Abstract
Background
Less is known about the risk factors for acute respiratory distress syndrome (ARDS) in sepsis patients diagnosed according to sepsis 3.0 criteria. Moreover, the risk factors for ARDS severity remain unclear.
Methods
We retrospectively collected the characteristics of sepsis patients from the intensive care unit of the First Affiliated Hospital of China Medical University from January 2017 to September 2018. Logistic regression was used in determining the risk factors.
Results
529 patients with sepsis were enrolled and 179 developed ARDS. The most common infection sites were acute abdominal infection (n = 304) and pneumonia (n = 117). Multivariate analysis showed that patients with pancreatitis with local infection (odds ratio [OR], 3.601; 95% confidence interval [CI], 1.429–9.073, P = 0.007), pneumonia (OR 3.486; 95% CI 1.890–6.430, P < 0.001), septic shock (OR 2.163; 95% CI 1.429–3.275, P < 0.001), a higher sequential organ failure assessment (SOFA) score (OR 1.241; 95% CI 1.155–1.333, P < 0.001) and non-pulmonary SOFA score (OR 2.849; 95% CI 2.113–3.841, P < 0.001) were independent risk factors for ARDS. Moreover, pneumonia is associated with increased severity of ARDS (OR 2.512; 95% CI 1.039–6.067, P = 0.041).
Conclusions
We determined five risk factors for ARDS in sepsis patients. Moreover, pneumonia is significantly associated with an increased severity of ARDS.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) remains a major clinical syndrome in the intensive care unit (ICU). It had been reported that 10.4% of total ICU-admitted patients developed ARDS. Moreover, nearly 40% of ARDS patients were underdiagnosed [1]. Sepsis, defined as life-threatening organ dysfunction caused by a dysregulated host response to either pneumonia or non-pulmonary infection, is a common risk factor for ARDS. Patients with sepsis-induced ARDS were reported to have a poorer prognosis than those without ARDS [2, 3] due to the lack of effective treatment strategies [4,5,6,7,8]. In accordance with the rapid deterioration of such clinical conditions, clinicians have shifted the management paradigm to prevent the development of ARDS [9]. Potential risk factors for ARDS have been reported in previous studies [10,11,12,13,14], but there is still a lack of large-sample studies on risk factors for ARDS in ICU sepsis patients diagnosed by updated sepsis 3.0 criteria [15]. Moreover, to our knowledge, the risk factors related to the severity of ARDS in ICU sepsis patients diagnosed according to sepsis 3.0 criteria have not been reported to date.
In this study, we evaluated risk factors for ARDS and those associated with ARDS severity in sepsis patients in ICU diagnosed according to sepsis 3.0 criteria.
Methods
Study population
We retrospectively collected the clinical records of adult (≥ 18 years old) patients with sepsis who were admitted to the ICU of the First Affiliated Hospital of China Medical University from January 1st, 2017 to September 30th, 2018. Sepsis was diagnosed in accordance with the Third International Consensus Definitions for Sepsis and Septic Shock [15]. Exclusion criteria were as follows: (1) patients who underwent cardiopulmonary resuscitation (CPR); (2) patients with advanced solid or hematological tumors; (3) patients currently diagnosed with cirrhosis; (4) patients who underwent organ transplantation; and (5) patients with regular administration of hormones or immunosuppressors.
Data collection and outcomes
Clinical records were collected regarding demographic characteristics, including age, sex, underlying disease, smoking and drinking history, and site of infection of each patient. The baseline acute physiology and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA) score, SOFA score excluding respiratory function (non-pulmonary SOFA score), and presence of septic shock before the development of ARDS were used to assess the severity of disease. We also included baseline laboratory data, such as PH, lactate (Lac), serum creatinine (Scr)and platelet value (PLT). The mechanical ventilation hours, length of ICU stay, length of hospital stay, and ICU mortality were recorded in detail. The 28-day mortality and 90-day mortality were assessed during follow-up. Sepsis patients were divided into 2 groups based on whether they developed ARDS. ARDS was diagnosed according to Berlin definition [16]. In terms of patients with ARDS, we further classified them into 3 groups based on the severity according to Berlin definition which is based on the oxygenation index (mild: 200 mmHg < PaO2/FiO2 ≤ 300 mmHg, moderate: 100 mmHg < PaO2/FiO2 ≤ 200 mmHg, severe: PaO2/FiO2 ≤ 100 mmHg) [16].
Statistical analysis
All categorical variables were presented as numbers with percentages, and all continuous variables were presented as medians with interquartile ranges (IQRs). In analyzing sepsis patients, we used the Mann–Whitney U test to compare continuous variables and the chi-square or Fisher’s exact test to compare categorical variables. Logistic regression analysis was performed using covariates that were significant (P < 0.05) on univariate analysis to identify risk factors for ARDS; a forward stepwise multivariate logistic regression model was used to screen independent predictors for ARDS. In analyzing patients who developed ARDS, we used the Kruskal–Wallis H test to compare continuous variables and the chi-square test for categorical variables. Ordered polytomous logistic regression was used to confirm the independent risk factors for the severity of ARDS. We used IBM SPSS for Windows version 24.0 for all statistical analyses.
Results
Baseline characteristics
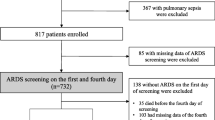
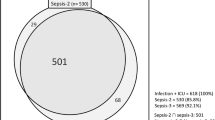
A total of 555 patients who met the inclusion criteria were screened, and 529 patients were enrolled (Fig. 1). The most frequent source of sepsis sites were the acute abdominal infection (n = 304), followed by the pneumonia (n = 117) and hepatobiliary system infection (n = 39). The distribution of infection sites is demonstrated in Fig. 2. The incidence of septic shock was 46%. A total of 179 (34%) patients developed ARDS during ICU stay. The median age of ARDS patients was 66 (IQR 53–76), 134 (75%) were males. 6% had a history of drinking and 18% had a history of smoking. Additionally, 350 (66%) of the septic patients did not develop ARDS.
According to a comparison of the patients without ARDS to sepsis-induced ARDS patients, there were significant differences in the variables including age, sex, comorbid cancer, infection site (pneumonia, acute abdominal infection, pancreatitis with local infection, skin and soft tissue infection), emergency admission, septic shock, SOFA score, non-pulmonary SOFA score and APACHE II score (P < 0.05) (Table 1).
Comparison of prognosis between ARDS and non-ARDS patients
The median length of mechanical ventilation was 114 (IQR 46–250) hours versus 34 (IQR 13–113) hours in ARDS patients and non-ARDS patients (P < 0.001), respectively. The median length of ICU stay was 7 (IQR 4–15) days versus 4 (IQR 2–8.25) days (P < 0.001). Moreover, both the ICU mortality rate (23% versus 10%) and 28-day mortality rate (47% versus 24%) were significantly higher in the ARDS group than in the non-ARDS group (P < 0.001). The analysis results of prognostic factors are listed in Table 2.
Risk factors for ARDS
A total of thirteen variables (Table 1) differed significantly between ARDS patients and non-ARDS patients. We took into account the clinical significance of the variables and finally screened out nine (sex, age, emergency admission, pneumonia, pancreatitis with local infection, septic shock, SOFA score, non-pulmonary SOFA score and APACHE II score) by univariate analysis (Table 3). Five variables remained significant after multivariate analysis (Table 4). Among them, pneumonia [odds ratio (OR), 3.486; 95% confidence interval (CI), 1.890—6.430, P < 0.001], pancreatitis with local infection (OR 3.601; 95% CI 1.429—9.073, P = 0.007), septic shock (OR 2.163; 95% CI 1.429—3.275, P < 0.001), SOFA score (OR 1.241; 95% CI 1.155—1.333, P < 0.001) and non-pulmonary SOFA score (OR 2.849; 95% CI 2.113—3.841, P < 0.001) were independent risk factors for ARDS in sepsis patients.
Risk factors for the severity of ARDS
Among the 179 ARDS patients, 39 (22%) were diagnosed with mild ARDS, 109 (61%) were diagnosed with moderate ARDS and 31 (17%) were diagnosed with severe ARDS. Variables that showed significant differences between ARDS and non-ARDS patients were included in the comparison among the three groups with different severities of ARDS. As a result, patients with pneumonia (mild ARDS vs moderate ARDS vs severe ARDS, 15% vs 24% vs 55%, P < 0.001) and higher SOFA score (7.92 vs 9.35 vs 9.58, P = 0.014) were more likely to develop severe ARDS. On the contrary, acute abdominal infection was the main cause of mild and moderate ARDS compared to severe ARDS (62% vs 51% vs 26%, P = 0.010). Prognostic factors showed significant differences in 28-day mortality rate (mild ARDS vs moderate ARDS vs severe ARDS, 25.6% vs 42.2% vs 41.9%, P = 0.023) and 90-day mortality rate (30.8% vs 43.1% vs 61.3%, P = 0.038) among the three groups (Table 5). Ordinal multivariate logistic regression revealed that patients with pneumonia (OR 2.512; 95% CI 1.039–6.067; P = 0.041) had significant correlation with increased severity of ARDS (Fig. 3).
Forest plot for risk factors of the severity of ARDS. Pneumonia remains the only significant risk factor for increased severity of ARDS in patients with sepsis (OR 2.512, 95% CI 1.039–6.067, p = 0.041). Other risk factors enrolled in the analysis include emergency admission (OR 0.685, 95% CI 0.311–1.513, p = 0.349), acute abdominal infection (OR 0.926, 95% CI 0.411–2.088, p = 0.852), SOFA (OR 1.093, 95% CI 0.985–1.212, p = 0.093). ARDS, acute respiratory distress syndrome; OR, odds ratio; CI, confidence interval; SOFA, sequential organ failure assessment
Prognosis of pulmonary ARDS
We further analyzed the prognostic profile of ARDS patients between pneumonia and non-pulmonary infection. Significant differences were found in length of mechanical ventilation (170 h vs 105.5 h, P = 0.013), length of ICU stay (11 days vs 7 days, P = 0.007), ICU mortality rate (39.6% vs 16.7%, P = 0.001), 28-day mortality rate (62.3% vs 33.3%, P < 0.001) and 90-day mortality rate (64.2% vs 34.9%, P < 0.001) (Table 6).
Discussion
In this study, we reported a rate of 34% of ARDS incidence in sepsis patients diagnosed according to sepsis 3.0 criteria. We also compared the clinical characteristics between patients with or without ARDS. We determined five risk factors for ARDS in sepsis patients. Furthermore, we figured out for the first time that pneumonia is significantly associated with an increased severity of ARDS. Our study adds additional evidence about sepsis patients who are at high risk of developing ARDS.
The 34% incidence of ARDS was the highest in the literature to date [10,11,12,13,14]. Possible reasons include: (1) the increased severity of sepsis in our cohort, as 46% patients developed septic shock, which was also a higher rate than that in previous studies [17]; (2) the improved clinical recognition of ARDS at our center. The 28-day mortality rate of ARDS patients from our cohort was 47%, which is similar to the 28-day mortality rate of sepsis-induced ARDS patients in a previous study (42%) [13], reflecting that our data is consistent and convincible.
Our study found a younger median age in patients with sepsis-induced ARDS (66 years vs. 70 years, P < 0.05). We noticed that the results of 3 studies, including ours, showed that sepsis patients who developed ARDS were younger than those who did not (P < 0.05), although age was not an independent factor for developing ARDS [10, 11]. ARDS is a clinical syndrome characterized by severe inflammatory responses accompanied by immune activation [18]. A set of functional and structural changes in the immune system, such as a decreased response to vaccination and inflammation response, are thought to be crucial components of aging [19]. This decreased response might be the underlying reason why the median age of ARDS patients is lower than that of non-ARDS patients. Further studies may focus on this interesting clinical phenomenon in critically ill patients.
Our study reported a significantly worse prognosis of patients with sepsis-induced ARDS compared with those without ARDS, regarding to the length of mechanical ventilation, length of ICU stay, and 28-day mortality, which is consistent with the data in the literature. Researchers reported that sepsis-induced ARDS leads to prolonged recovery from lung injury and delayed extubation [20, 21]. Moreover, the progression to ARDS is associated with an increased risk of in-hospital death in sepsis patients [22, 23]. Therefore, it is urgent to determine the risk factors of sepsis patients who developed ARDS.
We confirmed that septic shock is an independent risk factor for ARDS, which is consistent with previous studies [10, 11]. Septic shock is a severe form of sepsis in which the underlying circulatory, cellular, and metabolic abnormalities are enough to substantially increase the risk of death. Septic shock has long been recognized as a trigger factor for acute lung injury (ALI) and ARDS [14]. Iscimen et al. reported that 44% of septic shock patients developed ALI in their ICU [14], which was basically consistent with the rate in our ICU (48.6%). Patients with septic shock should undergo a more precise respiratory function assessment.
Moreover, we revealed a strong relationship between the infection site and the development of ARDS in sepsis patients, including pneumonia and pancreatitis with local infection. It has been reported that pneumonia is the most common source of infection in ARDS patients and also a risk factor for ARDS [24, 25]. In this case, ARDS may be caused by both direct lung injury and indirect systemic inflammatory response of sepsis. In patients with pancreatitis, ARDS is thought to be caused by severe systemic inflammatory response that leads to increased permeability of the endothelial and epithelial barriers, with leakage of protein-rich exudates into the alveolar space and interstitial tissues, thereby affecting oxygenation and gas exchange [26,27,28].
We also confirmed that SOFA and non-pulmonary SOFA scores were significantly associated with an increased risk of ARDS. Apart from SOFA, other assessment scores including lung injury prediction score (LIPS)[29], early acute lung injury (EALI) score[30], and APACHE II score have been reported to be associated with the development of ARDS. Studies of patients in the emergency department have shown that APACHE II score is an independent risk factor for ARDS in sepsis patients [10, 22], while SOFA score plays an independent role in bacteremia patients in ICU [12] which is inconsistent with our result. This may be explained by the fact that most patients admitted to the emergency department are at an early stage of the disease process and have not yet developed organ failure, while ICU patients have already developed complex organ dysfunction. This may remind clinicians to pay more attention to respiratory function in patients with other organ dysfunction, such as acute kidney injury or coagulation dysfunction.
Furthermore, we confirmed an association between pneumonia and increased severity of ARDS. Moreover, patients with pneumonia-induced ARDS showed significantly worse PaO2/FiO2 index, longer duration of mechanical ventilation and higher mortality rate. In the case of pneumonia, the pulmonary defense system can trigger an immune response to microbes, resulting in profound local and systemic inflammatory responses that may develop into ARDS, or severe ARDS [31, 32]. Nam et al. reported a significantly higher rate of pneumonia patients among 28-day non-survivors with ARDS caused by bacteremia-induced sepsis in Korea [11]. However, other studies showed no relationship between pneumonia and increased mortality [24, 33, 34]. Indeed, these results may not be directly comparable due to different inclusion criteria. In addition, this may be due to the differences in populations between the studies, such as Asian and non-Asian. We hypothesize that beneficial measurements and interventions should be implemented more aggressively in pneumonia patients to reduce the progression to severe ARDS, which may enhance the prognosis of ARDS caused by pulmonary sepsis.
ARDS is a clinical syndrome with a high rate of under-diagnosis. Up to 40% of ARDS patients cannot be clinically recognized quickly enough [1]. A better understanding of the risk factors for ARDS in sepsis patients will improve the ability of clinicians to identify ARDS as early as possible. Moreover, several studies have shifted the emphasis from clinical risk factors to biomarkers for development of ARDS, which is also a strategy for better precision medicine for ARDS. [35,36,37] Larger studies should be carried out to provide appropriate interventions for specific patients.
The limitations of this study are as follows: 1. Our study is a retrospective study, and the homogeneity of the data cannot be guaranteed, which may affect the results of the study. 2. The severity of the disease in the selected population is high, which cannot represent the clinical characteristics of all patients with sepsis–induced ARDS. 3. This study is a single-center study, and the results cannot be generalized to all patients with sepsis-induced ARDS or all the patients with severe ARDS.
Conclusion
Our study in the northeastern China showed that pneumonia, pancreatitis with local infection, septic shock, SOFA score and non-pulmonary SOFA score were independent risk factors for the development of ARDS in sepsis patients. In addition, pneumonia was significantly related to increased severity of ARDS and increased mortality. Multicenter, prospective studies are needed to better understand this severe clinical syndrome with high heterogeneity.
Availability of data and materials
The data could be shared if readers contact the authors and explain why they would like to access the data and material. Corresponding author should be contacted if someone wants to request the data from this study.
References
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
Estenssoro E, Dubin A, Laffaire E, Canales H, Saenz G, Moseinco M, Pozo M, Gomez A, Baredes N, Jannello G, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–6.
Cochi SE, Kempker JA, Annangi S, Kramer MR, Martin GS. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc. 2016;13(10):1742–51.
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart L, Blood Institute ACTN. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–36.
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, de Boisblanc B, Connors AF Jr, Hite RD, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75.
Martinez O, Nin N, Esteban A. Prone position for the treatment of acute respiratory distress syndrome: a review of current literature. Arch Bronconeumol. 2009;45(6):291–6.
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–16.
Gong MN, Thompson BT. Acute respiratory distress syndrome: shifting the emphasis from treatment to prevention. Curr Opin Crit Care. 2016;22(1):21–37.
Seethala RR, Hou PC, Aisiku IP, Frendl G, Park PK, Mikkelsen ME, Chang SY, Gajic O, Sevransky J. Early risk factors and the role of fluid administration in developing acute respiratory distress syndrome in septic patients. Ann Intensive Care. 2017;7(1):11.
Nam H, Jang SH, Hwang YI, Kim JH, Park JY, Park S. Nonpulmonary risk factors of acute respiratory distress syndrome in patients with septic bacteraemia. Korean J Intern Med. 2019;34(1):116–24.
Iriyama H, Abe T, Kushimoto S, Fujishima S, Ogura H, Shiraishi A, Saitoh D, Mayumi T, Naito T, Komori A, et al. Risk modifiers of acute respiratory distress syndrome in patients with non-pulmonary sepsis: a retrospective analysis of the FORECAST study. J Intensive Care. 2020;8:7.
Li S, Zhao D, Cui J, Wang L, Ma X, Li Y. Prevalence, potential risk factors and mortality rates of acute respiratory distress syndrome in Chinese patients with sepsis. J Int Med Res. 2020;48(2):300060519895659.
Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–22.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Saran R. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42(12):1980–9.
Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 2020;11:1722.
Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. 2018;463:21–6.
Dvorscak MB, Lupis T, Adanic M, Saric JP. Acute respiratory distress syndrome and other respiratory disorders in sepsis. Acta Med Croatica. 2015;69(3):167–75.
Sheu CC, Gong MN, Zhai R, Chen F, Bajwa EK, Clardy PF, Gallagher DC, Thompson BT, Christiani DC. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest. 2010;138(3):559–67.
Mikkelsen ME, Shah CV, Meyer NJ, Gaieski DF, Lyon S, Miltiades AN, Goyal M, Fuchs BD, Bellamy SL, Christie JD. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock. 2013;40(5):375–81.
Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–70.
Sheu CC, Gong MN, Zhai R, Bajwa EK, Chen F, Thompson BT, Christiani DC. The influence of infection sites on development and mortality of ARDS. Intensive Care Med. 2010;36(6):963–70.
Fujishima S, Gando S, Daizoh S, Kushimoto S, Ogura H, Mayumi T, Takuma K, Kotani J, Yamashita N, Tsuruta R, et al. Infection site is predictive of outcome in acute lung injury associated with severe sepsis and septic shock. Respirology. 2016;21(5):898–904.
Shields CJ, Winter DC, Redmond HP. Lung injury in acute pancreatitis: mechanisms, prevention, and therapy. Curr Opin Crit Care. 2002;8(2):158–63.
Jacobs ML, Daggett WM, Civette JM, Vasu MA, Lawson DW, Warshaw AL, Nardi GL, Bartlett MK. Acute pancreatitis: analysis of factors influencing survival. Ann Surg. 1977;185(1):43–51.
Raghu MG, Wig JD, Kochhar R, Gupta D, Gupta R, Yadav TD, Agarwal R, Kudari AK, Doley RP, Javed A. Lung complications in acute pancreatitis. JOP. 2007;8(2):177–85.
Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, Kojicic M, Kashyap R, Thakur S, Thakur L, Herasevich V, Malinchoc M, Gajic O. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J. 2011;37(3):604–9.
Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–43.
Delclaux C, Azoulay E. Inflammatory response to infectious pulmonary injury. Eur Respir J Suppl. 2003;42:10s–4s.
Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51.
Sevransky JE, Martin GS, Mendez-Tellez P, Shanholtz C, Brower R, Pronovost PJ, Needham DM. Pulmonary vs nonpulmonary sepsis and mortality in acute lung injury. Chest. 2008;134(3):534–8.
Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, Ware LB. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151(4):755–63.
Zheng F, Pan Y, Yang Y, Zeng C, Fang X, Shu Q, Chen Q. Novel biomarkers for acute respiratory distress syndrome: genetics, epigenetics and transcriptomics. Biomark Med. 2022.
Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T, Ragazzi R, Ruggeri P, Hansel TT, Caramori G, et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (Lond). 2019;16:1.
Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, May AK, Calfee CS, Matthay MA. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17(5):R253.
Acknowledgements
We acknowledge all the patients who enrolled in this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
YS and XL contributed the conception of the study, data collection and drafted the manuscript; LW contributed significantly to statistical analysis; SY helped to collect data. XM also contributed the conception of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by Ethics Committee of the First Affiliated Hospital of China Medical University. All the patients or their family who enrolled in this study have signed an informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, Y., Wang, L., Yu, S. et al. Risk factors for acute respiratory distress syndrome in sepsis patients: a retrospective study from a tertiary hospital in China. BMC Pulm Med 22, 238 (2022). https://doi.org/10.1186/s12890-022-02015-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02015-w