Abstract
Background
Chronic respiratory failure (CRF) can be treated at home with non-invasive ventilation (NIV) and/or long-term oxygen (LTOT). The prevalence of these treatments is largely unknown. We aimed to clarify the prevalence and indications of the treatments, and the three-year mortality of the treated patients in the Helsinki University Hospital (HUH) area in Finland.
Methods
In this retrospective study we analyzed the prevalence of adult CRF patients treated with NIV and/or LTOT on 1.1.2018 and followed these patients until 1.1.2021. Data collected included the underlying diagnosis, patient characteristics, information on treatment initiation and from the last follow-up visit, and mortality during the three-year follow-up. Patients with home invasive mechanical ventilation or sleep apnea were excluded.
Results
On 1.1.2018, we had a total of 815 patients treated with NIV and/or LTOT in the Helsinki University Hospital (HUH) area, with a population of 1.4 million. The prevalence of NIV was 35.4 per 100,000, of LTOT 24.6 per 100,000 and of the treatments combined 60.0 per 100,000. Almost half, 44.5%, were treated with NIV, 41.0% with LTOT, and 14.4% underwent both. The most common diagnostic groups were chronic obstructive pulmonary disease (COPD) (33.3%) and obesity-hypoventilation syndrome (OHS) (26.6%). The three-year mortality in all patients was 45.2%. In the COPD and OHS groups the mortality was 61.3% and 21.2%. In NIV treated patients, the treatment durations varied from COPD patients 5.3 years to restrictive chest wall disease patients 11.4 years. The age-adjusted Charlson co-morbidity index (ACCI) median for all patients was 3.0.
Conclusions
NIV and LTOT are common treatments in CRF. The prevalence in HUH area was comparable to other western countries. As the ACCI index shows, the treated patients were fragile, with multiple co-morbidities, and their mortality was high. Treatment duration and survival vary greatly depending on the underlying diagnosis.
Similar content being viewed by others
Background
Respiratory failure can be classified to two main types, hypercapnic and hypoxemic respiratory failure. Home mechanical ventilation (HMV) is a well-established treatment for hypercapnic chronic respiratory failure (CRF). It can be delivered either as non-invasive ventilation (NIV) with a mask or mouthpiece, or invasively via a tracheostomy (home invasive mechanical ventilation = HIMV). Hypoxemic CRF patients are treated with long-term oxygen treatment (LTOT). If the patient has both hypercapnia and hypoxemia the treatments can be combined. Various underlying diseases can cause CRF. Common diseased leading to hypercapnic CRF are chronic obstructive pulmonary disease (COPD), neuromuscular diseases (NMD), restrictive chest wall diseases (RCWD), and obesity-hypoventilation syndrome (OHS). Hypoxemic CRF in turn is most commonly seen in interstitial lung diseases, diseases leading to cor pulmonale and COPD with emphysema.
NIV treatment has been shown to improve patients symptoms and health status, and also survival in OHS, amyotrophic lateral sclerosis (ALS) and RCWD [1]. In COPD, the benefits have been debatable. However, NIV has recently been shown to improve survival and reduce readmissions and exacerbations [2,3,4]. There are international guidelines for initiation of NIV for COPD [5, 6] and OHS [7,8,9], but these guidelines do not cover all the diseases for which NIV is commonly used. In real life, the patients using NIV are a heterogeneous group with different underlying causes for hypercapnic CRF, different comorbidities and highly variable life expectancies. LTOT is recommended in international and Finnish guidelines for patients with chronic, severe resting hypoxemia [10,11,12]. In palliative care LTOT is commonly used for hypoxemic patients whose dyspnea is relieved with oxygen treatment.
The prevalence of non-invasive ventilation has been growing strongly over the last few decades [1, 13]. Home ventilators have taken great technical leaps and their costs have decreased [14]. A growing number of studies reports benefits on telemonitoring NIV home titration and follow-up [15]. Patient groups treated with NIV have also changed. Previously, RCWD patients were the predominant group, but now COPD and OHS patients are often the largest patient groups [16]. In Finland, as in many other countries, the current prevalence of NIV and LTOT is largely unknown. In 1.1.2019 the prevalence of HIMV in Finland was 2.0 per 100,000 for the whole country. In the Helsinki University Hospital area the prevalence was 1.5 per 100,000 [17]. The HIMV patients comprise only a tiny minority of all HMV patients. The aim of this study was to clarify the prevalence of NIV and LTOT in the Helsinki University Hospital area, consisting of a population of 1.4 million [18]. We also wanted to analyze the characteristics of NIV and LTOT patients and their three-year mortality.
Methods
Study design and statistical analysis
This study was a register-based retrospective, cross-sectional study. We included adult patients undergoing NIV and/or LTOT treatment on 1.1.2018 in the study. Under 16 years old patients treated with NIV and/or LTOT, and all patients with HIMV were excluded. Also, patients with only sleep apnea (i.e., without concomitant CRF), treated with NIV or adaptive servo ventilator (ASV), were excluded. On January 1st 2018 we had 22 patients treated with HIMV, roughly 170 sleep apnea only patients treated with NIV and 130 treated with ASV.
Information collected from the patient records included prevalence of NIV and/or LTOT on 1.1.2018, basic patient characteristics and clinical data, i.e. age, diagnosis, treatment modality and duration, and data on treatment initiation and on the last follow-up visit from 1.1.2018 to 1.1.2020. The three-year mortality, from 1.1.2018 to 1.1.2021, and age-adjusted Charlson co-morbidity index (ACCI) [19, 20], on 1.1.2018, were calculated.
We divided the patients into eight diagnostic groups: COPD, NMD, RCWD, OHS, interstitial lung disease (ILD), heart disease, cancer, and miscellaneous. In the NMD group, 38.1% of the patients had ALS. The miscellaneous group was very heterogenous including patients with e.g. bronchomalacia, pulmonary hypertension and bronchiectasis. Due to the small number of patients, the heart and cancer patients are included in the miscellaneous group in the tables. The patients who underwent LTOT with their NIV were included in the NIV group, if not otherwise clearly stated. Consequently, the LTOT group consists of patients who underwent LTOT only. Spirometry and BMI were maximum ± one year from the diagnosis. In the ACCI a higher score indicates a higher mortality risk.
Patient data were collected from all the seven pulmonary departments of the HUH area hospitals. On 31.12.2017 the adult population of the HUH area was 1.4 million people. According to Finnish legislation, patients’ consent is not required for register studies in Finland [21]. Thus, only the ethics committee approval was required. The Medical Ethics Committee of the Hospital District of Helsinki University approved the study protocol (study number HUS/88/2018). Statistical analysis was conducted using SPSS (IBM SPSS Statistics, version 25). Results are shown mainly as N (%) or mean values ± SD. We considered P values < 0.05 statistically significant. Diagnostic groups’ comparison was done by t-test, Mann–Whitney test, and chi-square test.
Results
Prevalence
On January 1st 2018 there were in total 815 CRF patients being treated with NIV or LTOT or both. The prevalence of NIV and/or LTOT was 60.0 per 100,000 inhabitants. For NIV treatment, the prevalence was 35.4 per 100,000. This includes patients who underwent LTOT with their NIV. The prevalence for LTOT only was 24.6 per 100,000. The prevalence was greatest in the COPD group and lowest in the RCWD group (Table 1).
Patient characteristics
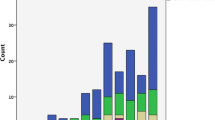
The patient characteristics are presented in Tables 2 and 3. The largest diagnostic groups were COPD and OHS (Fig. 1). The specific characteristics of COPD patients are shown in Table 4. According to the GOLD severity classification, 38.7% of our patients had severe and 33.6% very severe COPD. The majority of OHS patients had concomitant sleep apnea (81.3%) and their average apnea-hypopnea index (AHI) was 64.6 ± 33.2. The patients were on average 68.2 ± 14.4 years old, with a slight male predominance (53.0%), and with a history of 33.2 ± 18.7 pack years. The mean predicted FEV1 was 51.4 ± 21.2% and FVC 65.6 ± 21.2%. Average BMI was 35.9 ± 13.3, ranging from 10.0 to 89.9 kg/m2. The median ACCI was 3.0. Half of the treatment initiations were performed electively, 28.3% at a pulmonary ward and 21.0% at a pulmonary outpatient clinic, and the rest during an acute exacerbation. NIV and LTOT treatment amounts in diagnostic groups are shown in Fig. 2.
Diagnostic groups of all 815 NIV and LTOT patients on January 1st 2018. NIV, non-invasive ventilation; LTOT, long-term oxygen treatment; COPD, chronic obstructive pulmonary disease; NMD, neuromuscular disease; RCWD, restrictive chest wall disease; OHS, obesity-hypoventilation syndrome; ILD, interstitial lung disease; Misc, miscellaneous
Amount of NIV and LTOT treatments in diagnostic groups on 1.1.2018. NIV, non-invasive ventilation; LTOT, long-term oxygen treatment; COPD, chronic obstructive pulmonary disease; NMD, neuromuscular disease; RCWD, restrictive chest wall disease; OHS, obesity-hypoventilation syndrome; ILD, interstitial lung disease; Misc, miscellaneous
Mortality
During the three-year follow-up (1.1.2018–1.1.2021), 45.2% of the patients died (Fig. 3). The mortality was highest in the ILD (68.1%) and COPD (61.3%) groups. In COPD patients, the mortality was greater in the non-obese patients (BMI ≤ 30 kg/m2) compared to the obese patients (BMI ≥ 30 kg/m2), 60.4% and 50.8%, but it did not reach statistical significance (p = 0.105). However, the BMI was missing for 45.8% of the COPD patients. In cancer and heart disease groups, the mortality was 82.9% and 64.4%. However, in these groups the treatment was largely palliative as 62.2–74.3% had a do not resuscitate (DNR) decision. If these two groups were excluded, then the overall three-year mortality was 42.0%.
Comparing treatment modalities, mortality in LTOT patients was 69.3% and in NIV patients 28.3%. If the deceased heart disease and cancer patients were excluded from the LTOT group, then the mortality in LTOT patients was 53.3%. When NIV patients were divided into NIV only and NIV with LTOT groups, their mortalities were 22.9% and 44.9%. Over half of the patients died in a hospital (61.7%), with a minority dying at home (23.0%), in a palliative care center (10.0%), or in a sheltered home (5.3%).
Treatment durations and settings, blood gas values
The age of the patients at treatment initiation and treatment durations are shown in Table 3. On 1.1.2018 the average NIV duration for the living patients was 7.8 ± 6.2, and for the deceased 5.1 ± 3.9 years before death. For NIV treated COPD patients, the corresponding figures were for the living 5.8 ± 3.0, and for the deceased 4.4 ± 3.6 years. For LTOT patients, the durations were 7.1 ± 5.4 and 3.7 ± 2.9 years.
The baseline arterial and transcutaneous blood gas values are shown for all patients, COPD and OHS groups in Table 5. For LTOT only patients the initial partial pressure of arterial blood oxygen (PaO2) was 7.2 ± 1.1 kPa and PaCO2 was 5.3 ± 1.1 kPa. At the follow-up visit the NIV treated patients’ PaCO2 was 5.8 ± 0.9 kPa, but the information was missing from 65% of the patients.
The mean ± SD daily NIV use was 7.5 ± 4.3 h/night. The majority (72.9%) of the patients used NIV ≥ 90% of the nights; the median value for used nights was 99.0%. In surviving COPD patients, the mean ± SD daily NIV use was 7.8 ± 3.7 h/night and 78.0% used NIV ≥ 90% of the nights. For the deceased, the corresponding values were 5.7 ± 4.4 h/night, 64.3% used NIV ≥ 90% of the nights. However, information on NIV use was missing in 22% (hours/night) and 25% (nights used) of the patients. The mean (± SD) LTOT oxygen flow was 2.4 ± 1.3 l/min. Information on LTOT daily use was not available.
The majority (73.4%) of patients had NIV in pressure support mode (Fig. 4). The mean (± SD) initiation pressures (cmH2O) were IPAP 13.7 ± 3.1, and EPAP 6.7 ± 2.4, and at the last control IPAP 15.2 ± 3.6 and EPAP 7.5 ± 2.6, respectively.
NIV-modes at treatment initiation. COPD, chronic obstructive pulmonary disease; NMD, neuromuscular disease; RCWD, restrictive chest wall disease; OHS, obesity-hypoventilation syndrome; ILD, interstitial lung disease; Misc, miscellaneous; NIV, non-invasive ventilation. *Misc group includes heart disease and cancer patients
Discussion
In our retrospective study, the prevalence of NIV was 37.0 per 100,000 inhabitants and of LTOT 24.6 per 100,000 in the Helsinki University Hospital area on 1.1.2018. Altogether, the prevalence was 60.0 per 100,000 and we had 815 patients in a population of 1.4 million. The three-year mortality was high at 45.2%, but depended largely on the underlying disease.
Over the last few decades, NIV prevalence has grown rapidly and the diagnostic groups treated with NIV have changed. Previously, chronic hypercapnic respiratory failure due to mainly restrictive diseases, e.g. chest wall disorders, post-polio syndrome, sequelae of tuberculosis, and neuromuscular disorders, was treated with NIV [22]. In our study, COPD and OHS were the largest diagnostic groups treated with NIV, and restrictive diseases and NMD were minorities, 6.5% and 12.3%, respectively. This is in line with the Lake Geneva area study and with the Swedish national register for patients on Long Term Oxygen Therapy and Home Mechanical Ventilation (Swedevox) [16, 23].
The prevalence for NIV and LTOT treatments in HUH are comparable to other recent studies. The NIV prevalence in Geneva, Switzerland was 37.9 per 100,000 inhabitants (2017) [16], in Norway 47 per 100,000 (2019, Norwegian National registry for long-term ventilation) and in Sweden 33 per 100,000 (2018) [23]. In Sweden the prevalence of LTOT was 22.8 per 100,000 (2018) and in Denmark 48.1 per 100,000 (2010) [23, 24].
In many countries the prevalence for LTOT is greater than the NIV prevalence. However, studies on LTOT prevalence are more scarce and many of them are older than the NIV studies. In Sweden, the Swedevox register follows the prevalences of NIV and LTOT yearly. The Swedish LTOT prevalence was at its peak at 2014 (27 per 100,000) and has slightly decreased during the recent years (22.8 per 100,000, 2018). During the same time period, the NIV prevalence has in turn risen from 29 to 33 per 100,000 [23]. We presume the trend is similar in Finland. The prevalences of both treatments vary also largely in Sweden and in different HUH hospitals, probably also nationally in Finland. The guidelines for LTOT are similar in Finland compared to international guidelines [10,11,12] and they are conscientious followed. In Finland, continuous smoking is a contraindication to LTOT, but NIV can be initiated despite smoking. If the patient has hypoxemia and hypercapnia, the patient is primarily given NIV alone and if the hypoxemia is not improved then LTOT is added to NIV. LTOT is not given alone to patients with hypercapnia. In most of the Finnish hospitals only pulmonologist can prescribe LTOT. These regulations and practices might explain our lower amount of LTOT treatments, especially in the COPD patient group.
The overall mortality was high in our population, 45.2% in three years. The highest mortality was in the cancer, ILD, and heart disease groups. This is expected, as the diseases generally have low prognosis. In our study these patients had high ACCI scores, and the majority had made a DNR decision. As the number of cancer and heart disease patients was small in our study, these numbers should be considered only indicative. Our NIV patients’ mortality, 28.3%, was in line with a recent, large European study, where the three-year mortality was 31.3.% [25]. Their overall survival was better (6.6 vs. our 5.1 years), but their follow-up time was longer, and the study included 10.5% sleep apnea patients.
The survival benefit of NIV in COPD patients with hypercapnic chronic respiratory failure is not yet established, as previous randomized studies have been inconsistent. However, recent studies have shown positive impact on COPD patients’ long-term survival with NIV. In studies, NIV-treated COPD patients’ one year mortality was 12–28% and in control group 33–46% [2, 26], and three and four year mortalities, 43.9–45.5%[27,28,29].
Treatment durations and survival of our COPD patients were in line with other studies even though our patients were older (on average 74.5 vs 62.2–70.6 years) [2, 26,27,28,29], and had many co-morbidities. Our patients’ hypercapnia was similar to many studies [2, 27, 28]; in only one study was the hypercapnia markedly lower (48.5 mmHg = 6.5 kPa) [29]. Many of these studies were done with high-intensity NIV. Compared to these studies, our NIV pressures were remarkably lower, average follow-up IPAP 14.4 ± 2.7 cmH20 and EPAP 7.1 ± 2.3 cmH20. Despite the lower pressures, the PaCO2 decreased to on average 5.8 kPa. Due to the missing information, retrospective nature of the study, and the limited number of patients, these findings should be interpreted with caution.
There were some differences between our hypercapnic COPD patients treated with NIV and hypoxemic COPD patients treated with LTOT. The NIV-treated patients’ mortality (55.4 vs 69.8%) and ACCI (3.8 ± 2.4 vs 5.1 ± 2.9) were lower than LTOT patients. The NIV patients were younger (72.3 vs 76.1 years), more obese (BMI 30.8 vs 28.1 kg/m2), and unsurprisingly had a greater hypercapnia (7.9 vs 5.4 kPa). Interestingly, surviving COPD patients with NIV had better adherence to the treatment than those who deceased. It can be speculated whether the higher mortality was caused by lower NIV adherence or whether these patients already had more severe morbidity during the initiation, which led to lower adherence and higher mortality.
As with NIV, the effect of LTOT on mortality is also still under debate. In the 1980s two studies showed that LTOT reduced mortality in COPD patients with severe resting hypoxemia [30, 31]. These results led to several guidelines. In present guidelines, e.g. GOLD and the ATS guideline 2020 [11, 32], supplementary oxygen is recommended for COPD patients with severe resting hypoxemia and in the ATS guideline also for severe exertional hypoxemia. The guidelines do not suggest LTOT in mild to moderate chronic resting hypoxemia.
In a large international study in 2016, LTOT treated hypoxemic COPD patients had a three-year mortality of 19% [33]. This is a clearly better survival rate than in our study. These patients were younger (68.4 vs. 76.1 years), but with a lower FEV1 (34.4 vs 43.2% of predicted). In Sweden the one-year mortality was more similar to ours, about 40% [23]. However, the median LTOT durations were shorter in the Swedish and Danish studies, 1.4 and 1.5 years compared to our 4.9 years [23, 24].
The second largest patient group in our study was OHS. Our patients were slightly more hypercapnic (PaCO2 8.0 vs. 5.7–7.9 kPa), but otherwise the patients' characteristics were similar compared to other studies [16, 34, 35]. As expected, the mortality of our OHS patients was the lowest of the diagnostic groups (21.2%) and comparable to earlier studies, in which the five-year mortality ranged from 11% to 22.7% [8, 34,35,36,37].
Since 2018 the guidelines for OHS have changed. Now continuous positive airway pressure (CPAP) is considered the first-line treatment for ambulatory patients with OHS and concomitant severe sleep apnea [7,8,9]. This will diminish the amount of NIV treatments in OHS patients in coming years. In our study, over 80% of the OHS patients had concomitant sleep apnea. But as patients treated with CPAP were excluded, we were not able to compare these two treatment modes.
Treatment initiation and follow-up protocols varied greatly in our area’s clinics. Half of the initiations were performed electively (49.3%). Earlier studies have shown NIV home initiations to be non-inferior to in-hospital initiations [38]. The main reason for these variances between clinics is the different resources at the wards and outpatient clinics. We did not specifically evaluate the effect of initiation unit on the treatment results or on mortality, but no large differences were seen. Lately, due to the COVID-19 pandemic and developments in remote monitoring systems, both the initiations and follow-ups have shifted more to the outpatient clinics in our hospitals. In our opinion, the most significant factors affecting the success of the NIV treatments are the clinical skills of the medical staff and efficient treatment initiation and follow-up protocol, not the type of initiation unit itself.
Our study’s strength is the population size, 815 patients in a population of 1.4 million. This is one of the largest prevalence studies recently performed. Also, the prevalence is very reliable. In Finland all NIV and LTOT treatments are coordinated from special health care, therefore, the risk of missing patients is low. All equipment is loaned to patients free of charge. Thus, patient’s financial situation rarely affects the treatment decisions. As the study included an unselected population of patients, our study provides real-life practical information to physicians treating chronic respiratory failure.
The largest limitation in our study is the observational cross-sectional study design, which prevents analyses on treatment efficacy or on the initiation or follow-up protocols. We acknowledge that the treatment initiation criteria might have differed slightly due to patient heterogeneity and clinical decisions of the treating physicians. Due to real-life study design some values were missing from patient records. Our observations might not be directly generalizable to all other countries, where the treatment financing and patient demographics differ.
Conclusions
Chronic respiratory failure is commonly treated with home NIV and/or LTOT and its prevalence has been rising rapidly. In our large, retrospective study the prevalence in Helsinki University Hospital area of NIV was 35.4 per 100,000, of LTOT 24.6 and of the treatments combined 60.0 per 100,000. The treated patients had multiple co-morbidities and their three-year mortality was high, 45.2%. It might be speculated that in the future the number of NIV treated hypercapnic CRF patients will continue to rise due to developments in NIV treatments and prolonged survival of these patients. On the other hand, the OHS patients’ altered treatment protocol of CPAP as first-line treatment might diminish the number of their NIV treatments.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author, P. Kotanen. The data are not publicly available due to the data containing information that could compromise the privacy of research participants.
Abbreviations
- ABG:
-
Arterial blood gas test
- ACCI:
-
Age-adjusted Charlson co-morbidity index
- ALS:
-
Amyotrophic lateral sclerosis
- AHI:
-
Apnea–hypopnea index
- ASV:
-
Adaptive servo ventilator
- BMI:
-
Body mass index
- CRF:
-
Chronic respiratory failure
- COPD:
-
Chronic obstructive pulmonary disease
- CPAP:
-
Continuous positive airway pressure
- DNR:
-
Do not resuscitate
- FEV1 :
-
Forced expiratory volume
- FVC:
-
Forced vital capacity
- HUH:
-
Helsinki University Hospital
- HIMV:
-
Home invasive mechanical ventilation
- HMV:
-
Home mechanical ventilation
- ILD:
-
Interstitial lung disease
- LTOT:
-
Long-term oxygen treatment
- NIV:
-
Non-invasive ventilation
- NMD:
-
Neuromuscular disease
- PaCO2:
-
Partial pressure of arterial blood carbon dioxide
- Pa02:
-
Partial pressure of arterial blood oxygen
- PtcCO2:
-
Transcutaneous partial pressure of carbon dioxide
- OHS:
-
Obesity-hypoventilation syndrome
- RCWD:
-
Restrictive chest wall diseases
References
Hannan LM, Dominelli GS, Chen Y, Reid WD, Road J. Systematic review of non-invasive positive pressure ventilation for chronic respiratory failure. Respir Med. 2014;108:229–43.
Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705.
Murphy PB, Rehal S, Arbane G, Bourke S, Calverley PMA, Crook AM, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177–86.
Altintas N. Non-invasive positive pressure ventilation in chronic respiratory failure due to COPD. COPD J Chronic Obstr Pulm Dis. 2016;13(1):110–21.
Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E, et al. European Respiratory Society Guideline on Long- term Home Non-Invasive Ventilation for Management of Chronic Obstructive Pulmonary Disease. Eur Respir J. 2019;54:1901003.
Owens RL, Drummond MB, MacRea M, Branson RD, Celli B, Coleman JM, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease: an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(4):E74-87.
Mokhlesi B, Masa JF, Afshar M, Balachandran JS, Brozek JL, Dweik RA, et al. Evaluation and management of obesity hypoventilation syndrome an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2019;200(3):E6-24.
Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga MÁ. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28(151):1–14. https://doi.org/10.1183/16000617.0097-2018.
NICE. Obstructive sleep apnoea/ hypopnoea syndrome and obesity hypoventilation syndrome in over 16s NICE guideline [Internet]. NICE guideline. 2021 [cited 2022 Jan 21]. www.nice.org.uk/guidance/ng202
Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax. 2015;70:i1–43.
Jacobs SS, Krishnan JA, Lederer DJ, Ghazipura M, Hossain T, Tan AYM, et al. Home oxygen therapy for adults with chronic lung disease, an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):E121–41.
Harju T, Kankaanranta H, Katajisto M, Kilpeläinen M, Lehtimäki L, Lehto J, et al. Keuhkoahtaumatauti [Internet]. Käypä hoito. 2020 [cited 2021 Dec 11]. https://www.kaypahoito.fi/hoi06040
Wijkstra P, Duiverman M. Home mechanical ventilation: a fast-growing treatment option in chronic respiratory failure. Chest. 2020;158(1):26–7. https://doi.org/10.1016/j.chest.2020.03.020.
Piper AJ. Advances in non-invasive positive airway pressure technology. Respirology. 2020;25:372–82.
Jiang W, Wang L, Song Y. Titration and follow-up for home noninvasive positive pressure ventilation in chronic obstructive pulmonary disease: the potential role of telemonitoring and the Internet of things. Clin Respir J. 2021;15:705–15.
Cantero C, Adler D, Pasquina P, Uldry C, Egger B, Prella M, et al. Long-term noninvasive ventilation in the Geneva Lake Area: indications, prevalence, and modalities. Chest. 2020;158(1):279–91. https://doi.org/10.1016/j.chest.2020.02.064.
Kotanen P, Kreivi H-R, Vainionpää A, Laaksovirta H, Brander P, Siirala W. Home invasive mechanical ventilation in Finland in 2015–2019. ERJ Open Res. 2020;6(4):00223–2020. https://doi.org/10.1183/23120541.00223-2020.
The national statistical institution in Finland. Statistics Finland [Internet]. [cited 2022 May 27]. https://www.stat.fi/
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
Finnish Ministry of Justice. Decree of data protection act 5.12.2018/1050 [Internet]. [cited 2022 Feb 24]. https://www.finlex.fi
Leger P, Bedicam JM, Cornette A, Reybet-Degat O, Langevin B, Polu JM, et al. Nasal intermittent positive pressure ventilation: Long-term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105(1):100–5. https://doi.org/10.1378/chest.105.1.100.
Ekström M, Palm A, Theorell-Haglöw J, Midgren B. Swedevox [Internet]. Date last accessed: 17.11.2021. [cited 2022 Apr 21]. www.ucr.uu.se/swedevox
Ringbaek TJ, Lange P. Trends in long-term oxygen therapy for COPD in Denmark from 2001 to 2010. Respir Med. 2014;108(3):511–6. https://doi.org/10.1016/j.rmed.2013.10.025.
Patout M, Lhuillier E, Kaltsakas G, Benattia A, Dupuis J, Arbane G, et al. Long-term survival following initiation of home non-invasive ventilation: a European study. Thorax. 2020;75(11):965–73.
Frazier WD, Murphy R, van Eijndhoven E. Non-invasive ventilation at home improves survival and decreases healthcare utilization in medicare beneficiaries with Chronic Obstructive Pulmonary Disease with chronic respiratory failure. Respir Med. 2021;177: 106291. https://doi.org/10.1016/j.rmed.2020.106291.
Rantala HA, Leivo-Korpela S, Kettunen S, Lehto JT, Lehtimäki L. Survival and end-of-life aspects among subjects on long-term noninvasive ventilation. Eur Clin Respir J. 2020;8(1):1840494. https://doi.org/10.1080/20018525.2020.1840494.
Blankenburg T, Benthin C, Pohl S, Bramer A, Kalbitz F, Lautenschläger C, et al. Survival of hypercapnic patients with COPD and obesity hypoventilation syndrome treated with high intensity non invasive ventilation in the daily routine care. Open Respir Med J. 2017;11(1):31–40.
Borel JC, Pepin JL, Pison C, Vesin A, Gonzalez-Bermejo J, Court-Fortune I, et al. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology. 2014;19(6):857–65.
Noctural Oxygen Therapy Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. Ann Intern Med. 1980;93:391–8.
Stuart-Harris C, Bishop JM, Clark TJH. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–6.
Global Initiative for Chronic Obstructive Lung Disease. Date last accessed: 17.11.2021. www.goldcopd.org
Carone M, Antoniu S, Baiardi P, Digilio VS, Jones P, Bertolotti G. Predictors of mortality in patients with COPD and chronic respiratory failure: The Quality-of-Life Evaluation and Survival Study (QuESS): a three-year study. COPD J Chronic Obstr Pulm Dis. 2016;13(2):130–8. https://doi.org/10.3109/15412555.2015.1067294.
Castro-Ãnón O, De Llano LAP, De La Fuente SS, Golpe R, Marote LM, Castro-Castro J, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS ONE. 2015;10(2):1–12.
Borel JC, Burel B, Tamisier R, Dias-Domingos S, Baguet JP, Levy P, et al. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLoS ONE. 2013;8(1):1–8.
Kreivi H-R, Itäluoma T, Bachour A. Effect of ventilation therapy on mortality rate among obesity hypoventilation syndrome and obstructive sleep apnoea patients. ERJ Open Res. 2020;6(2):00101–2019. https://doi.org/10.1183/23120541.00101-2019.
Priou P, Hamel JF, Person C, Meslier N, Racineux JL, Urban T, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest. 2010;138(1):84–90.
Duiverman ML, Vonk JM, Bladder G, Van Melle JP, Nieuwenhuis J, Hazenberg A, et al. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: A randomised controlled trial. Thorax. 2020;75(3):244–52.
Acknowledgements
Biostatistician Olavi Koivisto from Helsinki University, Finland, was consulted in one analysis considering the calculations on NIV usage.
Funding
The study was funded by the following non-profit organizations in Finland: the Research Foundation of Pulmonary Diseases in Finland, Varsinais-Suomen Sairaanhoitopiirin Hengitystukiyksikkö, the Väinö and Laina Kivi Foundation, Finnish Anti-Tuberculosis Association, Tampere Tuberculosis Foundation, and the Governmental subsidy for health sciences research.
Author information
Authors and Affiliations
Contributions
Study design: PK, PB, H-RK. Data Collection and analysis: PK. Written report: PK, PB, H-RK. PK takes responsibility of the whole work. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
According to Finnish legislation, patients’ consent is not required for register studies in Finland. The Medical Ethics Committee of the Hospital District of Helsinki University approved the study protocol (study number HUS/88/2018). All study methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kotanen, P., Brander, P. & Kreivi, HR. The prevalence of non-invasive ventilation and long-term oxygen treatment in Helsinki University Hospital area, Finland. BMC Pulm Med 22, 248 (2022). https://doi.org/10.1186/s12890-022-02044-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02044-5