Abstract
Introduction
Central airway obstruction (CAO) represents a pathological condition that can lead to airflow limitation of the trachea, main stem bronchi, bronchus intermedius or lobar bronchus.
Main body
It is a common clinical situation consensually considered under-diagnosed. Management of patients with CAO can be difficult and deciding on the best treatment approach represents a medical challenge. This work intends to review CAO classifications, causes, treatments and its therapeutic limitations, approaching benign and malign presentations. Three illustrative cases are further presented, supporting the clinical problem under review.
Conclusion
Management of CAO still remains a challenge. The available options are not always effective nor free from complications. A new generation of costume-tailored airway stents, associated with stem cell-based therapy, could be an option in specific clinical situations.
Similar content being viewed by others
Introduction
Central airway obstruction (CAO) is defined as a pathological condition that leads to airflow limitation of the trachea, main stem bronchi, bronchus intermedius or lobar bronchus. It represents an important clinical situation that affects both adults and children. The exact incidence is, to the authors knowledge, unknown, but is widely accepted by the medical community that this disease is underdiagnosed [1]. While a surgical approach is considered the gold-standard treatment for most clinical situations, especially in benign etiologies, multiple factors concerning the underlying disease, the degree and type of CAO, and the patient’s condition may discourage this option. Interventional bronchoscopy emerged more than 120 years ago with Gustav Killian's works in bronchoscopy, and has been validated as a compelling treatment with immediate effects in CAO for the last 40 years [2, 3]. Regarding CAO, the scientific and medical communities currently face several limitations that arise from the anatomical, structural, and physiological characteristics of the trachea and main bronchi. The normal adult trachea is usually a rigid 11-14 cm tube supported anteriorly by C-shaped cartilaginous rings and posteriorly by smooth muscle, and this structure as a whole has some, although restricted, mobility during the respiratory process [4]. In older patients or those with hyperinflation, the posterior membranous wall may collapse during expiration. Any variation on the trachea diameter during the normal respiration cycle or any endobronchial changes, such as injury or secretions, may impair the ventilatory dynamic and affect the Reynolds number (transition from laminar to turbulent flow with increase resistance), thus resulting in the need for higher-pressure difference to maintain a normal flow rate [5, 6]. This will be reflected in the development of symptoms, especially in patients with a marked reduction in the cross-sectional area.
CAO is caused by several disorders and the first step for its approach is a clear etiological evaluation (Table 1).
Congenital or acquired CAO may result from intrinsic stenosis or extrinsic compression, leading to fixed obstruction, or caused by cartilage or pars membranous flaccidity, with dynamic obstruction. In the early twentieth century, infection and trauma were the main causes of CAO. In the 60 s, with the advent of intensive care units and the common practice of prolonged orotracheal intubations, post-intubation tracheal stenosis (PITS) and post-tracheotomy (PSST) tracheal stenosis incidence increased. Malignant causes of CAO, which have been reported in up to 20–30% of lung cancers in older studies [7], do not reflect contemporary lung cancer epidemiology. These rates are now lower, with the progressive change in lung cancer incidence and prevalence, favouring peripheral adenocarcinoma development [8]. A recent retrospect analysis of 342 baseline CT scans of lung cancer revealed 13% of patients having CAO [9].
Since CAO has multiple etiologies and different expressions, a clear definition and classification is of utmost relevance, as to estimate prognosis and determine the best therapeutic options [7]. A classification system based on histologic findings, pathological mechanisms, dynamics, functional impairment severity of airway narrowing and extend of airway abnormalities is crucial. The multidisciplinary involvement has led to the development of several systems with distinct criteria. Regarding laringotracheal stenosis, they were first classified by Cotton in 1984 based on the cross-sectional area of the stenosis [10]. In 1987, Grundfast et al. using the same criteria, upgraded the system looking for the predictive outcome [11]. In 1992 McCaffrey proposed a classification based on the vertical extend of the stenosis and predicting decannulation centred on anatomic location and extend [12]. Grillo et al. in 1993 reviewed the anatomic location and the possibility of anastomosis. In 1994, the Myer-Cotton Airway Grading System for subglottic stenosis was created and became routinely used to predict interventional success [13]. The four degrees of this system consider an obstruction of 0–50% for grade I, 51–70% for grade II, 71–99% for grade III, and complete luminal obstruction for grade IV [14]. The proposal of Lano et al. in 1998 was based on subsites involved. All those studies were focused on surgical approaches and outcomes based mainly on treatable anatomic premises, excluding extensions beyond the trachea and the possibility of endobronchial treatment. In 2007, Freitag et al. published a classification system dividing stenosis into structural and dynamic types, in addition to categorizing its degree and location (Fig. 1A). This classification was designed for grading tracheal stenosis from a pulmonologist’s perspective, but the degree of severity was not justified clinically. On the other hand, a histological classification was not included [15].
In 2010, Murgu and Colt made a different proposal, where a qualitative and quantitative classification was considered [16]. The gaps observed in Freitag et al. classification were also filled (Fig. 1B). The idea of creating an ideal and reproductive classification for CAO has not yet been fully completed and consensus has not yet been reached. In the future, ongoing studies will standardize nomenclatures and find descriptors that include the degree of stenosis and patient´s functional status, histologic and morphologic types, extension, localization, and mechanism of obstruction.
The European Laryngological Society (ELS) published a consensus paper in 2015 that offered a new classification system of benign laryngo-tracheal stenosis (LTS) in adults and children [17]. It included a routine five-step endoscopic evaluation of patients, to assess the grade of stenosis (Cotton– Myer grade I–IV), number of subsites involved (“a–d” for one to four of supraglottic, glottic, subglottic, and tracheal segments involved), and an additional plus sign (“+”) for patients with severe airway or medical comorbidities. The focus of this classification was to access the surgical outcome of patients with LTS.
Etiology
Congenital CAO
In the adult population, congenital disease itself as a cause of CAO is a very rare condition [18]. When identified, it may result from posterior fusion of the tracheal rings. In children, it is frequently associated with congenital cardiovascular abnormalities, the most common being the compression of anterior tracheal wall for an anomalous bifurcation of the innominate artery [19].
Acquired CAO
Tracheobronchial trauma/iatrogenic
Acquired non-malignant CAO is most frequently caused by iatrogenic tracheal trauma after prolonged intubation or tracheostomy tube placement. It is estimated that the incidence of PITS ranges from 6 to 21% and PTS 0.6 to 21% [20, 21]. PITS occurs mostly in the anatomical area of the inflated cuff and is related to pressure induced ischemic injury of the mucosa and cartilage. On the other hand, the inflammation caused by a foreign body may result in granulation tissue followed by fibrosis. Web-ring stenosis is the most common form [22]. With low-pressure cuffs and maintaining a pressure under 30 mmHg, it was possible to decrease the incidence of post-intubation stenosis to 1% and of post-tracheotomy to 10–15% [23]. The duration of intubation also impacts the frequency and severity. PSST may be above the stoma, at the level of the stoma, at cuff site or at the tip of the cannula. It results from a process of tissue repairing and formation of granulation tissue around the stoma. The most common type is granulation tissue formation at the stoma site. Stenosis due to cartilage fracture, tracheomalacia and web-like can also be seen [24]. Factors associated with PITS and PSST are the level of stoma, previous tracheal procedure, low blood pressure, severe respiratory failure, sepsis, concomitant autoimmune disease, sleep apnea syndrome,obesity, diabetis,high-dose corticosteroid therapy previous radiation and intubation > 48 h [25]. Post-transplantation bronchial stenosis occurs in 5–30% of patients and results from all repair process of anastomosis [26]. These anastomosis are vulnerable for several reasons: rejection may occur, and immunosuppression often results in infection and/or ischemia, especially in the first 4 weeks, which is the necessary time for bronchial arteries to re-establish full perfusion. Other causes of tracheobronchial stenosis include lobectomy, and sleeve and tracheal resections [27].
Trauma may also arise from thermal and chemical injuries or treatment with radiotherapy, causing localized stenosis.
Foreign body aspiration is more common in children and elderly and can lead to acute CAO. If the foreign body is not large enough to significantly occlude the lumen, granulation may emerge and cause obstruction. If the foreign body has an organic nature, a chemical bronchitis can occur [28].
Infection
Tuberculosis is the most common cause of post-infection stenosis in adults, mainly in high-prevalence geographic regions. It is usually caused by endobronchial involvement or lymph node fistulation trough adjacent bronchi. Early diagnosis and treatment with anti-tuberculosis medications typically prevents this situation [29]. Laryngotracheobronchitis (croup) is an acute viral respiratory disease which occurs usually in children under 6 years old and is rare in adults. Acute bacterial tracheitis due to S. Aureus sometimes can occur as over-infection after a viral episode and may cause CAO [30]. Laryngeal scleroma is a chronic and progressive granulomatous infection caused by Klebsiella rhinoscleromatis, a gram-negative bacteria endemic in Africa, Asia and South America, and may cause airway obstruction [31]. Other conditions like diphtheria, syphilis and fungal infections, although rare, are recognized causes of CAO [32].
Inflammatory and auto-immune diseases
Granulomatosis with polyangiitis is a systemic disease characterized by necrotizing vasculitis and granulomas. The airway is involved in 15–55% of cases and can be the only manifestation in 25% of patients [33, 34]. Granulomatosis with polyangiitis may lead to subglottic, tracheal, or bronchial stenosis, and nasal sinus is frequently involved. Laboratory findings may include positive anti-neutrophil cytoplasmic autoantibodies (c-ANCA) but usually there is no link between inflammatory activity and positive c-ANCA. The mucosa’s biopsy is the gold standard method for diagnosis and usually reveals granulomatous inflammation and vasculitis in early disease, while fibrosis ensues later. Unfortunately it is positive in only 5–15% of cases [35]. Systemic immunosuppressive therapy and steroids are the mainstay treatment, but endoscopic management may be required.
Tracheobronchial amyloidosis results from the pathological deposition of amyloid in the proximity of the tracheal, bronchial gland acini and blood vessel walls. It may be present as an isolated manifestation or as part of a systemic disease. Tracheal involvement can range from diffuse lesions to masses. Pulmonary manifestations as persistent pleural effusions and parenchymal nodules can be present. Diffuse wall thickening, irregular narrowing of the lumen and calcifications may be seen during bronchoscopy. Diagnosis is determined by biopsy of the lesions showing positive red Congo staining with submucosal extracellular deposits of amyloid protein. There is no effective treatment and radiation therapy in addiction to interventional bronchoscopy techniques and surgery should be considered [36].
Sarcoidosis is a multisystem disorder characterized by noncaseating granulomatous inflammation. Although any organ can be involved, the disease most commonly affects the lungs and intrathoracic lymph nodes. The airway can be affected even in the absence of parenchymal involvement. Bronchial wall thickening caused by granulomas and interstitial fibrous tissue may result in smooth or irregular luminal narrowing. The compression due to mediastinal lymphadenopathy is rare. Bronchoscopic findings include cobblestone appearance of the mucosa originated by granuloma, ranging from single to multiple stenosis sites [37]. The diagnosis of sarcoidosis is established based on a compatible clinical picture, evidence of noncaseating granulomas on biopsy, and exclusion of other granulomatous disorders. Treatment with immunosuppressive therapy may be necessary in severe cases. Bronchoscopic procedures are required in some situations [38].
Relapsing polychondritis (RP) is a multisystemic autoimmune disease characterized by recurrent inflammatory episodes affecting cartilaginous structures. These structures include ears, nose, peripheral joints, larynx, and tracheobronchial tree. It is more likely between 40 and 50 years and is often associated with another pathologic conditions as systemic vasculitis or connective tissue disorders [30]. The evolution of the disease alternates between an active inflammatory phase and non-acute phase. During disease course, about half patients will exhibit airway involvement including subglottic stenosis, tracheobronchomalacia and tracheobronchial stenosis. Thorax computer tomography (CT) and bronchoscopy reveal major airway collapse, thickening of the trachea and proximal bronchi, thickened calcified cartilaginous rings and tracheal nodularity. The posterior tracheal membrane is normally spared [35]. Usually, specific clinical criteria and bronchoscopic evaluation of airway are enough to diagnose of RP. If a biopsy is performed, inflammatory changes in the cartilage are normally present. Medical management of RP consist in control the inflammatory process with steroids combined with methotexate, azatriopine or cyclophosphamide. Some studies propose the use of immunomodularity therapies as etanercept, infliximab or rituximab [39, 40]. Interventional bronchoscopy procedures such as dilating, and stenting may help in specific cases [41].
Tracheobronchopatia osteochondroplastica (TO) is a rare benign disease with unknown origin that affects the cartilage rings of the trachea and, in lesser extent, the main bronchi. It occurs over the age of 50 years, and it is characterized by cartilaginous or osseous nodules projecting into the airway lumen, causing serious deformity. In 10% of the cases, the tracheal lumen can be reduced up to 50%. CT scans and bronchoscopy show densely calcified cartilage nodules. If the appearance is typical, no biopsy is needed. TO is a slow benign disease and rarely complicates. In cases of more serious degrees of stenosis, endoscopic laser ablation or stent application may be required [41]
Tracheobronchomalacia
Tracheobronchomalacia (TBM) is a condition characterized by diffuse or localized loss of rigidity of the trachea and mainstem bronchi, leading to airway collapse. If the affected portion is intrathoracic, the airway obstruction will be accentuated during expiration. More rarely, the affected portion is extra-thoracic and is more accentuated during inspiration. Congenital TBM is described more frequently in children and indicates a defect of the tracheal wall. The disorder can persist into adult life and is designated idiopathic giant trachea or Mounier-Khun syndrome. TBM may also develop along with other congenital conditions such as cystic fibrosis, Marfan syndrome, Ehlers-Danlos syndrome or congenital trachea-esophageal fistula [42]. Acquired TBM is associated with a variety of conditions. Prolonged endotracheal intubation and tracheostomy trauma are the most frequent, but head and neck surgery, trauma, radiotherapy or inflammatory conditions must also be considered [43, 44]. The diagnosis is based on dynamic Three-dimensional CT images and direct bronchoscopic visualization to confirm significant narrowing of airway lumen during forced breathing manoeuvres [45]. Applying continuous positive airway pressure has been proposed as a possible treatment [44]. In patients with diffuse TBM, a silicone stent placement should be considered in preparation for a more definitive treatment such as tracheoplasty. Majed et al. purpose a self-expandable metallic stents (SEMS) trial as a bridge to surgery [46]. Other surgical approach consists in restoring the C shape of the cartilages which is lost in TBM attaching longitudinal strips of splinting material along the membranous walls of the intrathoracic trachea [47].
Tumours
Both malignant and non-malignant tumours may cause CAO, either from direct extension of a locally advanced disease or from extrinsic compression. Primary tracheobronchial tumours are infrequent and include adenoid cystic carcinomas, bronchial carcinoid tumours, primary squamous cell carcinoma of the trachea and adenocarcinoma of the trachea [48]. Much frequent are metastases from primary lung cancers. Metastasis from breast, renal, thyroid, ovarian, uterine, testicular carcinomas and melanoma may also occur. It is estimated that 20–30% of patients with primary lung cancer will develop CAO; of those, 35% will die in consequence of asphyxia, hemoptysis and post-obstructive pneumonia [49]. Anatomically, malignant CAO is divided into 3 groups: intra-luminal, extrinsic compression and mixed. The purpose of this classification is based on the type of interventional assessment. The interventional bronchoscopy with palliative intent should be considered in these patients which may benefit from measures to relive dyspnea [2, 50]. Recurrent papillomatosis, resulting from infection of the human papilloma virus, produces lesions with polypoid appearance which can affect larynx, trachea, and bronchi. Endoscopic interventions are an important component, as recurrence is common [51].
Extrinsic compression
Most frequently extrinsic compression in children arises from abnormalities of the aortic arch and are defined as vascular rings. Double aortic arch and right sided aortic arch with aberrant left subclavian artery are the most common. Compression of the trachea by large aortic or innominate artery aneurysms may occur. Mediastinal masses rarely cause serious airflow limitations. Approximately 40% of mediastinal masses are malignant and 25% are cystic [35]. Thymic neoplasms and lymphoma, both Hodgkin´s and non-Hodgkin´s, are also common, but neurogenic tumours and teratomas must be considered.
An analogous but rare situation can be caused by the tumefaction of mediastinal lymph nodes due to metastatic or infectious diseases. Involvement of the trachea and left side bronchus by advanced esophageal cancer is common and is associated with a poor prognosis. Retrosternal extension of diffuse goiter may cause extrathoracic or intrathoracic airway obstruction [52]. Laryngoceles, saccular cysts and parathyroid cysts may be also implicated in CAO [53]. Airway narrowing due to osteophytes has been reported [54]. Postpneumonectomy syndrome occurs due to a compression of the left main bronchus between the aortic arch and left pulmonary artery, following a right pneumonectomy [55]. Significant CAO can occur from other infectious or malignant diseases like hematomas, abscess formation, empyema, or other expanding lesions. In most cases the treatment should address the underlying cause, but urgent intervention may be needed in case of respiratory failure. Most of the times this includes airway stent deployment to restore and maintain luminal patency [56, 57].
Other causes
Idiopathic tracheal stenosis is a rare inflammatory process that develops frequently in the subglottis or in the upper third of the trachea. It occurs mainly in women, suggesting an important role of estrogens[58]. Other authors suggest that it may be associated with gastroesophageal reflux [59].
Clinical presentation
CAO is much less common compared to other lower airway diseases. However, the symptoms may be identical, which may lead to misdiagnosis. Because these symptoms can be insidious CAO may be obscured for a long time resulting in a delayed diagnosis and worst outcome. Clinical presentation will depend on the location, extension and degree of obstruction, speed of progression and underlying disease. Other conditions, such as the patient healthiness, will have an important role in progression and outcome. The main symptoms are dyspnea and stridor occurring in 54% of patients as initial complain [45]. These manifestations are more prominent during exercise but may progress to dyspnea at rest. The first usually occurs when the lumen is reduced to 8 mm and the second when it reaches less than 5 mm. Cough, wheezing, diminished sputum clearance and recurrent infection will emerge when it reaches this level of severity. Unlike wheezing, stridor occurs predominantly during expiration and is emphasized by hyperventilation. If the obstruction is mainly thoracic, both inspiratory and expiratory stridor will appear. Hoarseness can be heard and could be a signal of laryngeal involvement. Less common is an initial presentation with acute respiratory distress.
Diagnostic evaluation
When CAO is suspected, clinical investigations to look for the underlying diagnosis include, in the first instance, thorax CT and blood tests (inflammatory markers, auto immune diseases), lung function tests, other imaging exams [X-ray, CT, magnetic resonance imaging (MRI)] and bronchoscopy. Blood tests are frequently normal or with nonspecific changes, but inflammatory markers, white cell blood count or auto-immune screen can identify underlying diseases [35]. As the symptoms, physiological and measurable abnormalities in lung function tests frequently do not become evident until the airway lumen is < 8 mm, which corresponds to an obstruction of more than 80%. In these cases, a variable extra-thoracic obstruction shows flattering of the inspiratory loop, intrathoracic obstruction shows flattering of expiratory loop and fixed obstructions reveal flattering in both. On the other hand, if the cause of CAO is a lung tumour, an abnormal lung function test could not be adequately valorised because of the prevalence of lung cancer in chronic obstructive pulmonary disease (COPD) [60], whereas the lung function tests are usually anomalous. Unless the cause of CAO is an extra-thoracic compression caused by a vascular abnormality or tumour, X-ray usually gives few and unspecific information. The thoracic CT with tracheal protocols is the procedure of choice for imaging the upper airway, with a sensitivity of 97% [35]. With standard chest protocols, tracheal disease is easily underestimated. CT allows to identify the precise anatomical location of the lesion, its characteristics and extension, including the visualization of patency of the distal airway. Using an axial view, it is possible to evaluate the degree of stenosis. Extremely thin slices allow 3-D reconstructions, especially useful to provide pre-management volumetric analyses. Dynamic CT is effective for the diagnosis of TBM. MRI has usually limited value although it can be used to access the surrounding mediastinum, mostly the vascular structures [35]. A direct view of the airway is provided by both flexible and rigid bronchoscopy. They allow to directly observe the extension of airway narrowing, the characteristics of the mucosa, and the specific location. Samplings can be collected for microbiological and cytologic analyses. Because of the risk of precipitating acute and complete obstruction, flexible bronchoscopy should be performed judiciously and by trained physicians with appropriate equipment [61].
Although many different causes of CAO and a relatively well-defined protocol approach, its management is not straightforward nor consensual and the long-time outcomes are far from satisfactory in some cases, as shown in Table 2
A CT scan shows a narrowing in the mid trachea; B Endoscopic vision of subglottic; C endoscopic view of trachea after placement of stent, showing the patency of airway; D Distal view of stent; E, F Stent migration and partially obstructed by mucus. E, F, Courtesy of Dr. José Almeida – Centro Hospitalar de Vila Nova de Gaia, Portugal
Sequence of happenings observed in thoracic CT scan and in bronchoscopy: A thoracic aorta aneurism with tracheal contact; B 3-D reconstruction of thoracic aorta aneurism; C carina with signs of extrinsic compression; D stent in left main bronchus; E total occlusion of stent witch mucus; F patency of stent post laborious cleaning; G, F evidence of fistulization from LMB to mediastinum, contacting directly to aneurismatic sac
Discussion on CAO management and the role of tracheal stents
CAO cases must have a multidisciplinary team discussion to cover all possible treatments and to select the best therapeutic options. Therapy must be addressed according to the pathologic findings and to the patient´s health status. Definitive management may include both medical and surgical interventions. Possibilities embraces observation, drug therapy, radiation, tracheostomy, endoscopic treatment, and surgical management. Endoscopic treatment includes dilatation, laser resection and airway stenting [62]. As described before, several classification systems can be used to predict the success of the intervention, although further studies are needed to confirm its usefulness. In non-symptomatic or minimal symptomatic patients with stable and non-progressive stenosis, close observation is considered an adequate approach [63]. In the decision of management, according to Galluccui et al., the most important differentiation to be made is between simple and complex stenosis as it determines the success or failure of the endoscopic intervention [64]. Complex tracheal stenosis is associated with extensive scaring (> 10 mm) and varying degrees of cartilage involvement, circumferential contraction scaring, or tracheal stenosis associated to malacia or inflammation. Tracheal sleeve resection is considered to be the first-option and definitive treatment in complex tracheal stenosis [65]. Nevertheless, bronchoscopic management in selected patients is reported to have a high success rate [66, 67]. Thus, a short segment, membranous, benign, concentric and not involving cartilage stenosis can be consider for endoscopic treatment as first option [30]. It is also an option for patients with surgical ineligibility due to the stenosis characteristics, infection, or poor medical status. Complex stenosis may require stent placement. Endoscopic intervention can also work as a bridge for surgical treatment [64]. In the first clinical case, the patient had a complex stenosis. Although surgery could not be performed because of comorbidities, the decision based on the difficult mechanical ventilation, easily reaching high plateau pressures with low tidal volumes, was taken with the purpose of being only temporary. Prior to this scenario, the patient manifested mild symptoms for 2 years, and at the time, the option was the maintenance under close observation but without intervention. Interventional bronchoscopy options include the use of both rigid and flexible bronchoscopy. Both have advantages in different scenarios and should be seen as complimentary procedures [62, 68]. Guidelines do not favour rigid over flexible bronchoscopy, although the procedure with rigid bronchoscopy is considered safer. Through the flexible bronchoscope thermal and cryotherapy, balloon dilatations, micro-debridement and stenting can be performed. Several thermal techniques can be used [61]: (a) electrocautery, one of the most available and cheap, achieves an immediate outcome but requires a skilled user, also requires contact and FiO2 should be decreased; (b) argon-plasma coagulation is a non-contact technique capable to burn tissue and control bleeding. It has a penetration depth of 2-3 mm, which makes it a safe but longstanding technique; (c) LASER has supplanted other thermal techniques because of its minimum energy delivery and cut precision. CO2 laser is more precise but has a limited coagulation effect compared with Nd:Yag laser[69]. In contrast to heat therapies, contact cryotherapy has been also described as useful in CAO. It is exclusively used for endobronchial lesions and may require repeated procedures. Rigid bronchoscope debulking of lesions and scar tissue is also simple, effective and accomplished by suction under direct telescopic guidance [62, 68].
Several drugs used to prevent the complications associated to endoluminal wound healing have been assessed in the last decades and are still under investigation. The most used as co-adjuvant are corticosteroids [70] and mitomycin-c. A literature review in 2010 concluded that mitomycin-c may postpone but not prevent the recurrence of stenosis [71].
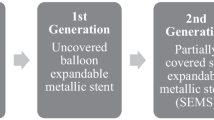
For patients with primary lung cancer which develop CAO, besides all interventional bronchoscopy techniques mention above, in some cases, additional stenting is required [68]. Airway stents are tube-shaped devices that are inserted into the airway to maintain patency. Stent placement is indicated in both intraluminal and extraluminal significant CAO and can be used in benign and malignant disease. Placing an airway stent is a difficult decision, and the operator must consider the benefit of its application, by preventing the airway re-occlusion, and also its disadvantages, mostly regarded with long-term associated complications [72, 73]. There are two main types of airway stents concerning the material they are made of: tubular stents made of silicone and SEMS. Metallic stents come with or without a silastic or polyurethane covering, which are used to minimize tumour overgrowth and tissue granulation [74]. The development of granulation tissue is one of the common occurrences in uncovered or partially covered stents, and its coverage was developed to limit this disadvantage. Indeed, in covered stents, granulation tissue tends to only be located at the extremities and its progression into the stent is rare [75]. Both types of stents can be placed through rigid bronchoscopy, although SEMS could also be placed with flexible bronchoscopy. As a foreign body, there are complications associated to endotracheal stents (Table 3).
Metallic stents, especially non-covered, should be considered permanent because of extensive granulation tissue and epithelialization. The longer the stent remains, the more difficult is its removal. Silicone stents are more easily replaced or removed [76]. Based on 2005 Food and Drug Administration warning, metallic stents are not recommended in benign airway strictures because of the reported extensive granulation tissue and stent fracture. Considering these recommendations, they should not be placed in patients where removal is considered in the future [32, 77]. In the 3 cases presented, disrupt of mucociliary clearance and mucus plugging were detected as clinical impairments. Infection aroused in 2 cases and migration in the first clinical case. Fistulation is a serious problem, and happens because of friction between stent and mucosa [78]. In TBM, stent insertion should be only applied in patients with symptomatic and severe obstruction (> 60%) and non-responsive to positive pressure ventilation. QOL and functional status are improved in short term in 70% and dyspnea in 90% of patients after silicone stenting [79, 80]. For patients with severe, diffuse and symptomatic TBM who had repeated stent migration, external stent fixation, tracheostomy or Montgomery T-tubes are a good option to improve symptoms or as a bridge to a more definitive treatment [79]. As the ideal stent is still not available, physicians should consider the full assortment of silicone and SEMS. The main advantages of silicon stents rely on their easy removal and repositioning, less granulation tissue formation and absence of tumour ingrowth. On the other hand, rigid bronchoscopy is needed for insertion, and they are more suitable to migrate. SEMS are easy to insert, as it can be performed by flexible bronchoscopy. Following, they are more stable and more effective in extreme extrinsic compression. The main disadvantages are the impossibility of repositioning or removal and tumour ingrowth [50], especially in the case of uncovered metallic stents. The ideal stent should be simple to insert, easily removed, capable to resist compression and to allow clearance of secretions, as well as easy to nail and avoid migration. New custom-made and bioabsorbable airway stents made of different biomaterials are under investigation and have been placed in humans. Polydioxanone is the one that has been most often used and for the longest period, with report of a patient successfully treated and free of intervention for 44 months [81, 82]. Other biomaterials are under investigation, as the polylactic acid, polyglycolic acid, polycaprolactone and polyamide[83, 84]
Outcomes
In malignant diseases, there is no controlled trial evidence regarding tracheal or bronchial intervention due to ethical concerns. However, it is consistently demonstrated in the literature that interventional bronchoscopy procedures with stenting improves symptoms of dyspnea, QOL and lung function [2, 85, 86]. Long term favourable outcomes are achieved with a combination of laser and gentle dilatation in benign tracheal stenosis [32, 64, 87]. Although the use of stents in benign clinical conditions is not consensual, a considerable number of supportive literature describes promising results [88]. According to Monnier et al., using the Myer-Cotton grading system, the success of interventions with CO2 laser is 92% in grade I, 46% in grade II and 13% in grade III [17]. Lim SY and colleagues, concerning PIS, demonstrated that the time relapsed between stenosis and first intervention is important, with positive outcomes of 90% for those with intervention within 6 months and 61% for those with longer intervention period [89]. According to Galluccio et al. the use of silicone stents as part of endoscopic intervention, achieved airway patency of 96% in simple stenosis and 69% in complex stenosis [64]. When stents are used in TBM, the complication rates are higher when the disease occurs with loss of cartilaginous support [64].
The role of tracheal tissue engineering in CAO
Airway tissue engineering is a field of Regenerative Medicine with the goal of developing biological substitutes that can restore, maintain, or improve tissue functions. When stenting is not possible, there are other options (Table 4) with pros and cons that range from autografting and allografting to the use of prosthetic materials. Prosthetic options have some limitations such as development of inflammation and incomplete host tissue integration. In the case of grafts, autografts are good options due to the absence of rejection and inflammation after application, however the availability of donor tissue is a limitation. Furthermore, allografts must be associated with immunosuppressive drugs. To overcome these constraints, alternatives associated with tissue engineering have been developed in recent years. In these approaches, therapies are based on the use of three essential elements: cells, growth factors and scaffolds in distinct combined approaches [90]. Different cell populations have already been extensively applied in this field. Tissue-engineered tracheal bioprosthesis coated with chondrocytes and polymers were used and implanted in vivo, and Mesenchymal stem cells (MSCs)-derived chondrocytes have also been considered as alternative cell sources, particularly in promoting tracheal cartilaginous tissue regeneration [91]. These cells allow biomechanically stable seeded scaffolds, but chondrocytes become infeasible after implantation. Epithelial cells were explored as an alternative, and scaffolds seeded with these cells demonstrated increased viability and promoted better tracheal regeneration in vivo [92]. To maximize the advantages of each cell type, a combination of epithelial cells cocultured with articular chondrocytes was tested, and good in vitro performance, as well as positive tissue regeneration in vivo, were observed [93]. The co-culture of bone marrow derived MSCs with fibroblasts and endothelial cells of the cartilage and umbilical vein, their aggregation in spheroids and subsequent 3D printing in an architecture similar to native tracheal tissues allowed the development of layers of epithelial tissue with good viability and vascularization [94]. The culture of Induced pluripotent stem cells in an air—liquid interface system allowed their differentiation into ciliated epithelial cells and non-ciliated club cells, making them fair candidates for application in tracheal regeneration [95]. Tissue engineered airway grafts seeded with isolated cells from umbilical cord blood, amniotic fluid or adipose tissue revealed to not be subjected to immune rejection and can provide mechanical characteristics compatible with tracheal physiology. Finally, the use of cells from fetal tissues allowed the synthesis of a cartilage rich in glycosaminoglycans, β-elastin and smooth muscle cells, with epithelial growth, smooth muscle having cilia movement and no ectopic growth or tumour development [90, 96]. The scaffolds to be used to promote regeneration of tracheal tissue must have some specific characteristics, namely: design that mimics the anatomical shape of the trachea or defects to be filled; mechanical strength and flexibility identical to native tracheal tissue to prevent collapse; porosity that allows good vascularization and cell proliferations. In addition, the scaffold must be biocompatible, biodegradable, non-toxic and non-immunogenic, to ensure a good cytocompatibility and to not trigger organic reactions and rejections [97]. To guarantee these characteristics, the scaffold manufacturing methods as well as their material are important decisions to consider. Various natural and synthetic materials have already been used, either alone or in combination, including silk, gelatin, polyglycolic acid, collagen, polycaprolactone and decellularized matrix [90]. Growth factors influence cell proliferation and differentiation by modulating the pro-regenerative environment at the site of administration, also ensuring cell growth and nutrition. The list of growth factors already used in tracheal tissue engineering include insulin-like growth factor, transforming growth factor, granulocyte colony-stimulating factor, vascular epithelial growth factor, basic fibroblast growth factor, epithelial growth factor and platelet-derived growth factor [90]. These growth factors can be administered both exogenously and loaded on the scaffold, but while the first approach is more advantageous in the case of minor injuries, endogenous administration is most appropriate in significant tracheal damages [98]. The best combination of cells, scaffolds and growth factors has not yet been defined, but tissue engineering has the potential to allow the development of alternative methods for effective tracheal reconstruction. The goal of these technologies is the development of a fully autologous engineered trachea that allows to overcome the limitations of the current synthetic and grafts options.
Conclusion
CAO represents an important clinical impairment caused by benign or malignant diseases. Surgical management remains the preferred approach but there are situations in which, whether due to patient limitation or the nature of the pathology, intervention bronchoscopy, with its accessory techniques such as laser, cryotherapy, mechanical dilation, or debridement, play a preponderant role. When it comes to the choice of a stent, several considerations must be considered. Silicone stents are cheaper, easily retrievable and can be repositioned as much times as necessary. On the other hand, they need for rigid bronchoscopy to be handled, are more suitable to migrate and interfere with mucociliary clearance. Metallic stents can be placed by flexible bronchoscopy, are usually more adaptable to an irregular surface and allow reepithelization. The same way, the tumour ingrowth can happen which make them almost impossible to be securely removed. Although an exciting new generation of personalized airway stents along with stem cell therapy is expected, this reality is far away from being achieved. A recent evolutional step was made when manufacturers offered modifications of their products according to patient´s needs, with rapid prototyping 3D printing techniques, allowing stents to be tailored to the individual´s airway in days or even hours [99]. If on one hand 3D printing technology could become accessible for physicians, on the other hand there are no certified manufacturers nor a direct access between parts. Maybe a creation of a software easy-to-use between hospitals and manufacturers could provide that answer but there are still ethical and legal issues to overcome [100].
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on request.
Abbreviations
- c-ANCA:
-
Cytoplasmic autoantibodies
- CAO:
-
Central airway obstruction
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computer tomography
- LMB:
-
Main left bronchus
- LTS:
-
Laryngo-tracheal stenosis
- MRI:
-
Magnetic resonance imaging
- MSCs:
-
Mesenchymal stem cells
- PITS:
-
Post-intubation tracheal stenosis
- PSST:
-
Post-tracheotomy tracheal stenosis
- QOL:
-
Quality of life
- RP:
-
Relapsing polychondritis
- SEMS:
-
Self-expandable metallic stents
- TBM:
-
Tracheobronchomalacia
- TO:
-
Tracheobronchopatia osteochondroplastica
References
Quach A, et al. Prevalence and underdiagnosis of airway obstruction among middle-aged adults in northern France: the ELISABET study 2011–2013. Respir Med. 2015;109(12):1553–61.
Stratakos G, et al. Survival and quality of life benefit after endoscopic management of malignant central airway obstruction. J Cancer. 2016;7(7):794–802.
Ost DE, et al. Therapeutic bronchoscopy for malignant central airway obstruction. Chest. 2015;147(5):1282–98.
Drevet G, Conti M, Deslauriers J. Surgical anatomy of the tracheobronchial tree. J Thorac Dis. 2016;8(Suppl 2):S121.
Brouns M, et al. Tracheal stenosis: a flow dynamics study. J Appl Physiol. 2007;102(3):1178–84.
Ko Y, et al. Changes in the flow-volume curve according to the degree of stenosis in patients with unilateral main bronchial stenosis. Clin Exp Otorhinolaryngol. 2015;8(2):161.
Ernst A, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278–97.
Abdel-Rahman O. Changing epidemiology of elderly small cell lung cancer patients over the last 40 years; a SEER database analysis. Clin Respir J. 2018;12(3):1093–9.
Daneshvar C, et al. Prevalence and outcome of central airway obstruction in patients with lung cancer. BMJ Open Respir Res. 2019;6(1):429.
Cotton RT. Pediatric laryngotracheal stenosis. J Pediatr Surg. 1984;19(6):699–704.
Grundfast KM, Morris MS, Bernsley C. Subglottic stenosis: retrospective analysis and proposal for standard reporting system. Ann Otol Rhinol Laryngol. 1987;96(1):101–5.
McCaffrey TV. Classification of laryngotracheal stenosis. The Laryngoscope. 1992;102(12):1335–40.
Myer CM III, O’connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994;103(4):319–23.
Song SA, et al. Reliability of peak expiratory flow percentage compared to endoscopic grading in subglottic stenosis. Laryngosc Investig Otolaryngol. 2020;5(6):1133–9.
Freitag L, et al. A proposed classification system of central airway stenosis. Eur Respir J. 2007;30(1):7–12.
Colt HG, Murgu SD. Interventional bronchoscopy from bench to bedside: new techniques for early lung cancer detection. Clin Chest Med. 2010;31(1):29–37.
Monnier P, et al. Preoperative assessment and classification of benign laryngotracheal stenosis: a consensus paper of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2015;272(10):2885–96.
Yoshimatsu Y, et al. Difficult intubation due to unknown congenital tracheal stenosis in the adult: a case report and literature review. J Thorac Dis. 2018;10(2):E93.
Mehta PA, Sharma G, Congenital pulmonary airway malformation. StatPearls, 2021.
Shenoy L, Nileshwar A. Postintubation tracheal stenosis: a devastating complication. Indian J Respir Care. 2019;8(2):69.
Roushdy MM, Abdel-Ghaffar HS, Saleh AEM. Factors affecting occurrence of post intubation and post tracheostomy tracheal stenosis in intensive care units. Egypt J Ear Nose Throat Allied Sci. 2020;21(1):1–4.
Ulusan A, et al. Surgical treatment of postintubation tracheal stenosis: a retrospective 22-patient series from a single center. Asian J Surg. 2018;41(4):356–62.
Puchalski J, Musani AI. Tracheobronchial stenosis: causes and advances in management. Clin Chest Med. 2013;34(3):557–67.
Shin B, et al. Clinical implications of differentiating between types of post-tracheostomy tracheal stenosis. J Thorac Dis. 2017;9(11):4413.
Songu M, Ozkul Y. Risk factors for adult postintubation tracheal stenosis. J Craniofac Surg. 2019;30(5):e447–50.
Murgu SD, et al. Central airway obstruction: benign strictures, tracheobronchomalacia, and malignancy-related obstruction. Chest. 2016;150(2):426–41.
Parshin A, et al. Long-term postoperative outcomes in patients with cicatricial tracheal stenosis depending on surgical approach. Khirurgiia. 2021;1:5–14.
Ng J, et al. Clinical features and treatment outcomes of airway foreign body aspiration in adults. J Thorac Dis. 2019;11(3):1056.
Lee KCH, et al. Long-term outcomes of tracheobronchial stenosis due to tuberculosis (TSTB) in symptomatic patients: airway intervention vs. conservative management. J Thorac Dis. 2020;12(7):3640.
Bugalho A. Management of subglottic stenosis and subglottic stenosis in systemic disease. In: Principles and practice of interventional pulmonology, 2013. Springer, p 409–20
Elwany S, et al. A myriad of scleroma presentations: the usual and unusual. Head Neck Pathol. 2020;14(3):588–92.
Bacon JL, Patterson CM, Madden BP. Indications and interventional options for non-resectable tracheal stenosis. J Thorac Dis. 2014;6(3):258–70.
Quinn KA, et al. Subglottic stenosis and endobronchial disease in granulomatosis with polyangiitis. Rheumatology. 2019;58(12):2203–11.
Terrier B, et al. Granulomatosis with polyangiitis: endoscopic management of tracheobronchial stenosis: results from a multicentre experience. Rheumatology. 2015;54(10):1852–7.
Fishman AP, Elias JA. Fishman’s pulmonary diseases and disorders. New Yrok: McGraw-Hill Medical; 2008.
Mamatha S, et al. Diagnostic validity of computed tomography thorax in comparison with fibreoptic bronchoscopy for endobronchial lesions. J Clin Diagn Res 2021;15(3).
Thillai M, et al. BTS clinical statement on pulmonary sarcoidosis. Thorax. 2021;76(1):4–20.
Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. 2021;385(11):1018–32.
Schräder C, Lohmann J. Successful therapy with etanercept in relapsing polychondritis. Z Rheumatol. 2010;69(4):356–8.
Abdwani R, Kolethekkat AA, Al Abri R. Refractory relapsing polychondritis in a child treated with antiCD20 monoclonal antibody (rituximab): first case report. Int J Pediatric Otorhinolaryngol. 2012;76(7):1061–4.
Wu X, et al. Long-term outcome of metallic stenting for central airway involvement in relapsing polychondritis. Ann Thorac Surg. 2019;108(3):897–904.
Wallis C et al., ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J 2019;54(3).
Buitrago DH, et al. Current concepts in severe adult tracheobronchomalacia: evaluation and treatment. J Thorac Dis. 2017;9(1):E57.
Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology. 2006;11(4):388–406.
Casas DB, et al. Non-malignant central airway obstruction. Archivos de Bronconeumología (English Edition). 2014;50(8):345–54.
Majid A, et al. Short-term use of uncovered self-expanding metallic airway stents for severe expiratory central airway collapse. Respiration. 2016;92(6):389–96.
Grillo HC. Surgery of the trachea and bronchi. 2004: PMPH-USA.
Sarihan S, et al., Radiotherapy in patients with trachea tumours: a retrospective study and literature review. Turk J Oncol, 2020;35(2).
Cavaliere S, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest. 1996;110(6):1536–42.
Semaan R, Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J Thorac Dis. 2015;7(Suppl 4):S352.
Rosado de Castro PH, et al. Recurrent respiratory papillomatosis. QJM Int J Med. 2018;111(11):823–4.
Madhusudhana R, et al. Multinodular goiter with retrosternal extension causing airway obstruction: management in intensive care unit and operating room. Indian J Crit Care Med. 2014;18(8):543.
Kara İ, et al. Bilateral laryngocele causing epiglottic deformity and upper airway obstruction. Turk Arch Otorhinolaryngol. 2019;57(2):99.
Varsak YK, Eryilmaz MA, Arbag H. Dysphagia and airway obstruction due to large cervical osteophyte in a patient with ankylosing spondylitis. J Craniofac Surg. 2014;25(4):1402–3.
Petrella F, Spaggiari L. Postpneumonectomy syndrome: An old challenge for new technologies. J Thorac Cardiovasc Surg. 2018;155(4):e139–40.
Ryu YJ, et al. Comparison of natural and Dumon airway stents for the management of benign tracheobronchial stenoses. Respirology. 2006;11(6):748–54.
Ayub A, Al-Ayoubi AM, Bhora FY. Stents for airway strictures: selection and results. J Thorac Dis. 2017;9(Suppl 2):S116.
Aravena C, et al. Idiopathic subglottic stenosis: a review. J Thorac Dis. 2020;12(3):1100.
Terra RM, et al. Idiopathic tracheal stenosis: successful outcome with antigastroesophageal reflux disease therapy. Ann Thorac Surg. 2008;85(4):1438–9.
Young RP, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–6.
Mitchell PD, Kennedy MP. Bronchoscopic management of malignant airway obstruction. Adv Ther. 2014;31(5):512–38.
Rosell A, Stratakos G. Therapeutic bronchoscopy for central airway diseases. Eur Respir Rev 2020;29(158).
Holden VK, Channick CL. Management of benign central airway obstruction. 2018.
Galluccio G, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg. 2009;35(3):429–33.
Siciliani A, Rendina EA, Ibrahim M. State of the art in tracheal surgery: a brief literature review. Multidiscip Respir Med. 2018;13(1):1–7.
Özdemir C et al. Endoscopic and surgical treatment of benign tracheal stenosis: a multidisciplinary team approach. Ann Thor Cardiovasc Surg 2018; p. oa. 18-00073.
Arami S, Jabbardarjani H, Masjedi M. Treatment of post-intubation tracheal stenosis with the Nd-YAG laser at the NRITLD. Crit Care. 2005;9(1):1–1.
Dunlap DG, et al. Interventional therapies for central airways. J Thorac Imaging. 2019;34(4):W49–59.
Gurău P. Flexible endoscopic laser surgery for early glottic carcinoma. Am J Otolaryngol. 2021;42(5):103020.
Zozzaro M, Harirchian S, Cohen EG. Flexible fiber CO2 laser ablation of subglottic and tracheal stenosis. Laryngoscope. 2012;122(1):128–30.
Veen EJ, Dikkers FG. Topical use of MMC in the upper aerodigestive tract: a review on the side effects. Eur Arch Otorhinolaryngol. 2010;267(3):327–34.
Iyoda A, et al. Contributions of Airway Stent for Long-term Outcome in Patients With Malignant Central Airway Stenosis or Obstruction. J Bronchol Intervent Pulmonol. 2021;28(3):228–34.
Chen D-F, et al. Long-term efficacy and safety of the Dumon stent for benign tracheal stenosis: a meta-analysis. J Thorac Dis. 2021;13(1):82.
McRae K. Tracheal stents. In: Cohen's comprehensive thoracic anesthesia, 2022. Elsevier, p. 741–56
Dahlqvist C et al. Fully covered metallic stents for the treatment of benign airway stenosis. Can Respir J 2016;2016.
McGrath EE, Warriner D, Anderson PB. Is stent insertion via flexible bronchoscopy a feasible alternative to surgery in inoperable thyroid related tracheobronchial stenosis? J Thorac Dis. 2013;5(3):302.
Gaafar AH, Shaaban AY, Elhadidi MS. The use of metallic expandable tracheal stents in the management of inoperable malignant tracheal obstruction. Eur Arch Otorhinolaryngol. 2012;269(1):247–53.
Saito Y, Imamura H. Airway stenting. Surg Today. 2005;35(4):265–70.
Ernst A, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest. 2007;132(2):609–16.
Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg. 2007;84(6):1870–7.
Lischke R, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg. 2011;40(3):619–24.
Liu K-S, et al. Experimental absorbable stent permits airway remodeling. J Thorac Cardiovasc Surg. 2011;141(2):463–8.
Wang H et al. Overview of degradable polymer materials suitable for 3d printing bio-stent. In: Advances in graphic communication, printing and packaging technology and materials. 2021. Springer, p. 815–21
Yeazel TR, Becker ML. Advancing toward 3D printing of bioresorbable shape memory polymer stents. Biomacromol. 2020;21(10):3957–65.
Saji H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. 2010;11(4):425–8.
Santos R, et al. Bronchoscopic palliation of primary lung cancer: single or multimodality therapy? Surg Endosc Other Intervent Techn. 2004;18(6):931–6.
Cavaliere S, Foccoli P, Toninelli C. Curative bronchoscopic laser therapy for surgically resectable tracheobronchial tumors: personal experience. J Bronchol Intervent Pulmonol. 2002;9(2):90–5.
Guibert N, Saka H, Dutau H. Airway stenting: Technological advancements and its role in interventional pulmonology. Respirology. 2020;25(9):953–62.
Lim SY, et al. Prognostic factors for endotracheal silicone stenting in the management of inoperable post-intubation tracheal stenosis. Yonsei Med J. 2012;53(3):565–70.
Dhasmana A, Singh A, Rawal S. Biomedical grafts for tracheal tissue repairing and regeneration “Tracheal tissue engineering: an overview.” J Tissue Eng Regen Med. 2020;14(5):653–72.
Lin C-H, Su J-M, Hsu S-H. Evaluation of type II collagen scaffolds reinforced by poly (ε-caprolactone) as tissue-engineered trachea. Tissue Eng Part C Methods. 2008;14(1):69–77.
Bae S-W, et al. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int J Mol Sci. 2018;19(6):1624.
Macchiarini P, et al. Clinical transplantation of a tissue-engineered airway. The Lancet. 2008;372(9655):2023–30.
Machino R, et al. Replacement of rat tracheas by layered, trachea-like, scaffold-free structures of human cells using a bio-3D printing system. Adv Healthc Mater. 2019;8(7):1800983.
Gomperts BN. Induction of multiciliated cells from induced pluripotent stem cells. Proc Natl Acad Sci. 2014;111(17):6120–1.
Knaneh-Monem H, et al. Differential epithelial growth in tissue-engineered larynx and trachea generated from postnatal and fetal progenitor cells. Biochem Biophys Res Commun. 2019;510(2):205–10.
Walles T. Bioartificial tracheal grafts: can tissue engineering keep its promise? Expert Rev Med Dev. 2004;1(2):241–50.
Dikina AD, et al. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomaterials. 2015;52:452–62.
Freitag L, et al. Towards individualized tracheobronchial stents: technical, practical and legal considerations. Respiration. 2017;94(5):442–56.
Guedes F, Maurício AC, Bugalho A. Central airway stenosis: opening the path. Archivos de Bronconeumologia, 2020.
Acknowledgements
This research was supported by Projects PEst-OE/AGR/UI0211/2011 from FCT, and COMPETE 2020, from ANI – Projetos ID&T Empresas em Copromoção, by the project “insitu.Biomas – Reinvent biomanufacturing systems by using an usability approach for in situ clinic temporary implants fabrication” with the reference POCI-01-0247-FEDER-017771, by the project “Print-on-Organs – Engineering bioinks and processes for direct printing on organs” with the reference POCI-01-0247-FEDER-033877, and by the project “Bone2Move – Development of “in vivo” experimental techniques and modelling methodologies for the evaluation of 4D scaffolds for bone defect in sheep model: an integrative research approach” with the reference POCI-01-0145-FEDER-031146. Mariana Vieira Branquinho (SFRH/BD/146172/2019), Ana Catarina Sousa (SFRH/BD/146689/2019), and Rui Damásio Alvites (SFRH/BD/116118/2016), acknowledge FCT, for financial support.
Author information
Authors and Affiliations
Contributions
FG, MVB, ACS, RDA, AB and ACM conceived the project and the main conceptual ideas and contributed to the writing of the manuscript. FG performed the procedures of the clinical cases. MVB and RDA designed the figures. ACM and AB supervised the discussion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guedes, F., Branquinho, M.V., Sousa, A.C. et al. Central airway obstruction: is it time to move forward?. BMC Pulm Med 22, 68 (2022). https://doi.org/10.1186/s12890-022-01862-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-01862-x