0.8 mL of mixed additive (Growth Supplement) and miscellaneous bacterial inhibitor (PANTA) was added to the BBL MGIL culture tube, and 0.5 mL of the treated sample was then injected into the MGIT culture tube. The inoculated MGIT culture tube was placed into a BACTEC MGIT-960 TB culture instrument, and negative or positive culture results were obtained by automatic scanning by the instrument.
Imaging and analysis of PTB patients
Patients with PTB were examined by spiral CT, and the imaging results were analyzed by two physicians who specialized in CT examination. The observation range was from the lung apex to the lung floor, and the CT imaging characteristics of PTB were recorded. CT imaging was combined with the clinical characteristics of patients to divide the patients into two groups: patients with lesions involving more than three lung fields; respiratory symptoms such as cough, expectoration and chest tightness; and a body temperature ≥ 39 °C in the severe TB group (Stage II). This group contained 1 patient with sputum smear + , 8 patients with sputum smear + + , 12 patients with sputum smear + + + and 13 patients with sputum smear + + + . In contrast, those with mild illness were classified into the mild group (Stage I).
Data sharing
The datasets generated and analyzed during the current study are available in the https://doi.org/10.6084/m9.figshare.19803757.v1 repository.
Statistical analysis
The data were analyzed using the statistical software GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, USA). The data are presented as the mean ± SEM. One-way ANOVA was used to perform comparisons, Pearson’s test was used to analyze correlations, and results with p values of < 0.05 were considered statistically significant.
Results
Basic clinical characteristics of selected subjects
The selected subjects were divided into the HC (control group) (n = 25) and PTB (n = 79) groups. Age, sex, TB severity, sputum smears and T lymphocyte subsets in the HC and PTB groups were analyzed (Table 1).
Detection and analysis of CD8+CD28− and CD4+CD25highTreg cells in the peripheral blood of PTB patients and HCs
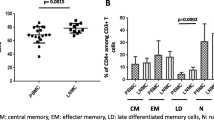
The percentages of CD4+CD25high and CD8+CD28−Treg cells in the peripheral blood of subjects in the HC group and PTB group were analyzed by flow cytometry (Fig. 1A). The results showed that the percentages of CD8+CD28−Treg cells and CD4+CD25highTreg cells in the PTB group were significantly higher than those in the HC group (Fig. 1B, C).
Gating strategy and frequencies of peripheral blood CD8+CD28− and CD4+CD25highTreg cells in the HC group and PTB group. A The gating strategies and representative results showing the frequencies of peripheral blood CD8+CD28− and CD4+CD25highTreg cells in the HC group and PTB group. B Statistical analysis of the frequencies of CD8+CD28− and CD4+CD25highTreg cells in the HC and PTB groups
Analysis of the correlation between the number of peripheral blood CD8+CD28− and CD4+CD25high Treg cells and the course of TB
The proportions of CD8+CD28− and CD4+CD25highTreg cells in stage I and stage II TB patients were compared with those in HCs, and the proportion of CD8+CD28−Treg cells in stage II patients was significantly higher than that in the HC group. The percentage of CD4+CD25highTreg cells in the Stage I and Stage II groups was higher than that in the HC group (Fig. 2A, B).
Correlation between the levels of peripheral blood CD8+CD28− and CD4+CD25highTreg cells and the disease course of patients with TB. A Statistical analysis of the frequencies of CD8+CD28−Treg cells among the CD8+ population was compared among the HC, PTB-Stage I and PTB-Stage II groups. B Statistical analysis of the frequencies of CD4+CD25highTreg cells among the CD4+ population was compared among the HC, PTB-Stage I and PTB-Stage II groups. C Statistical analysis of the frequencies of CD8+CD28− Treg cells among the CD8+ population was compared among the subgroups of PTB patients with different bacillary loads in their sputum. D Statistical analysis of the frequencies of CD4+CD25highTreg cells among the CD4+ population was compared among the subgroups of PTB patients with different bacillary loads in their sputum. + : 3–9 strips/100 visual fields, + + : 1–9 strips/10 visual fields, + + + : 1–9/field and + + + + : ≥ 10 strips/field
In addition, acid-fast staining of sputum smears was performed, and the amount of mycobacteria in PTB patients was used to divide the patients into four groups: + , + + , + + + and + + + . The percentages of CD8+CD28− and CD4+CD25highTreg cells were compared among patients in these four groups. The percentage of CD8+CD28−Treg cells was higher in the + + + group and + + + + group than in the + group. The percentage of CD8+CD28−Treg cells in the + + + + group was higher than that in the + + group. However, there were no differences in the proportions of CD4+CD25highTreg cells (Fig. 2B).
Analysis of the correlation between the levels of CD8+CD28− and CD4+CD25highTreg cells and lymphocyte subsets in the peripheral blood of patients with TB
The percentage of peripheral blood CD8+CD28−Treg cells in patients with TB was correlated with the levels of lymphocyte subsets. The results showed that the proportion of CD8+CD28−Treg cells was negatively correlated with the proportion of CD4+T cells in the PTB group but was positively correlated with the CD8+T-cell percentage in the PTB group and HC group (Fig. 3A–D and Additional file 1: Fig. S2 A–D). There were no relationships between the proportions of CD4+CD25high Treg cells and the levels of lymphocyte subsets in the PTB group and HCs (Fig. 3E–H and Additional file 1: Fig. S2 E–H).
Correlation between the frequencies of peripheral blood CD8+CD28− and CD4+CD25highTreg cells and lymphocyte subsets in the PTB groups. A–D Correlation between the frequencies of peripheral blood CD8+CD28−Treg cells and lymphocyte subsets in the PTB groups. E–H Correlation between the frequencies of peripheral blood CD4+CD25highTreg cells and lymphocyte subsets in the PTB groups
Discussion
Immunotherapy has been successful as a treatment for many diseases. Therefore, understanding the escape mechanism of TB and developing therapeutic methods for immune targets will provide ideas for the treatment of MDR-TB. At present, the immune escape mechanism of MTB has not been fully clarified, but it is thought that TB immunity is associated with type IV allergies and cellular immunity [16, 17]. With progress in TB immune research, an increasing number of scholars have found that the pathogenesis of TB is closely related to alveolar macrophages (AMs), T lymphocytes and related cytokines in vivo [18, 19]. Treg cells are important in maintaining immune balance and play important roles in autoimmune diseases, organ transplantation, bacterial and viral infections and the pathological states of various tumors [20,21,22,23].
An abnormal increase in the levels of CD4+CD25highTreg cells and the inhibition of the immune response to TB are important factors that cause low cellular immunity in patients with PTB and lead to prolonged and repeated attacks of PTB [2]. It has been shown that the number of CD4+CD25highTreg cells in TB patients is abnormally increased, which affects the duration and development of this disease by inhibiting effector immune cells [2]. In this study, the percentage of CD4+CD25highTreg cells in patients with PTB was examined and compared with that in healthy individuals, and the correlations between the levels of CD4+CD25highTreg cells, the levels of lymphocyte subsets and monocyte percentages were analyzed. The results showed that the percentage of CD4+CD25highTreg cells in the peripheral blood of PTB patients was higher than that in healthy individuals, suggesting that the initiation and continuation of the immune response amplified the number of CD4+CD25highTreg cells. As the body failed to clear MTB in time, the increase in the number of CD4+CD25highTreg cells further inhibited the effective anti-MTB immune response, resulting in an inadequate or insufficient response and leading to MTB escape and chronic infection. The results showed that there was no significant difference in the percentage of CD4+CD25highTreg cells among PTB patients with different grades of MTB, which indicated that the intensity of the MTB antigen and the deterioration of lung lesions had no significant effect on the number of CD4+CD25highTreg cells.
CD8+CD28−Treg cells are an important subgroup of Treg cells. Studies have shown that the number of CD8+CD28−Treg cells is increased in tumor patients, and these cells participate in the inhibition of the tumor-specific immune response and are closely related to tumor progression [3, 8]. John S Manavalan et al. [24] found the CD8+CD28−Tregs were Foxp3 positive in vitro and our study also found most CD8+Foxp3+T cells were CD28 negative (Additional file 1: Fig. S3), which prompt the CD8+CD28−Tregs have relationship with TGF-β and IL-10. In a study of viral pneumonia, it was found that CD8+Treg cells inhibited the function of CD8+T cells through IL-10 [25]. Another study found that CD8+CD28−Treg cells highly expressed type II immune response-related cytokines, such as IL-4, IL-10 and TGF-β [26]. The above studies suggest that CD8+CD28−Treg cells may play an important role in the formation of tuberculosis and granuloma. However, the distribution of these cells in PTB patients and the correlation with clinical symptoms and lymphocyte subsets are still unclear. Therefore, this study further examined the proportion of the CD8+CD28−Treg cell population in PTB patients compared with that in healthy individuals and analyzed the correlation between the numbers of these cells and lymphocyte subsets. The results showed that the percentage of CD8+CD28−Treg cells in the peripheral blood of PTB patients was higher than that in healthy individuals. In addition, the percentage of CD8+CD28−Treg cells in HCs and PTB patients correlated with the proportions of CD4+T cells and CD8+T cells, and this effect was different from that observed for the proportions of CD4+CD25highTreg cells, which was only associated with the proportions of CD8+T cells in PTB patients but not in HCs. This finding indicates that CD4+CD25highTreg cells belong to the induced cell population, and the proportion of induced cells increases with disease occurrence and progression and the initiation and continuation of the immune response, thus inhibiting the expansion of the immune response. As an intrinsic group of immune cells, CD8+CD28−Treg cells may regulate the proportions of each subgroup of immune cells. With the occurrence and progression of pneumonia, TB and other diseases and the initiation of the immune response, the proportion of these cells increases to limit an excessive immune response. However, in chronic infections such as TB, the abnormal increase in the number of these cells may inhibit an effective response. Further analysis showed that the percentage of CD8+CD28−Treg cells increased with the severity of PTB and the level of MTB excretion, which indicated that the intensity of MTB antigens and the degree of disease deterioration could affect the number of CD8+CD28− Treg cells.
Conclusions
In this study, the relationship between the CD8+CD28−Treg cell population and TB was analyzed systematically. The proportion of CD8+CD28− cells in the peripheral blood of PTB patients was significantly increased, and there was a correlation between the proportion of CD8+CD28−Treg cells and the immune function of TB patients. Further examination showed that unlike the number of CD4+CD25highTreg cells, the increase in the number of CD8+CD28−Treg cells was related to the progression of PTB, which may affect the ratio of immune cell subsets by inhibiting the immune response, resulting in the progression of PTB. These results suggest that the CD8+CD28−Treg cell population may be an important immunosuppressive subgroup in TB patients and may become an important target of immune therapy for TB.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the https://doi.org/10.6084/m9.figshare.19803757.v1 repository.
Abbreviations
- PTB:
-
Pulmonary tuberculosis
- Treg cells:
-
Regulatory T cells
- HC:
-
Healthy control
- MTB:
-
Mycobacterium tuberculosis
- TB:
-
Tuberculosis
- CMI:
-
Cell-mediated immunity
- DTH:
-
Delayed type hypersensitivity
- DCs:
-
Dendritic cells
- AMs:
-
Alveolar macrophages
- CT:
-
Computed tomography
References
Jeremiah C, Petersen E, Nantanda R, Mungai BN, Migliori GB, Amanullah F, Lungu P, Ntoumi F, Kumarasamy N, Maeurer M, et al. The WHO Global tuberculosis 2021 report: not so good news and turning the tide back to End TB. Int J Infect Dis. 2022. https://doi.org/10.1016/j.ijid.2022.03.011.
Cardona P, Cardona PJ. Regulatory T cells in mycobacterium tuberculosis Infection. Front Immunol. 2019;10:2139.
Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: are they players in autoimmunity? Autoimmun Rev. 2002;1(5):279–83.
Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3(3):237–43.
Elkord E, Sasidharan Nair V. T-regulatory cells in health and disease. J Immunol Res. 2018;2018:5025238.
Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167(3):1137–40.
Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210.
Huff WX, Kwon JH, Henriquez M, Fetcko K, Dey M. The evolving role of CD8+CD28−immunosenescent T cells in cancer immunology. Int J Mol Sci. 2019;20(11):2810. https://doi.org/10.3390/ijms20112810.
Martínez-Escribano JA, Hernández-Caselles T, Campillo JA, Campos M, Frías JF, García-Alonso A, Alvarez-López MR. Changes in the number of CD80(+), CD86(+), and CD28(+) peripheral blood lymphocytes have prognostic value in melanoma patients. Hum Immunol. 2003;64(8):796–801.
Chen F, Yang M, Song Q, Wu J, Wang X, Zhou X, Yuan Y, Song Y, Jiang N, Zhao Y, et al. Enhanced antitumor effects and improved immune status of dendritic cell and cytokine-induced killer cell infusion in advanced cancer patients. Mol Clin Oncol. 2017;7(5):903–10.
Huang L, Qiao G, Morse MA, Wang X, Zhou X, Wu J, Hobeika A, Ren J, Lyerly HK. Predictive significance of T cell subset changes during ex vivo generation of adoptive cellular therapy products for the treatment of advanced non-small cell lung cancer. Oncol Lett. 2019;18(6):5717–24.
Yang D, Wang X, Zhou X, Zhao J, Yang H, Wang S, Morse MA, Wu J, Yuan Y, Li S, et al. Blood microbiota diversity determines response of advanced colorectal cancer to chemotherapy combined with adoptive T cell immunotherapy. Oncoimmunology. 2021;10(1):1976953.
Fenoglio D, Dentone C, Signori A, Di Biagio A, Parodi A, Kalli F, Nasi G, Curto M, Cenderello G, De Leo P, et al. CD8(+)CD28(-)CD127(lo)CD39(+) regulatory T-cell expansion: a new possible pathogenic mechanism for HIV infection? J Allergy Clin Immunol. 2018;141(6):2220-2233.e2224.
Quan XQ, Xu C, Wang RC, Zhang CT, Zhang Q, Zhou HL. The relationship between Chlamydia pneumoniae infection and CD4/CD8 ratio, lymphocyte subsets in middle-aged and elderly individuals. Microb Pathog. 2020;149: 104541.
Minning S, Xiaofan Y, Anqi X, Bingjie G, Dinglei S, Mingshun Z, Juan X, Xiaohui J, Huijuan W. Imbalance between CD8(+)CD28(+) and CD8(+)CD28(-) T-cell subsets and its clinical significance in patients with systemic lupus erythematosus. Lupus. 2019;28(10):1214–23.
de Martino M, Lodi L, Galli L, Chiappini E. Immune response to mycobacterium tuberculosis: a narrative review. Front Pediatr. 2019;7:350.
Zhai W, Fengjuan W, Zhang Y, Yurong F, Liu Z. The immune escape mechanisms of mycobacterium tuberculosis. Int J Mole Sci. 2019;20(2):340. https://doi.org/10.3390/ijms20020340.
Bussi C, Gutierrez MG. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol Rev. 2019;43(4):341–61.
Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14(12):963–75.
He X, Liang B, Gu N. Th17/Treg imbalance and atherosclerosis. Dis Markers. 2020;2020:8821029.
Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, Paulos CM. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–69.
Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mole Sci. 2018;19(3):730. https://doi.org/10.3390/ijms19030730.
Rackaityte E, Halkias J. Mechanisms of Fetal T cell tolerance and immune regulation. Front Immunol. 2020;11:588.
Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, Marboe C, Mancini D, Cortesini R, Suciu-Foca N. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16(8):1055–68.
Yang N, Li Z, Jiao Z, Gu P, Zhou Y, Lu L, Chou KY. A Trichosanthin-derived peptide suppresses type 1 immune responses by TLR2-dependent activation of CD8(+)CD28(-) Tregs. Clin Immunol. 2014;153(2):277–87.
Eusebio M, Kuna P, Kraszula L, Kupczyk M, Pietruczuk M. Allergy-related changes in levels of CD8+CD25+FoxP3(bright) Treg cells and FoxP3 mRNA expression in peripheral blood: the role of IL-10 or TGF-beta. J Biol Regul Homeost Agents. 2014;28(3):461–70.
Acknowledgements
We thank all the patients, their families and support staff who participated in this study.
Funding
This work was funded by the National Natural Science Foundation, China, Grant number (81900577); the Science and Technology Plan of Jiangsu, China, Grant number (BZ2019017); Youth Science and Technology Project of Suzhou city (KJXW2020048); and the Science and Technology Plan of Suzhou, China, grant number (SKY2021061, SS2019074, GSWS2019067, SZYJTD201716 and LCZX202116).
Author information
Authors and Affiliations
Contributions
XY, LY, JCX, MJW, HC, YYS, BBG, ZJY and LNH conceived the study. XY, JCX and PJZ acquired and analyzed the data. JCX wrote the first draft of the manuscript. PJZ and PX contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research related to human use complied with all the relevant national regulations and institutional policies and in accordance with the tenets of the Helsinki Declaration and was approved by the Ethics Committee of the Fifth People’s Hospital of Suzhou and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Analysis of the relationship between CD25, Foxp3 and CD127 on the CD4+ and CD8+ T cells.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, X., Lin, Y., Chen, H. et al. Distribution and clinical significance of circulating CD8+CD28− regulatory T cells in the peripheral blood of patients with pulmonary tuberculosis. BMC Pulm Med 22, 291 (2022). https://doi.org/10.1186/s12890-022-02088-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02088-7