Abstract
Background
Better insight in patients’ prognosis can help physicians to timely initiate advance care planning (ACP) discussions with patients with chronic obstructive pulmonary disease (COPD). We aimed to identify predictors of mortality.
Methods
We systematically searched databases Embase, PubMed, MEDLINE, Web of Science, and Cochrane Central in April 2020. Papers reporting on predictors or prognostic models for mortality at 3 months and up to 24 months were assessed on risk-of-bias. We performed a meta-analysis with a fixed or random-effects model, and evaluated the discriminative ability of multivariable prognostic models.
Results
We included 42 studies (49–418,251 patients); 18 studies were included in the meta-analysis. Significant predictors of mortality within 3–24 months in the random-effects model were: previous hospitalization for acute exacerbation (hazard ratio [HR] 1.97; 95% confidence interval [CI] 1.32–2.95), hospital readmission within 30 days (HR 5.01; 95% CI 2.16–11.63), cardiovascular comorbidity (HR 1.89; 95% CI 1.25–2.87), age (HR 1.48; 95% CI 1.38–1.59), male sex (HR 1.68; 95% CI 1.38–1.59), and long-term oxygen therapy (HR 1.74; 95% CI 1.10–2.73). Nineteen previously developed multicomponent prognostic models, as examined in 11 studies, mostly had moderate discriminate ability.
Conclusion
Identified predictors of mortality may aid physicians in selecting COPD patients who may benefit from ACP. However, better discriminative ability of prognostic models or development of a new prognostic model is needed for further large-scale implementation.
Registration: PROSPERO (CRD42016038494), https://www.crd.york.ac.uk/prospero/.
Similar content being viewed by others
Key message
-
This article describes a systematic review that describes the predictors of mortality in patients with chronic obstructive pulmonary disease. The results indicate that the previous hospitalizations for acute exacerbation, cardiovascular mortality, male sex, long-term oxygen therapy, and other multicomponent prognostic models could aid physicians in timely advance care planning.
-
Multiple predictors of mortality can aid physicians in timely advance care planning in patients with chronic obstructive pulmonary disease. However, combining these predictors in prognostic models with adequate performance requires more research.
Background
Advance care planning (ACP) allows patients and their physicians to make plans for future healthcare [1]. In ACP, patients’ goals and preferences regarding future medical treatment and care are discussed. ACP is aimed at improving the quality of care for chronically ill patients, especially those who are nearing their end of life. This also holds for patients with advanced stages of chronic obstructive pulmonary disease (COPD), which is the third leading cause of mortality globally [2].
ACP discussions are not yet standard practice for patients with COPD [3]. Physicians seem to find it important to identify the patients who qualify for such discussions, but they are often uncertain about when to start them [4]. This uncertainty may be due to the gradual functional decline that patients with COPD typically experience [5]. That gradual decline is often interrupted by acute exacerbations, which may not only lead to hospitalization but are also associated with an increased in-hospital mortality risk [6]. Mortality rates ranged from 1.8 to 20.4% within three months after a hospitalization, and from 18.8 to 45.4% for the period from three to 24 months after a hospitalization [7].
Internationally, several quality frameworks strongly advise the initiation of ACP for patients in the last year of life, although patients’ preferences may differ regarding the start of ACP. Advance care planning is probably most efficient when started at least several months before the patient dies. Better and timely insight in patients’ mortality risk may thus support physicians in the timely initiation of ACP discussions. Several predictors of mortality have been studied for COPD, such as age, gender, body mass index, comorbidity, and forced expiratory volume in one second (FEV1) [7]. But consensus on its use in multivariable prognostic model has not been reached. We aimed to provide an overview of predictors and prognostic models for mortality within 3–24 for patients with COPD. Furthermore, we studied the discriminative ability of the multicomponent prognostic models for mortality.
Methods
Search strategy and selection process
The study protocol for this review and meta-analysis (also including a search for advanced cancer) on predictors of mortality in patients with COPD was published on PROSPERO with registration number CRD42016038494; link https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=38494. One researcher (CO) and an information specialist from the Erasmus MC Medical Library developed the search strategy, which consisted of the terms “obstructive airway disease”, “COPD”, “prediction”, and “mortality”. They performed the search in the following databases in April 2020: Embase, PubMed, MEDLINE, Web of Science, and Cochrane Central (Additional file 1: e-Table S1). All identified papers were downloaded into a reference management program.
Studies reporting on single predictors as well as studies reporting on prognostic models were eligible. Both observational and experimental studies were included if they: studied patients with COPD; studied mortality within a follow-up period of 3–24 months; presented risk estimates (hazard ratio, odds ratio, or relative risk) with corresponding standard errors of predictors or presented the performance (model discrimination: the ability to distinguish survivors and non-survivors, measured with the c-statistic or area under the receiver operating characteristic curve [AUC]; and model calibration: observed versus predicted risk of mortality) of prognostic models; and were published in English in the year 2000 or later. We excluded studies that examined predictors outside the follow-up period of 3–24 months and systematic reviews and meta-analyses.
CO initially screened the titles of all papers, followed by independent screening of the abstracts by CO and SAD. The decision to include a study was based on full texts, which were requested from its first author if unavailable. Disagreements regarding inclusion of studies were solved by discussion or by involvement of two other researchers (CCDR and AH). The researchers also hand-searched the reference lists of included studies and of systematic reviews to include other relevant studies.
Data extraction
CO and SAD independently extracted information from each included study on first author, publication year, design, population (sample size, age, sex, average FEV1), follow-up period, mortality rate, risk estimates and standard errors, and if available, the discriminative ability of a prognostic model. All means or medians were derived with standard deviations or interquartile range, respectively. In case different studies examined the same study data, we selected the most recent publication. In addition, when studies reporting estimates of mortality risk covered different follow-up periods, we selected the longest follow-up.
Two researchers independently scored all studies on risk-of-bias with a customized appraisal checklist that consisted of the Quality in Prognosis Studies tool, complemented with items from the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies checklist (Additional file 1: e-Table S2) [8, 9]. Studies were scored as low, moderate, or high risk-of-bias based on six domains: study participation, study attrition, predictors, outcome, statistical analysis, confounding, and, if applicable, prognostic model performance. The researchers resolved any disagreements in the risk-of-bias scoring through discussion. This study was reported according to the Preferred Reporting Items for Systematic and Meta-analyses (PRISMA) guideline [10].
Data analysis
A meta-analysis was performed, in which we pooled predictors with the same measuring units or cut-offs, from multivariable analyses [11]. We calculated the overall risk estimate and standard error in a fixed-effects model for predictors pooled from two studies, and a random-effects model for predictors pooled from three or more studies. For predictors in a random-effects model, we calculated the between-study heterogeneity with the I2-statistic [12]. A heterogeneity of 0–30% was considered as insignificant, 40–60% as moderate, and > 60% as substantial. We examined the possibility to perform an additional meta-analysis with low risk-of-bias studies only. All meta-analyses were performed using Cochrane’s Review Manager Version 5.3.
Furthermore, we summarized the variables and discriminative ability of multivariable prognostic models. Discriminative ability is generally expressed in terms of the AUC or c-statistic, ranging from 0.5 (no discrimination) to 1 (perfect discrimination) [13]. Funnel plots were used to detect publication bias [14].
Results
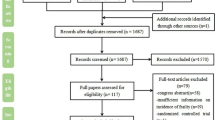
Our search resulted in 6,436 studies after removing duplicates, of which we excluded 6,325 after screening titles and abstracts (Fig. 1). Based on the full texts of the remaining 111 studies, we included 42 studies for a qualitative synthesis, of which 18 studies were included in the meta-analysis for different predictors and eleven studies reported on multicomponent prognostic models. Forty studies were observational cohort studies and two studies were secondary analyses of randomized controlled trials. The studies had sample sizes ranging from 49–418,251 patients’ mean or median age ranging from 53.0–81.7 years, follow-up periods ranging from 6–24 months, and mortality rates ranging from 5.1–65.0% (Table 1). Six studies were scored as having a low risk-of-bias, 28 as having a moderate risk-of-bias, and eight as having a high risk-of-bias (Table 2).
Five potential predictors were studied with the fixed-effects model due to the low number of studies (Fig. 2). Of those predictors, having diabetes (HR 2.69; 95% CI 1.67–4.33) significantly increased the likelihood that a patient would die. Additionally, lower hemoglobin levels (HR 0.77; 95% CI 0.64–0.92) significantly decreased the likelihood of survival. Six-minute walking distance, dyspnea, and blood urea nitrogen were not significantly associated with mortality. Six of the 10 potential predictors that were analyzed with the random-effects model were significantly associated with mortality (Fig. 3 and Additional file 1: e-Fig. S1). Hospitalization for acute exacerbation of COPD in the previous 12 or 24 months (pooled from 6 studies; HR 1.97; 95% CI 1.32–2.95) and readmission within 30 days of discharge from the hospital (pooled from 4 studies; HR 5.01; 95% CI 2.16–11.63) significantly increased the risk of mortality. Other significant predictors were age, male sex, and long-term oxygen therapy. The presence of cardiovascular comorbidity was also significantly associated with mortality, but the Charlson comorbidity index score was not. Body mass index, FEV1, and partial pressure of carbon dioxide in the arterial blood (PaCO2) were also not significantly associated with mortality. Due to the limited number of low risk-of-bias studies, a meta-analysis with those studies only was not possible. The between-study heterogeneity was insignificant, i.e. I2 0%, for predictors age, male sex, FEV1, or PaCO2. There was moderate heterogeneity, i.e. I2 40–60%, for cardiovascular comorbidity, long-term oxygen therapy, or hospitalization for acute exacerbation of COPD. Predictors that showed substantial between-study heterogeneity, i.e. I2 > 60%, were body mass index, Charlson comorbidity index score, or readmission within 30 days of discharge. Funnel plots showed no evidence of major publication bias (Additional file 1: e-Fig. S2). A list of the variables that were excluded from the meta-analysis is presented in Additional file 1: e-Table S3.
Summary forest plot of pooled hazard ratios for mortality with a random-effects model. CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; HR, hazard ratio; PaCO2, partial pressure of carbon dioxide in the arterial blood. *In the previous 12 or 24 months
Eleven studies reported on 19 different multicomponent prognostic models for mortality within a period of 3–24 months, of which the ADO, BODE, BODEX, CODEX, and DOSE models were most studied. (Table 3 and Additional file 1: e-Table S4). These models partly included overlapping variables (e.g. FEV1, body mass index, dyspnea, previous exacerbations) in various combinations. All prognostic models had a moderate discriminative ability with an AUC or c-statistic ranging between 0.6–0.8 (Table 3). The models were studied for various follow-up periods, but showed comparable discriminative abilities. Model calibration was not reported for the prognostic models.
Discussion
This systematic review and meta-analysis aimed to identify predictors of mortality within 3–24 months in patients with COPD. We found eight predictors that were significantly associated with mortality. Four of these predictors are related to patient demographics or history (age, sex, diabetes, and cardiovascular comorbidity), three to the underlying pulmonary disease (long-term oxygen therapy, previous hospitalization for acute exacerbation of COPD, and readmission within 30 days), and one to laboratory tests (haemoglobin). Overall, all significant predictors in our study seem to be readily obtainable, by taking the patient’s medical history and by performing simple blood tests. Similar to our findings, a review by Singanayagam et al. [7] found that age, diabetes, and cardiovascular comorbidity were associated with mortality within 3–24 month [7]. However, they found sex and long-term oxygen therapy to be associated with 3-month mortality and not with 3–24 month mortality as it is the case in our study. Further, low body mass was associated with mortality during longer follow-ups of 4-year and 17-year in studies by, respectively, Landbo et al. [15] and Schols et al. [16]. Additionally, dyspnea severity, which was not found to be a significant predictor in our study, was associated with 5-year mortality in a study by Nishimura et al. [17].
We also reviewed existing prognostic models for prediction of mortality in patients with COPD. The studies that reported on prognostic models did not provide the relevant effect sizes and associated standard errors of the individual variables. Therefore, the variables from those studies could not be included in our meta-analysis. Interestingly, some of the predictors included in prognostic models were not significant in our meta-analysis, such as dyspnea, body mass index, Charlson comorbidity index, 6-min walking distance, and FEV1. Of the predictors that were significantly associated with mortality in our meta-analysis, only age, previous hospitalization for acute exacerbation of COPD, and hemoglobin level were also included in one or more prognostic models, namely the ADO, BODEX, CODEX, COPD Prognostic Score, or ProPal-COPD models. Overall, the majority of the existing prognostic models had a moderate discriminative ability (AUC 0.6–0.8). Additionally, in a meta-analysis of the 6-min walking distance in 14,497 patients with COPD, Celli et al. (2016) found an AUC to predict mortality of 0.71 and 0.70 at 6 and 12 months, respectively [18]. No study reported on the calibration of the models, which is needed to judge their applicability in a specific clinical setting. Studies validating prognostic models for predicting mortality in COPD and their methodological quality should therefore be improved.
The surprise question (‘Would you be surprised if this patient died in the next year?’), which is usually recommended to be used to identify patients who are likely to die within a period of 12 months, has not been thoroughly studied for COPD yet [19]. The surprise question was only included in the ProPal-COPD model [20]. This model was one of the two models with a good discriminative ability (AUC > 0.8). Although this finding should be interpreted with some caution due to the small sample size of the study, it suggests that the physician’s clinical judgement might be important in the prediction of mortality.
This systematic review had several limitations. Firstly, some variables were pooled from only two studies, which is not ideal for a meta-analysis. We could not pool some studies in the meta-analysis because of incomplete reporting of data. Additionally, some variables could not be included in the meta-analysis because the studies did not use uniform methods for categorization of the outcomes. Furthermore, due to the low number of studies that had a low risk-of-bias based on our customized appraisal tool, we could not perform a meta-analysis with only low risk-of-bias studies. In addition, the quality of the studies was limited by the lack of data about the loss to follow-up and handling of missing values. Studies on prognostic factors could decrease study bias by reporting the number of missing values, how those values were analyzed, and the number of patients lost to follow-up. Secondly, there was substantial heterogeneity across studies for the predictors body mass index, Charlson comorbidity index, and readmission, which may be caused by the different follow-up periods, ranging between 6 and 24 months, and different study populations regarding measured FEV1 levels. Additionally, for the Charlson comorbidity index, the heterogeneity could be especially explained by the results from the small study of Navarro et al., which were discrepant to the results of the larger ones, possibly indicating selection bias. Although the I2, which is an indicator for statistical heterogeneity, was insignificant or moderate for most predictors, the pooled overall prediction effect should be interpreted with caution. Lastly, we only included published studies, especially studies from 2000 onward, whereby we might have missed some predictors of mortality.
Conclusion
This systematic review and meta-analysis provide an overview of predictors and multicomponent prognostic models for mortality within 3–24 months for patients with COPD. We conclude that mortality within 3–24 months is to a certain extent predictable. The existing models showed overall moderate discriminative ability, but no information on model calibration was available. We therefore suggest that there is a need for improvement in the validation of prognostic models. A more accurate prediction of mortality might give physicians more certainty in timely initiating ACP in patients with COPD. Further prognostic research should include physician’s clinical prediction of mortality based on the ‘surprise question’.
Availability of data and materials
The data is included in the Supplementary Material and will not be shared separately.
References
Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, van der Heide A, Heyland DK, Houttekier D, Janssen DJA, Orsi L, Payne S, Seymour J, Jox RJ, Korfage IJ, European Association for Palliative C. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543-e51.
Global Strategy for the Diagnosis MaPoC. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2006 revision. Available from: https://www.who.int/respiratory/copd/GOLD_WR_06.pdf.
Jabbarian LJ, Zwakman M, van der Heide A, Kars MC, Janssen DJA, van Delden JJ, Rietjens JAC, Korfage IJ. Advance care planning for patients with chronic respiratory diseases: a systematic review of preferences and practices. Thorax. 2018;73(3):222–30.
Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158(21):2389–95.
Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ. 2005;330(7498):1007–11.
Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–6.
Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–9.
Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–37.
Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, Reitsma JB, Collins GS. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925–31.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–61.
Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–7.
Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–40.
Celli B, Tetzlaff K, Criner G, Polkey MI, Sciurba F, Casaburi R, Tal-Singer R, Kawata A, Merrill D, Rennard S, Consortium CBQ. The 6-minute-walk distance test as a chronic obstructive pulmonary disease stratification tool. Insights from the COPD biomarker qualification consortium. Am J Respir Crit Care Med. 2016;194(12):1483–93.
White N, Kupeli N, Vickerstaff V, Stone P. How accurate is the “Surprise Question” at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med. 2017;15(1):139.
Duenk RG, Verhagen C, Bronkhorst EM, Djamin RS, Bosman GJ, Lammers E, Dekhuijzen P, Vissers K, Engels Y, Heijdra Y. Development of the ProPal-COPD tool to identify patients with COPD for proactive palliative care. Int J Chron Obstruct Pulmon Dis. 2017;12:2121–8.
Abu Hussein N, Ter Riet G, Schoenenberger L, Bridevaux PO, Chhajed PN, Fitting JW, Geiser T, Jochmann A, Joos Zellweger L, Kohler M, Maier S, Miedinger D, Schafroth Török S, Scherr A, Siebeling L, Thurnheer R, Tamm M, Puhan MA, Leuppi JD. The ADO index as a predictor of two-year mortality in general practice-based chronic obstructive pulmonary disease cohorts. Respiration. 2014;88(3):208–14.
Almagro P, Soriano JB, Cabrera FJ, Boixeda R, Alonso-Ortiz MB, Barreiro B, Diez-Manglano J, Murio C, Heredia JL, Working Group on Copd SSoIM. Short- and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145(5):972–80.
Ankjærgaard KL, Rasmussen DB, Schwaner SH, Andreassen HF, Hansen EF, Wilcke JT. COPD: mortality and readmissions in relation to number of admissions with noninvasive ventilation. COPD J Chronic Obstructive Pulm Dis. 2017;14(1):30–6.
Bélanger M, Couillard S, Courteau J, Larivée P, Poder TG, Carrier N, Girard K, Vézina FA, Vanasse A. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045–54.
Bloom CI, Ricciardi F, Smeeth L, Stone P, Quint JK. Predicting COPD 1-year mortality using prognostic predictors routinely measured in primary care. BMC Med. 2019;17(1):73.
Cheng J, Hu G, Li Y, Wei L, Zhou Y, Ran P. Prognostic role of nt-probnp for in-hospital and 1-year mortality in patients with acute exacerbations of COPD. Int J COPD. 2020;15:57–67.
Coleta KD, Silveira LVA, Lima DF, Rampinelli EA, Godoy I, Godoy I. Predictors of first-year survival in patients with advanced COPD treated using long-term oxygen therapy. Respir Med. 2008;102(4):512–8.
Duenk RG, Verhagen C, Dekhuijzen P, Vissers K, Engels Y, Heijdra Y. The view of pulmonologists on palliative care for patients with COPD: a survey study. Int J Chron Obstruct Pulmon Dis. 2017;12:299–311.
Duman D, Aksoy E, Agca MC, Kocak ND, Ozmen I, Akturk UA, Gungor S, Tepetam FM, Eroglu SA, Oztas S, Karakurt Z. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. [Erratum appears in Int J Chron Obstruct Pulmon Dis. 2016;11:417; PMID: 27013872]. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–78.
Edwards L, Perrin K, Wijesinghe M, Weatherall M, Beasley R, Travers J. The value of the CRB65 score to predict mortality in exacerbations of COPD requiring hospital admission. Respirology. 2011;16(4):625–9.
Eriksen N, Vestbo J. Management and survival of patients admitted with an exacerbation of COPD: Comparison of two Danish patient cohorts. Clin Respir J. 2010;4(4):208–14.
Fan VS, Curtis JR, Tu SP, McDonell MB, Fihn SD. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest. 2002;122(2):429–36.
García-Sanz MT, Cánive-Gómez JC, Senín-Rial L, Aboal-Viñas J, Barreiro-García A, López-Val E, González-Barcala FJ. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(3):636–45.
Gavazzi A, De Maria R, Manzoli L, Bocconcelli P, Di Leonardo A, Frigerio M, Gasparini S, Humar F, Perna G, Pozzi R, Svanoni F, Ugolini M, Deales A. Palliative needs for heart failure or chronic obstructive pulmonary disease: results of a multicenter observational registry. Int J Cardiol. 2015;184:552–8.
Gudmundsson G, Gislason T, Lindberg E, Hallin R, Ulrik CS, Brondum E, Nieminen MM, Aine T, Bakke P, Janson C. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res. 2006;7:109.
Guerrero M, Crisafulli E, Liapikou A, Huerta A, Gabarrus A, Chetta A, Soler N, Torres A. Readmission for acute exacerbation within 30 days of discharge is associated with a subsequent progressive increase in mortality risk in COPD patients: a long-term observational study. PLoS ONE. 2016;11(3):e0150737.
Hallin R, Gudmundsson G, Ulrik CS, Nieminen MM, Gislason T, Lindberg E, Brondum E, Aine T, Bakke P, Janson C. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2007;101(9):1954–60.
Ho TW, Tsai YJ, Ruan SY, Huang CT, Lai F, Yu CJ. In-hospital and one-year mortality and their predictors in patients hospitalized for first-ever chronic obstructive pulmonary disease exacerbations: a nationwide population-based study. PLoS ONE. 2014;9(12).
Hoong JM, Ferguson M, Hukins C, Collins PF. Economic and operational burden associated with malnutrition in chronic obstructive pulmonary disease. Clin Nutr. 2017;36(4):1105–9.
Horita N, Koblizek V, Plutinsky M, Novotna B, Hejduk K, Kaneko T. Chronic obstructive pulmonary disease prognostic score: a new index. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(2):211–8.
Hu G, Wu Y, Zhou Y, Wu Z, Wei L, Li Y, Peng G, Liang W, Ran P. Prognostic role of D-dimer for in-hospital and 1-year mortality in exacerbations of COPD. Int J COPD. 2016;11(1):2729–36.
Man SFP, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61(10):849–53.
Marin JM, Alfageme I, Almagro P, Casanova C, Esteban C, Soler-Cataluna JJ, de Torres JP, Martinez-Camblor P, Miravitlles M, Celli BR, Soriano JB. Multicomponent indices to predict survival in COPD: the COCOMICS study. Eur Respir J. 2013;42(2):323–32.
Martinez FJ, Han MK, Andrei AC, Wise R, Murray S, Curtis JL, Sternberg A, Criner G, Gay SE, Reilly J, Make B, Ries AL, Sciurba F, Weinmann G, Mosenifar Z, DeCamp M, Fishman AP, Celli BR, National Emphysema Treatment Trial Research G. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med. 2008;178(5):491–9.
Martinez-Rivera C, Portillo K, Muñoz-Ferrer A, Martínez-Ortiz ML, Molins E, Serra P, Ruiz-Manzano J, Morera J. Anemia is a mortality predictor in hospitalized patients for copd exacerbation. COPD J Chronic Obstructive Pulm Dis. 2012;9(3):243–50.
Morales DR, Flynn R, Zhang J, Trucco E, Quint JK, Zutis K. External validation of ADO, DOSE, COTE and CODEX at predicting death in primary care patients with COPD using standard and machine learning approaches. Respir Med. 2018;138:150–5.
Navarro A, Costa R, Rodriguez-Carballeira M, Yun S, Lapuente A, Barrera A, Acosta E, Vinas C, Heredia JL, Almagro P. Prognostic assessment of mortality and hospitalizations of outpatients with advanced chronic obstructive pulmonary disease. Usefulness of the CODEX index. Rev Clin Esp. 2015;215(8):431–8.
Neo HY, Xu HY, Wu HY, Hum A. Prediction of poor short-term prognosis and unmet needs in advanced chronic obstructive pulmonary disease: use of the two-minute walking distance extracted from a six-minute walk test. J Palliat Med. 2017;20(8):821–8.
Niksarlioǧlu EY, Ergan Arsava B, Uǧur Demir A, Topeli Iskit A, Çöplü L. Risk factors associated with mortality of COPD patients hospitalised for exacerbation. Turk Toraks Derg. 2013;14(4):134–40.
Park S, Lee SJ, Shin B, Lee SJ, Kim SH, Kwon WC, Kim J, Lee MK. The association of delta neutrophil index with the prognosis of acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20(1).
Pascual-Guardia S, Badenes-Bonet D, Martin-Ontiyuelo C, Zuccarino F, Marín-Corral J, Rodríguez A, Barreiro E, Gea J. Hospital admissions and mortality in patients with COPD exacerbations and vertebral body compression fractures. Int J COPD. 2017;12:1837–45.
Philip J, Lowe A, Gold M, Brand C, Miller B, Douglass J, Sundararajan V. Palliative care for patients with chronic obstructive pulmonary disease: exploring the landscape. Intern Med J. 2012;42(9):1053–7.
Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23(1):28–33.
Puhan MA, Siebeling L, Zoller M, Muggensturm P, ter Riet G. Simple functional performance tests and mortality in COPD. Eur Respir J. 2013;42(4):956–63.
Ranieri P, Bianchetti A, Margiotta A, Virgillo A, Clini EM, Trabucchi M. Predictors of 6-month mortality in elderly patients with mild chronic obstructive pulmonary disease discharged from a medical ward after acute nonacidotic exacerbation. J Am Geriatr Soc. 2008;56(5):909–13.
Renom F, Yanez A, Garau M, Rubi M, Centeno MJ, Gorriz MT, Medinas M, Ramis F, Soriano JB, Alvar A. Prognosis of COPD patients requiring frequent hospitalization: role of airway infection. Respir Med. 2010;104(6):840–8.
Shin B, Kim SH, Yong SJ, Lee WY, Park S, Lee SJ, Lee SJ, Lee MK. Early readmission and mortality in acute exacerbation of chronic obstructive pulmonary disease with community-acquired pneumonia. Chronic Respir Dis. 2019;16.
Slenter RHJ, Sprooten RTM, Kotz D, Wesseling G, Wouters EFM, Rohde GGU. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2013;85(1):15–26.
Stolz D, Kostikas K, Blasi F, Boersma W, Milenkovic B, Lacoma A, Louis R, Aerts JG, Welte T, Torres A, Rohde GGU, Boeck L, Rakic J, Scherr A, Hertel S, Giersdorf S, Tamm M. Adrenomedullin refines mortality prediction by the BODE index in COPD: the "BODE-A" index. [Erratum appears in Eur Respir J. 2014 Dec;44(6):1718]. Eur Respir J. 2014;43(2):397–408.
Yohannes AM, Baldwin RC, Connolly MJ. Predictors of 1-year mortality in patients discharged from hospital following acute exacerbation of chronic obstructive pulmonary disease. Age Ageing. 2005;34(5):491–6.
Zhan ZW, Chen YA, Dong YH. Comparative performance of comorbidity measures in predicting health outcomes in patients with chronic obstructive pulmonary disease. Int J COPD. 2020;15:335–44.
Zimmermann M, Traxler D, Bekos C, Simader E, Mueller T, Graf A, Lainscak M, Marčun R, Košnik M, Fležar M, Rozman A, Korošec P, Klepetko W, Moser B, Ankersmit HJ. Heat shock protein 27 as a predictor of prognosis in patients admitted to hospital with acute COPD exacerbation. Cell Stress Chaperones. 2020;25(1):141–9.
Acknowledgements
We thank researcher Hester F. Lingsma for her assistance with the study design, and Gerdien B. de Jonge for her assistance with the search strategy.
Funding
This study was funded by The Netherlands Organization for Health Research and Development (ZonMw) [grant number 844001209].The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
CO, SAD, DN, AH, and CCDR contributed substantially to the study design, data-analysis, and data interpretation. CO drafted the initial manuscript. CO had full access to all the data in the study and takes full responsibility for the integrity of the data, the accuracy of the data-analysis, and the decision to submit for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. van der Rijt reports grants from The Netherlands Organization for Health Research and Development. The remaining authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary tables and figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Owusuaa, C., Dijkland, S.A., Nieboer, D. et al. Predictors of mortality in chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med 22, 125 (2022). https://doi.org/10.1186/s12890-022-01911-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-01911-5