Abstract
Background
A balloon occlusion technique is suggested for use in cryobiopsy for interstitial lung diseases because of the bleeding risk. However, it may interfere with selection of the involved bronchus for peripheral pulmonary lesions (PPLs). A two-scope technique, in which two scopes are prepared and hemostasis is started using the second scope immediately after cryobiopsy, has also been reported. This study aimed to evaluate the safety and diagnostic utility of transbronchial cryobiopsy using the two-scope technique for PPLs.
Methods
Data of patients who underwent conventional biopsy followed by cryobiopsy using the two-scope technique for PPLs from November 2019 to March 2021 were collected. The incidence of complications and risk factors for clinically significant bleeding (moderate to life-threatening) were investigated. Diagnostic yields were also compared among conventional biopsy, cryobiopsy, and the combination of them.
Results
A total of 139 patients were analyzed. Moderate bleeding occurred in 25 (18.0%) patients without severe/life-threatening bleeding. Although five cases required transbronchial instillation of thrombin, all bleeding was completely controlled using the two-scope technique. Other complications included two pneumothoraces and one asthmatic attack. On multivariable analysis, only ground-glass features (P < 0.001, odds ratio: 9.30) were associated with clinically significant bleeding. The diagnostic yields of conventional biopsy and cryobiopsy were 76.3% and 81.3%, respectively (P = 0.28). The total diagnostic yield was 89.9%, significantly higher than conventional biopsy alone (P < 0.001).
Conclusions
The two-scope technique provides useful hemostasis for safe cryobiopsy for PPLs, with a careful decision needed for ground-glass lesions.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Lung cancer is the leading cause of cancer-related deaths worldwide. Since the widespread use of low-dose computed tomography (CT) screening has promised to reduce lung cancer mortality, the detection and diagnosis rates of peripheral pulmonary lesions (PPLs) have increased [1, 2]. PPLs have a high incidence of early-stage lung cancer and can also represent a wide variety of benign conditions [3]. Therefore, precise pathological confirmation for PPLs with high-quality tissue specimens obtained using non-surgical biopsy is preferred to avoid unnecessary surgical resections [4, 5]. In addition, even in the case of advanced-stage disease, accounting for 65% of non-small cell lung cancer (NSCLC) [6], adequate amounts of tissue specimens without crush artifacts are required, because identification of the tumor’s immunohistochemical and molecular characteristics is necessary for its appropriate management [7, 8]. However, obtaining large and well-preserved specimens using conventional biopsy during bronchoscopy, a minimally invasive and well-established procedure for diagnosing PPLs, can be difficult and leads to severe clinical limitations for determining treatment plans for both early and advanced stages [9, 10].
Cryobiopsy is emerging as a relatively new technique for collecting larger tissue specimens with fewer crush artifacts during bronchoscopy compared to conventional biopsy [11, 12]. Its diagnostic utility for interstitial lung diseases (ILDs) and endobronchial malignancies has been reported [13, 14]. Moreover, the utility of the quantitative and pathological advantages of cryobiopsy for treatment plans in lung cancer may facilitate the use of cryobiopsy for PPLs [15]. However, the technique has been acknowledged to be challenging for PPLs, because the optimal technique to control bleeding caused by cryobiopsy is unknown. Bleeding is one of the main complications associated with cryobiopsy [16]. Although the prophylactic balloon occlusion technique has generally been used as a hemostatic technique for cryobiopsy of ILDs [17], preventive placement of the balloon during the procedure is detrimental in PPLs because it interferes with bronchoscope manipulation and guiding of the devices such as radial endobronchial ultrasound (R-EBUS), guide sheath (GS), and the cryoprobe into the correct bronchus route toward the targeted lesion. Thus, other ideal and feasible techniques for both bleeding control and endoscopic maneuverability are required to use cryobiopsy for PPLs. In addition, to reduce severe hemorrhage associated with cryobiopsy for PPLs, it is essential to confirm the risk factors for bleeding.
The two-scope technique is an alternative method of hemostasis for cryobiopsy of ILDs; it has established safety and diagnostic utility [18]. It allows physicians to control bleeding promptly and manipulate devices flexibly during the procedure. This study evaluated the safety and diagnostic utility of transbronchial cryobiopsy using the two-scope technique for PPLs. To further assess safety with respect to bleeding, predictive factors for clinically significant bleeding due to cryobiopsy were also examined.
Methods
Patients
The medical records of consecutive patients who underwent cryobiopsy using the two-scope technique for PPLs suspected to be lung cancer at Osaka City University Hospital between November 2019 and March 2021 were retrospectively reviewed. The study was approved by the Ethics Committee of Osaka City University Hospital (protocol no. 2021-056). Written informed consent was obtained from all patients prior to bronchoscopy.
Bronchoscopy equipment and conventional biopsy procedure
All bronchoscopies were performed using a bronchoscope (BF-P260F/BF-P290 as a thin scope or BF-1T260/BF-1TQ290 as a thick scope, Olympus, Tokyo, Japan) in combination with an R-EBUS probe (UM-S20-17S or UM-S20-20R, Olympus) and a cryoprobe (1.9 mm 20402-040 or 2.4 mm 20402-032, Erbe Elektromedizin GmbH, Germany) under local anesthesia with a combination of pethidine hydrochloride and midazolam for conscious sedation. A 2.55-mm GS kit (K-203, Olympus) was used when the thick scope was selected. A 1.9-mm cryoprobe was used through the working channel of the thin scope or the GS combined with the thick scope. A 2.4-mm cryoprobe was used with the thick scope after removing the GS. Virtual bronchoscopy (VB) and virtual fluoroscopy (Ziostation2®, Ziosoft Ltd., Tokyo, Japan) were created using thin-section CT (TSCT) images [19, 20]. The type of bronchoscope and the size of cryoprobe were selected by each operator based on CT images and VB navigation. Though treatment with antiplatelet or anticoagulant agents was discontinued before bronchoscopy, bridging anticoagulation therapy was performed in patients with high thromboembolic risk [21].
Before the procedures, all patients were intubated fiberoptically with an 8.0- or 8.5-mm uncuffed endotracheal tube (Portex® Siliconised PVC Oral/Nasal Uncuffed Tracheal Tube, Smiths Medical, Minneapolis, MN, USA). An R-EBUS probe was inserted through the working channel of the bronchoscope and advanced towards the target PPL by referring to VB, virtual fluoroscopy, and X-ray fluoroscopy (VersiFlex VISTA®, Hitachi, Japan). When assessing the location of the R-EBUS probe against the target lesion, the images were categorized as “within”, “adjacent to”, or “invisible” [22]. After detecting the target lesion, conventional biopsies using a forceps (FB-15C-1 or FB-231D, Olympus) or an aspiration needle (NA-1C-1, Olympus) were performed. Forceps biopsies were repeated 5–10 times to collect tissue samples [23, 24]. The occurrence of pneumothorax was routinely checked by fluoroscopy at every biopsies.
The two-scope technique for cryobiopsy
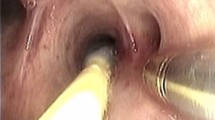
After completing conventional biopsies, cryobiopsy using the two-scope technique was subsequently performed at the same sites as the conventional biopsies. The technique was modified a little to a previously reported method to suit cryobiopsy for PPLs [18] (Fig. 1). After guiding the cryoprobe through the bronchoscope towards the target lesion, the tip of the scope was wedged as close as possible to the target lesion under fluoroscopic guidance. The connector of the scope was then detached from the light source of an endoscope system while the operator kept the cryoprobe in place. Next, an assistant connected the second thick scope to control bleeding to the light source. The suction tube was also detached from the first scope for cryobiopsy and connected to the second scope for hemostasis. The final adjustment of the cryobiopsy site was made by matching the views of anterior–posterior and 45-degree right/left anterior oblique between X-ray fluoroscopy and virtual fluoroscopy. After the assistant was ready for hemostasis, the cryoprobe was cooled for 3–6 s. The tissue sample attached to the frozen tip was removed by extracting the cryoprobe together with the first scope by the operator. The second scope was then immediately inserted towards the cryobiopsy site by the assistant, and the patient was simultaneously rotated to the biopsy-side down lateral decubitus position. Hemostatic agents were injected when bronchoscopic suction alone was insufficient to stop bleeding. The collected tissue samples were thawed in normal saline and fixed in formalin. Rapid on-site cytologic evaluation was optional. The number of conventional biopsies and cryobiopsies was determined during the procedure in the discretion by the operator and the assistant, based on the amount of bleeding after each biopsy, the size of the obtained tissue sample, the results of the rapid on-site cytologic evaluation, and whether next-generation sequencing analysis was performed. Chest radiography was routinely performed at least until the next day to assess complications.
Transbronchial cryobiopsy using the two-scope technique for a peripheral pulmonary lesion. After passing the cryoprobe to the target lesion, the bronchoscope’s tip is wedged and fixed into the involved bronchus under X-ray fluoroscopy by the operator (arrowhead) (a). The bronchoscope for cryobiopsy is detached from the light source while the operator (arrowhead) keeps the cryoprobe with the bronchoscope in place, and an assistant (arrow) connects the second thick scope for hemostasis to the light source and suction tube (b). Cryobiopsy is performed under fluoroscopic guidance by the operator (arrowhead), and an assistant (arrow) immediately inserts the second thick scope through the endotracheal tube (c). An assistant controls bleeding, and the patient is simultaneously rotated to the biopsy-side down lateral decubitus position (d)
Complications
The following complications associated with the procedure were reviewed: bleeding, pneumothorax, pulmonary infection, hypoxia, respiratory failure, and other life-threatening events. The severity of bleeding was determined on a scale of four steps according to the standardized definitions of bleeding after transbronchial lung biopsy [25]: mild, bleeding requiring less than 1 min of suctioning or wedging of the bronchoscope; moderate, bleeding requiring more than 1 min of suctioning or wedging or instillation of cold saline, diluted vasoactive substances, or thrombin; severe, bleeding requiring selective intubation using an endotracheal tube or balloon/bronchial blocker for less than 20 min; and life-threatening, bleeding requiring persistent selective intubation for more than 20 min, blood transfusion, bronchial artery embolization, or intensive care therapy. Moreover, moderate to life-threatening was defined as clinically significant bleeding.
Diagnostic criteria
The final diagnosis was determined based on the histological findings. Histological findings of malignancy were considered diagnostic. Cases of benign conditions were considered diagnostic when specific benign findings (i.e., granuloma, fibrosis, and inflammation) on histology or positive microbiological cultures were found and the subsequent clinical courses were compatible after a 6-month follow-up period. Cases without significant histological and/or microbiological findings and that decreased in size after at least 6 months of follow-up by CT were regarded as having inflammation. Patients who could not be diagnosed using bronchoscopy were diagnosed using surgical biopsy, repeat bronchoscopy, or CT-guided biopsy. Diagnostic yield was defined as the percentage of diagnostic cases.
Assessment and statistics
To evaluate the safety and diagnostic utility, the incidence of complications and diagnostic yield were respectively compared between conventional biopsies and cryobiopsy using the McNemar-Bowker test for categorical data. In terms of diagnostic utility, the total diagnostic yield, which was the combination of each biopsy technique, was also compared with that of conventional biopsy alone. The diagnostic yield of conventional biopsy and cryobiopsy for each R-EBUS image obtained during the procedure was also compared.
Furthermore, factors affecting the occurrence of clinically significant bleeding (moderate, severe, and life-threatening) related to the procedure were analyzed using Fisher’s exact test. The clinical variables analyzed were chosen from those previously reported as risk factors for bleeding and empirically predicted factors as follows: patient age, sex, body height (≤ 170 cm or > 170 cm) [16], body mass index (≤ 25 kg/m2 or > 25 kg/m2) [26], lesion diameter (≤ 20 mm or > 20 mm), lobar position (right upper lobe/left upper segment, right middle lobe/left lingula, or bilateral lower lobes), location area (outer or inner), lesion appearance on CT [solid nodule or ground-glass nodule (GGN)], presence of the bronchus sign (positive or negative), the R-EBUS findings obtained (within, adjacent to, or invisible), presence of comorbid conditions [chronic obstructive pulmonary disease (COPD), ILD, and bronchial asthma], perioperative heparin bridging therapy (performed or not), cryoprobe size (1.9 mm or 2.4 mm), number of cryobiopsies performed (1 or ≥ 2), freezing time (≤ 5 s or > 5 s) [27], and sample size obtained by cryobiopsy (≤ 15 mm2 or > 15 mm2). Lesion diameter was determined based on the longest diameter on axial TSCT images. Location area was designated as “outer” if the lesion was in the outer-third ellipse or “inner” if the lesion was within the inner or middle third ellipse [19]. A GGN was defined as a peripheral pulmonary lesion with an area of increased attenuation and retention of the underlying vessels and bronchi on TSCT images [28]. The bronchus sign was a finding of the involved bronchus leading to the target lesion on TSCT [29]. The sample size was assessed using Olympus OlyVIA (version 3.2, Olympus) to enclose the biopsy section after scanning the hematoxylin and eosin (HE)-stained slides using a virtual slide system (VS-120, Olympus). These cut-offs were determined based on either our clinical experience (sample size) or previous reports.
Selected variables with P < 0.20 in univariable analyses were used in the analysis of multivariable logistic regression. Descriptive statistics expressed are numbers, percentages, and medians (range). All statistical tests were two-sided, and P < 0.05 was considered significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [30].
Results
During the study period, 141 patients underwent transbronchial cryobiopsy using the two-scope technique for PPLs. Of them, cryobiopsy could not be completed in two cases because the tip of the cryoprobe could not be passed to the target lesion. The remaining 139 patients were enrolled and analyzed; a summary of patients’ characteristics are shown in Table 1. The median (range) size was 25.8 (8.7–72.7) mm. Most of the PPLs were located in the right upper lobe/left upper segment (50.4%) and at the outer area (61.8%). Lesions appearing on CT as solid nodules and as GGNs were seen at rates of 74.1% and 25.9%, respectively. Moreover, 20.1% had respiratory comorbidities (11.5% COPD, 4.3% ILD, and 4.3% bronchial asthma), and 3.6% received preoperative bridging anticoagulation therapy.
The results of bronchoscopy are shown in Table 2. The median (range) procedure time was 28.2 (9.9–55.5) minutes, and the overall diagnostic yield was 89.9%. Figure 2 shows a representative case of successful transbronchial cryobiopsy using the technique for primary lung cancer.
Representative case of transbronchial cryobiopsy using the two-scope technique for a peripheral pulmonary lesion. Computed tomography evaluation of an 82-year-old woman showing a part-solid ground-glass nodule (GGN) in the right upper lobe. CT shows it is located at right S3 (a). The target lesion is approached using a thin scope guided by virtual bronchoscopy displaying the bronchus route “B3aiiαy” toward the target lesion (b). Radial endobronchial ultrasound (R-EBUS) showing a blizzard sign (c). The biopsies using forceps and cryoprobe are performed with reference to fluoroscopy and R-EBUS imaging (d). Cryobiopsy causes moderate bleeding that is stopped using bronchoscopic suctioning in 2 min (e). Adenocarcinoma is revealed by only the cryobiopsy specimen (hematoxylin–eosin stain, × 200) (f)
Regarding complications, conventional biopsies were not associated with any complications (Table 3). Although mild and moderate bleeding occurred in 42.4% and 18.0% of the patients, respectively, there were no cases of severe or life-threatening bleeding. Of the cases with moderate bleeding, five patients (3.6%) required thrombin instillation. The hemostatic bronchoscope entirely controlled all moderate bleeding episodes without requiring any other intervention. Cryobiopsy caused pneumothorax in two patients (1.4%), both of whom required thoracic drainage, an asthma attack in one patient (0.7%), and hypoxemia requiring oxygen inhalation for several minutes in three patients (2.2%). Of the three patients with hypoxemia, two were overlapped and caused by moderate bleeding, and the other was due to an asthma attack. There were no cases of pneumonia, lung abscesses, respiratory failure, or other severe complications.
The clinical factors associated with clinically significant bleeding (moderate, severe, and life-threatening) with cryobiopsy are shown in Table 4. In univariable analyses, the ground-glass feature of the lesion was a significant factor for clinically significant bleeding (47.2% vs. 7.8%, P < 0.001). Sample size over 15 mm2 also tended to have a higher bleeding rate, but there was no significant difference (27.8% vs. 14.6%, P = 0.084). In multivariable analysis, only the ground-glass feature of the lesion had a significant effect (OR 9.30, 95% CI 3.40–25.40, P < 0.001) on the clinically significant bleeding rate compared to a solid feature. There were no significant differences between GGNs and solid nodules in the following factors: visualization yields on R-EBUS, within, adjacent to, and invisible in 30.6% (11/36) vs. 39.8% (41/103), 66.7% (24/36) vs. 57.3% (59/103), and 2.8% (1/36) vs. 2.9% (3/103), respectively, P = 0.66; median (range) number of cryobiopsy samples taken, 1 (1–2) vs. 1 (1–3), P = 0.26; and median (range) specimen size of cryobiopsy, 11.2 (3.3–29.1) vs. 11.4 (5.2–23.5), P = 0.87.
Table 5 presents the final histological diagnoses and diagnostic yields of each biopsy technique. Most of the PPLs were finally diagnosed as adenocarcinoma (61.2%). The diagnostic yields of conventional biopsy and cryobiopsy were 76.3% and 81.3%, respectively (P = 0.28). Of them, 8.6% (12 cases) were diagnosable only by conventional biopsy, and 13.7% (19 cases) were diagnosable only by cryobiopsy. When each biopsy technique was combined, the total diagnostic yield was 89.9%, which was significantly higher than conventional biopsy alone (P < 0.001). Although there was no significant difference in the diagnostic yield between conventional biopsy and cryobiopsy when R-EBUS images were “within” (conventional biopsy 49/52, 94.2% vs. cryobiopsy 44/52, 84.6%; P = 0.18) and “invisible” (conventional biopsy 1/4, 25.0% vs. cryobiopsy 1/4, 25.0%; P-value not available), the diagnostic yield of cryobiopsy was significantly higher than that of conventional biopsy when R-EBUS images was “adjacent to” (conventional biopsy 56/83, 67.4% vs. cryobiopsy 68/83, 81.9%; P = 0.019). In the 14 nondiagnostic cases, the diagnosis was established by surgery in six patients, repeat bronchoscopy in three patients, and CT-guided needle biopsy in one patient. The remaining four were clinically suspected to have lung cancer but were being followed-up on CT without surgery at the patients’ request.
Discussion
The present study showed that the two-scope technique combined with cryobiopsy provides high safety, good hemostatic capacity, and high diagnostic yield (89.9%), which is associated with minimal complications from cryobiopsy for PPLs. Of 139 patients who underwent cryobiopsy using the two-scope technique, all bleeding episodes were completely controlled without additional bleeding control interventions, although moderate bleeding occurred in 25 patients (18.0%). Moreover, the lesion appearance on CT was shown to be a predictor of clinically significant bleeding after cryobiopsy, which may be helpful when selecting a biopsy technique.
Bleeding is a worrisome complication of cryobiopsy for PPLs, similar to ILDs [15,16,17]. In the present study, all bleeding episodes during cryobiopsy were controlled using the two-scope technique. This technique has the following advantages over the balloon method in terms of bleeding control. First, it allows easier hemostasis even in the bilateral upper lobe bronchi and B6, where balloon insertion is difficult [17]. Second, since there is no risk of balloon migration, further efficient endoscopic hemostasis can be achieved by combining it with postural change. Third, the amount of bleeding can be checked endoscopically in real-time, and hemostatic agents can be promptly added. Five cases with moderate bleeding required hemostatic agents and were easily controlled by prompt thrombin instillation in the present study. On the other hand, unlike ILD, cryobiopsy can be avoided in places with large blood vessels such as bronchial arteries by referring to R-EBUS images routinely checked in PPLs [31]. Furthermore, several (3–6) cryobiopsies from multiple areas and lobes are required to reduce sampling error in ILDs, whereas 1–2 specimens from one area are clinically sufficient for diagnosis of lung cancer and next-generation sequencing [32, 33]. In addition to effective hemostasis with the technique, the above differences in clinical backgrounds may have led to differences in the degree of bleeding between PPLs and ILDs with cryobiopsy. Accordingly, the results suggest that bleeding related to cryobiopsy for PPLs can be controlled using the two-scope technique. However, the preparation and immediate implementation of bleeding control interventions such as bronchial artery embolization and surgery are necessary for urgent life-threatening bleeding due to cryobiopsy [34].
One interesting finding was that the lesion appearance on CT was significantly associated with clinically significant bleeding. The present study did not find any significant relationship between clinically significant bleeding and short height and sex, previously reported as risk factors for bleeding with cryobiopsy of ILDs [16]. The present findings are difficult to explain by referring to previous reports on bronchoscopy. However, this is in line with several previous reports concerning CT-guided biopsy that identified the ground-glass feature of the lesion as an independent predictive factor for more hemorrhagic complications [35,36,37,38]. These reports suggested that this is due to the preserved bronchovascular structures and less compact histological nature of GGNs, which show minimal histological changes, such as adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic-predominant adenocarcinoma; preserved bronchovascular structures with less tumor invasion can increase the risk of iatrogenic access between an injured bronchus and pulmonary vessels associated with biopsy. Furthermore, the lack of compactness can reduce the compression effect on the biopsy site by the lesion itself [35]. These hypotheses can similarly explain our findings that the ground-glass feature of the lesion was a predictor of higher bleeding risk associated with cryobiopsy, which may help physicians decide whether to perform cryobiopsy.
Regarding other complications, pneumothorax occurred in 1.4% (2/141), whereas previous studies have reported a high incidence of pneumothorax in ILDs [39]. These two cases had PPLs located near the visceral pleura. The two-scope technique is free from the balloon blocker’s obstacle, allowing physicians to insert instruments into the correct bronchial route and obtain samples from the precise location for PPLs referring to VB and R-EBUS. Accordingly, the incidence rate of pneumothorax was lower than that previously reported for ILDs, and the complication rate, except for bleeding related to cryobiopsy, was maintained at the same level as in conventional biopsies. We presume that the complication rate of cryobiopsy for PPLs is comparable to that of conventional forceps biopsy, except for bleeding.
In the current study, most of the PPLs were detected using R-EBUS (97.1%, 135/139); however, the diagnostic yield of conventional biopsy remained at 76.3%. Thus, the diagnostic value of conventional biopsy has been insufficient due to its disadvantages (e.g., small crushed tissue specimen with artifacts, forceps’ weak mechanical strength of grasping, and collection site being limited in a forward direction). In contrast, cryobiopsy can harvest large specimens of good quality in a 360° manner laterally by freezing the surrounding peripheral lung tissue. Accordingly, 19 cases (13.7%) were diagnosed only by cryobiopsy, and the diagnostic yield when R-EBUS image was “adjacent to” and the overall diagnostic yield (89.9%) improved compared to conventional biopsy alone. Thus, cryobiopsy is considered a useful biopsy technique for diagnosing PPLs. However, 12 cases (8.6%) were diagnosed only be conventional biopsy. Presumably, the hardness of the cryoprobe tip caused misalignment in selecting the peripheral bronchus near the target lesion in these cases. The hardness caused interruption of cryobiopsy in two cases. Therefore, we assume that conventional biopsy and cryobiopsy play complementary roles in diagnosing PPLs. It may be better to perform both biopsy techniques to improve bronchoscopy diagnostic yield in PPLs at this stage. A thinner flexible cryoprobe, which has been recently developed and has shown good diagnostic utility in animal studies, may overcome the problems caused by the cryoprobe’s hardness and improve the diagnostic yield of cryobiopsy for PPLs in the future [40].
This study had several limitations. First, it was retrospective, non-randomized, and conducted at a single institution, increasing the probability of patient selection bias. Furthermore, multiple analyses may have occurred in multivariable analysis. It was difficult to estimate the risk factors for bleeding caused by cryobiopsy from previous bronchoscopy reports using conventional biopsy, which resulted in the increased number of clinical factors included in the analysis. Second, a direct comparison between the two-scope technique and the balloon occlusion method for cryobiopsy was not performed in this study. Finally, cryobiopsy was performed after conventional biopsy in all cases. The effect of conventional biopsy on bleeding related to cryobiopsy was not accurately evaluated. Further prospective studies are required to determine the optimal bleeding control technique for cryobiopsy for PPLs.
Conclusions
The two-scope technique is a feasible hemostatic technique for transbronchial cryobiopsy for PPLs, providing safety and high diagnostic performance. We suggest that physicians should consider evaluating the lesion appearance on CT as a predictive factor for clinically significant bleeding related to cryobiopsy for PPLs.
Availability of data and materials
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Abbreviations
- CT:
-
Computed tomography
- PPLs:
-
Peripheral pulmonary lesions
- NSCLC:
-
Non-small cell lung cancer
- ILD:
-
Interstitial lung disease
- R-EBUS:
-
Radial endobronchial ultrasound
- GS:
-
Guide sheath
- VB:
-
Virtual bronchoscopy
- TSCT:
-
Thin-section computed tomography
- GGN:
-
Ground-glass nodule
- COPD:
-
Chronic obstructive pulmonary disease
References
Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, Jones GC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980–91.
Flores R, Bauer T, Aye R, Andaz S, Kohman L, Sheppard B, Mayfield W, Thurer R, Smith M, Korst R, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg. 2014;147(5):1619–26.
Field JK, Smith RA, Aberle DR, Oudkerk M, Baldwin DR, Yankelevitz D, Pedersen JH, Swanson SJ, Travis WD, Wisbuba II, et al. International association for the study of lung cancer computed tomography screening workshop 2011 report. J Thorac Oncol. 2012;7(1):10–9.
Barta JA, Henschke CI, Flores RM, Yip R, Yankelevitz DF, Powell CA. Lung cancer diagnosis by fine needle aspiration is associated with reduction in resection of nonmalignant lung nodules. Ann Thorac Surg. 2017;103(6):1795–801.
Lim W, Ridge CA, Nicholson AG, Mirsadraee S. The 8(th) lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg. 2018;8(7):709–18.
Korpanty GJ, Graham DM, Vincent MD, Leighl NB. Biomarkers that currently affect clinical practice in lung cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol. 2014;4:204.
Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9(6):499–506.
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Ishikawa Y, Wistuba I, Flieder DB, Franklin W, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137(5):668–84.
Kim L, Tsao MS. Tumour tissue sampling for lung cancer management in the era of personalised therapy: what is good enough for molecular testing? Eur Respir J. 2014;44(4):1011–22.
Hetzel J, Hetzel M, Hasel C, Moeller P, Babiak A. Old meets modern: the use of traditional cryoprobes in the age of molecular biology. Respiration. 2008;76(2):193–7.
Yarmus L, Akulian J, Gilbert C, Illei P, Shah P, Merlo C, Orens J, Feller-Kopman D. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: a pilot safety study. Chest. 2013;143(3):621–6.
Schumann C, Hetzel J, Babiak AJ, Merk T, Wibmer T, Moller P, Lepper PM, Hetzel M. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg. 2010;140(2):417–21.
Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S, Ryu JH, Fruchter O, Piciucchi S, Dubini A, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95(3):188–200.
Matsumoto Y, Nakai T, Tanaka M, Imabayashi T, Tsuchida T, Ohe Y. Diagnostic outcomes and safety of cryobiopsy added to conventional sampling methods: an observational study. Chest. 2021;160:1890–901.
Hetzel J, Eberhardt R, Petermann C, Gesierich W, Darwiche K, Hagmeyer L, Muche R, Kreuter M, Lewis R, Ehab A, et al. Bleeding risk of transbronchial cryobiopsy compared to transbronchial forceps biopsy in interstitial lung disease—a prospective, randomized, multicentre cross-over trial. Respir Res. 2019;20(1):140.
Colella S, Haentschel M, Shah P, Poletti V, Hetzel J. Transbronchial lung cryobiopsy in interstitial lung diseases: best practice. Respiration. 2018;95(6):383–91.
Sriprasart T, Aragaki A, Baughman R, Wikenheiser-Brokamp K, Khanna G, Tanase D, Kirschner M, Benzaquen S. A single US center experience of transbronchial lung cryobiopsy for diagnosing interstitial lung disease with a 2-scope technique. J Bronchol Interv Pulmonol. 2017;24(2):131–5.
Matsumoto Y, Izumo T, Sasada S, Tsuchida T, Ohe Y. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J. 2017;11(2):185–92.
Nakai T, Izumo T, Matsumoto Y, Tsuchida T. Virtual fluoroscopy during transbronchial biopsy for locating ground-glass nodules not visible on X-ray fluoroscopy. J Thorac Dis. 2017;9(12):5493–502.
Herman DD, Thomson CC, Brosnhan S, Patel R, Trosini-Desert V, Bilaceroglu S, Poston JT, Liberman M, Shah PL, Ost DE, et al. Risk of bleeding in patients undergoing pulmonary procedures on antiplatelet or anticoagulants: a systematic review. Respir Med. 2019;153:76–84.
Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, Murayama M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126(3):959–65.
Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N, Oizumi S, Nishimura M. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132(2):603–8.
Takeyasu Y, Yoshida T, Motoi N, Teishikata T, Tanaka M, Matsumoto Y, Shinno Y, Okuma Y, Goto Y, Horinouchi H, et al. Feasibility of next-generation sequencing (Oncomine DX Target Test) for the screening of oncogenic mutations in advanced non-small-cell lung cancer patients. Jpn J Clin Oncol. 2021;51(7):1114–22.
Folch EE, Mahajan AK, Oberg CL, Maldonado F, Toloza E, Krimsky WS, Oh S, Bowling MR, Benzaquen S, Kinsey CM, et al. Standardized definitions of bleeding after transbronchial lung biopsy: a Delphi consensus statement from the Nashville working group. Chest. 2020;158(1):393–400.
Suemitsu R, Sakoguchi T, Morikawa K, Yamaguchi M, Tanaka H, Takeo S. Effect of body mass index on perioperative complications in thoracic surgery. Asian Cardiovasc Thorac Ann. 2008;16(6):463–7.
Ing M, Oliver RA, Oliver BG, Walsh WR, Williamson JP. Evaluation of transbronchial lung cryobiopsy size and freezing time: a prognostic animal study. Respiration. 2016;92(1):34–9.
Kim TJ, Lee JH, Lee CT, Jheon SH, Sung SW, Chung JH, Lee KW. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol. 2008;190(1):234–9.
Gaeta M, Pandolfo I, Volta S, Russi EG, Bartiromo G, Girone G, La Spada F, Barone M, Casablanca G, Minutoli A. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol. 1991;157(6):1181–5.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Inomata M, Kuse N, Awano N, Tone M, Yoshimura H, Jo T, Minami J, Takada K, Muto Y, Fujimoto K, et al. Utility of radial endobronchial ultrasonography combined with transbronchial lung cryobiopsy in patients with diffuse parenchymal lung diseases: a multicentre prospective study. BMJ Open Respir Res. 2021;8(1):e000826.
Ravaglia C, Wells AU, Tomassetti S, Gurioli C, Gurioli C, Dubini A, Cavazza A, Colby TV, Piciucchi S, Puglisi S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med. 2019;19(1):16.
Tone M, Inomata M, Awano N, Kuse N, Takada K, Minami J, Muto Y, Fujimoto K, Kumasaka T, Izumo T. Comparison of adequacy between transbronchial lung cryobiopsy samples and endobronchial ultrasound-guided transbronchial needle aspiration samples for next-generation sequencing analysis. Thorac Cancer. 2021;12(2):251–8.
DiBardino DM, Haas AR, Lanfranco AR, Litzky LA, Sterman D, Bessich JL. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an academic medical center. Ann Am Thorac Soc. 2017;14(6):851–7.
Song YS, Park CM, Park KW, Kim KG, Lee HJ, Shim MS, Goo JM. Does antiplatelet therapy increase the risk of hemoptysis during percutaneous transthoracic needle biopsy of a pulmonary lesion? AJR Am J Roentgenol. 2013;200(5):1014–9.
Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology. 2014;271(1):291–300.
Tai R, Dunne RM, Trotman-Dickenson B, Jacobson FL, Madan R, Kumamaru KK, Hunsaker AR. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology. 2016;279(1):287–96.
Hwang EJ, Park CM, Yoon SH, Lim HJ, Goo JM. Risk factors for haemoptysis after percutaneous transthoracic needle biopsies in 4,172 cases: focusing on the effects of enlarged main pulmonary artery diameter. Eur Radiol. 2018;28(4):1410–9.
Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease: a systematic review and metaanalysis. Ann Am Thorac Soc. 2016;13(10):1828–38.
Yarmus LB, Semaan RW, Arias SA, Feller-Kopman D, Ortiz R, Bosmuller H, Illei PB, Frimpong BO, Oakjones-Burgess K, Lee HJ. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest. 2016;150(2):329–36.
Acknowledgements
The authors thank Dr. Kazuto Hirata for his clinical advice to carry out the study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
TN performed the procedures and designed the study, acquired, analyzed, and interpreted the data, and wrote the initial draft of the manuscript. YM contributed to the supervision of the procedures, the study design, interpretation of the data, and editing of the manuscript. YK and MO performed the pathological diagnoses. TW and KA drafted and edited the manuscript. KS contributed to statistical analyses. KO, YM, AO, and KS performed the procedures. TK assisted in the preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Osaka City University Hospital (protocol no. 2021-056), and the study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All participants provided written, informed consent prior to participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakai, T., Watanabe, T., Kaimi, Y. et al. Safety profile and risk factors for bleeding in transbronchial cryobiopsy using a two-scope technique for peripheral pulmonary lesions. BMC Pulm Med 22, 20 (2022). https://doi.org/10.1186/s12890-021-01817-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01817-8