Abstract
Background
Supplemental oxygen delivered with standard oxygen therapy (SOT) improves exercise capacity in patients with idiopathic pulmonary fibrosis (IPF). Although high-flow nasal cannula oxygen therapy (HFNC) improves oxygenation in other respiratory diseases, its impact on exercise performance has never been evaluated in IPF patients. We hypothesized that HFNC may improve exercise capacity in IPF subjects compared to SOT.
Methods
This was a prospective, crossover, pilot randomized trial that compared both oxygenation methods during a constant submaximal cardiopulmonary exercise test (CPET) in IPF patients with exertional oxygen saturation (SpO2) ≤ 85% in the 6-min walking test. The primary outcome was endurance time (Tlim). Secondary outcomes were muscle oxygen saturation (StO2) and respiratory and leg symptoms.
Results
Ten IPF patients [71.7 (6) years old, 90% males] were included. FVC and DLCO were 58 ± 11% and 31 ± 13% pred. respectively. Tlim during CPET was significantly greater using HFNC compared to SOT [494 ± 173 vs. 381 ± 137 s, p = 0.01]. HFNC also associated with a higher increase in inspiratory capacity (IC) [19.4 ± 14.2 vs. 7.1 ± 8.9%, respectively; p = 0.04], and a similar trend was observed in StO2 during exercise. No differences were found in respiratory or leg symptoms between the two oxygen devices.
Conclusions
This is the first study demonstrating that HFNC oxygen therapy improves exercise tolerance better than SOT in IPF patients with exertional desaturation. This might be explained by changes in ventilatory mechanics and muscle oxygenation. Further and larger studies are needed to confirm the benefits of HFNC in IPF patients and its potential usefulness in rehabilitation programs.
Similar content being viewed by others
Background
Idiopathic Pulmonary Fibrosis (IPF) is a progressive, chronic and fibrosing interstitial pneumonia with a poor prognosis [1]. IPF is clinically characterized by exertional dyspnea and hypoxemia, which is secondary to VA/Q mismatching and diffusing capacity limitation, and markedly worsens during exercise due mainly to the latter mechanism [2]. However, other factors including ventilatory inefficiency and peripheral muscle dysfunction can also contribute to exercise limitation in these patients [3]. Moreover, as a consequence of the associated restrictive ventilatory pattern, patients present a rapid and shallow breathing that becomes even more evident during exercise. This leads to a high minute ventilation at the expense of an elevated respiratory rate, with a progressive reduced capacity to increase tidal volume (VT), reflected by a low inspiratory capacity (IC) [4].
Although some small studies have demonstrated the presence of muscle dysfunction in IPF patients [5, 6] its causes are still poorly understood. As it occurs in chronic obstructive pulmonary disease (COPD) they probably would include physical inactivity due to exertional dyspnea, malnutrition, poor oxygen delivery, drug-induced myopathy and ageing [7]. In this regard, Wickerson et al. [8] recently demonstrated a clear muscle deoxygenation in peripheral muscles of IPF patients even at low workloads. However, oxygen therapy using standard delivery devices (nasal cannula, Venturi masks) (SOT) has recently been demonstrated to improve exercise capacity, increasing endurance time, reducing blood oxygen desaturation and dyspnea and even improving skeletal muscle metabolism in IPF patients [9, 10]. Nevertheless, it is broadly recognized in clinical practice that SOT has important limitations to achieving optimal levels of oxygenation in such patients during exercise [11]. It is worth noting, however, that the use of high-flow nasal cannula (HFNC) has become common in recent years for the treatment of patients with acute non-hypercapnic respiratory failure [12]. This system can deliver heated and humidified oxygen at high flow rates (up to 60 L/min), achieving high inspiratory fractions of oxygen (FIO2) [12], and producing higher physiological benefits compared with SOT devices [13]. Moreover, in subjects with stable COPD and severe ventilatory limitation for instance, the use of HFNC can better improve endurance time and blood oxygen saturation during exercise than SOT [14]. In contrast, these higher benefits did not appear to be present in a previous study that compared HFNC and SOT in an heterogeneous group that included different interstitial lung diseases [15]. However, there is a complete lack of previous studies assessing the potential advantages of HFNC in IPF in particular. Another aspect that has not been evaluated to date is the impact of HFNC on skeletal muscle oxygenation, and its relationship with potential improvements in exercise tolerance in IPF patients. Near-infrared spectroscopy (NIRS) has emerged in the last decade as a non-invasive method to assess skeletal muscle blood flow and oxygenation, thus approaching this tissue metabolic status in several respiratory diseases [16], including IPF [8]. We hypothesized that HFNC oxygen therapy could be more efficient than SOT in improving exercise tolerance in IPF patients with exertional desaturation. Therefore, we designed a crossover trial with the objective of comparing the effect of these two oxygen supplementation methods on endurance time (Tlim) in patients with stable IPF. Secondary end-points were peripheral muscle oxygen saturation (StO2) during exercise as well as dyspnea and leg symptoms during exertion.
Methods
Study participants
Patients with IPF diagnosis, according to the 2018 international consensus guidelines [1], were consecutively recruited from specialized Interstitial Lung Disease (ILD) clinics in two tertiary teaching hospitals. Eligible patients were those reaching a mean pulse oximeter oxygen saturation (SpO2) ≤ 85% (WirstOx2 TM Model 3150 Oximeter. Nonin Medical, INC. Plymouth, MN, USA) during the 6-min walking test (6MWT) performed at room air [17]. Exclusion criteria were fibrotic ILD other than IPF, coexistence of COPD, asthma or moderate-to-severe pulmonary hypertension [18], and inability to perform a cardiopulmonary exercise test (CPET) due to osteo-articular or cognitive limitations.
Study design
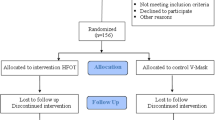
This was a randomized crossover clinical trial conducted in three visits (Fig. 1). Patients were included from March 2019 to February 2020 and were subsequently randomized. The study was approved by the local ethics committee, carried out according to the principles of the Declaration of Helsinki for human investigations and registered as a clinical trial (NCT04564664). All participants signed the appropriate informed consent prior to their inclusion.
An initial screening visit was performed according to the study protocol. Sociodemographic and clinical variables were also collected including GAP (Gender-Age-Physiology) and body mass (BMI) indices, individual comorbidities, Charlson comorbidity index and treatments received. Conventional pulmonary function tests and the 6MWT were performed according to international guidelines [17, 19, 20]. In addition, quadriceps and hand-grip strength were also measured following the previously described methodology [21], and the highest value of at least three correct maneuvers was chosen for both measurements.
First exercise evaluation
To assess maximum exercise capacity (WRmax) and to determine the FIO2 (Venturi mask) necessary to always maintain a SpO2 > 85%, a symptom-limited incremental CPET was performed by all patients. Subjects were randomized consecutively with a 1:1 allocation sequence to perform the first CPET with either HFNC or SOT.
Second and third evaluation
Following randomization all individuals performed two consecutive, submaximal CPET (75% of their WRmax) with at least 24 h apart. Half of them started with the HFNC O2 supplementation and the remaining with SOT.
Definitions and variables
The above mentioned three CPET were performed using a cycloergometer (Ergoline Medical Graphics Corporation, St. Paul, MN, USA) and a gas analyzer (Cardiorespiratory Diagnostic System, Ultimaseries TM, MediGraphics, Orlando, FL, USA). The tests were interrupted if adverse effects such as chest pain, changes in the electrocardiographic record or desaturation reaching SpO2 < 80% appeared despite oxygen supplementation.
Symptom-limited incremental CPET
This was performed to evaluate the patient’s WRmax according to the ATS/ACCP standardization statement [22]. In this regard, a conventional increasing protocol (10 Watts/min) was used and subjects had to maintain a 50–60 rpm constant speed at all time. As previously mentioned, the appropriate FIO2 needed to maintain a SpO2 > 85% was obtained.
Submaximal CPET with SOT
This was performed using a conventional Venturi system at the FIO2 chosen in the incremental CPET.
Submaximal CPET with HFNC
This exercise test was also carried out at the FIO2 obtained in the incremental CPET using an AIRVO2 device in this case (Optiflow TM, Fisher and Paykel, New Zealand), with flows ranging between 40 and 60 l/min, heat and humidification. The endurance time (Tlim) for the latter two tests was defined as the point at which the patient was unable to maintain 60 rpm speed despite very active encouragement and was measured in seconds.
Vital and ventilation variables during all CPETs
SpO2 and heart rate (HR) were monitored with a pulse oximeter (Biox, OHMEDA, Madison, WI, USA) and blood pressure was assessed every 2 min. In addition, respiratory rate (RR) and tidal volume (VT) were recorded from the above mentioned exercise system. Inspiratory capacity (IC) maneuvers were carried out at the beginning and immediately following the end of the tests. The same was done for dyspnea and leg fatigue assessment through Borg scales.
Leg muscle saturation (StO2)
Leg muscle saturation (StO2) was also monitored using NIRS, (NIRO-200NX, Hamamatsu, Japan) during all CPET procedures in 7 patients, and specific data obtained at rest, during free-pedaling, submaximal exercise and recovery were further analyzed. The system was calibrated before each study following manufacturer’s recommendations and the output frequency was 1 Hz in all cases, with the sensor being placed on the left quadriceps (vastus medialis) [16]. Two specific values were considered for the analysis of StO2 behavior. On the one hand, its mean value during submaximal exercise (75% WRmax) and on the other, the isotime value at task failure. Moreover, two derived variables were also evaluated: exercise induced StO2 fall (i.e. Mean StO2 at submaximal exercise − StO2 at rest) and exercise unloading-induced rebound (i.e. StO2 in the recovery phase − mean value at submaximal exercise).
Sample size estimation and statistical analysis
Based on previous studies, a sample size of at least 8 subjects was required to detect a mean difference in Tlim equal or higher than 33% between HFNC and SOT [23], considering a 20% dropout rate [15, 23, 24]. Categorical variables were expressed as frequencies and continuous variables as mean and standard deviation (SD) or median and 25, 75 percentiles (p25–p75). Paired t-test was used for comparisons between the two O2 supplementation methods in normally distributed variables, and Wilcoxon test for those with non-normal distribution. Correlation analysis was performed using Spearman correlation coefficient. A p value < 0.05 was always considered as statistically significant. The analysis was performed using the IBM SPSS statistics pack for Windows (Version 23.0, IBM Corp., Armonk, NY, USA).
Results
Participant characteristics
The study flow diagram is detailed in Fig. 1. A total of 10 patients were finally enrolled in our crossover trial from March 2019 to January 2020. No adverse events were registered during any of the CPET performances, and all patients completed the trial. Baseline characteristics are summarized in Table 1. Subjects walked an average of 436 m (around 90% pred.) and presented a mean SpO2 of 81% in the 6MWT. Mild pulmonary hypertension as approximated by echocardiography was present in 4 patients. Peripheral muscle strength was preserved. During the incremental CPET patients achieved a mean workload of 81 watts (64% pred.) and the mean FIO2 needed to maintain SpO2 over 85% was 0.3. Ventilatory and cardiocirculatory characteristics of this incremental CPET are summarized in the Additional file 1: Table S1. No significant differences were present between those patients randomized to initiate the submaximal CPET with HFNC and those who began with SOT.
Primary and secondary outcomes
Table 2 compares most relevant parameters obtained between submaximal CPETs with HFNC or SOT. As stablished in the previous incremental CPET, FIO2 was 0.33 ± 0.07 for both supplementation methods. It is worth noting that Tlim was significantly greater (30%) during exercise with HFNC when compared with SOT (shown in Fig. 2). Differences in Tlim between both O2 supplementation methods inversely correlated with the mean SpO2 observed in the 6MWT (r = − 0.705, p = 0.02). Absolute differences between both supplementation methods in Tlim were also directly related to those observed in SpO2 at task failure in submaximal CPETs (r = 0.85, p = 0.002), showing a similar tendency with differences in mean StO2 obtained during submaximal exercise (r = 0.607, p = 0.148, respectively). No other correlations were found between Tlim and the remaining variables.
In addition, a higher StO2 was observed with HFNC compared to SOT during free-pedaling exercise with a similar tendency for its mean value 75% WRmax and isotime (shown in Fig. 3, and in the Additional file 2: Table S2). Moreover, although no changes were observed in the breathing pattern, a significantly higher percentage of improvement was observed in IC under HFNC if compared with SOT. Finally, no differences between the two oxygen devices were found in symptoms (either dyspnea or leg discomfort) at the end of the submaximal exercise test.
Peripheral muscle oxygen saturation (StO2) measured by NIRS during CPET performance with both oxygen devices (n = 7). StO2 muscle oxygen saturation, NIRS near-infrared spectroscopy device, CPET cardiopulmonary exercise test, HFNC high-flow nasal cannula. *p < 0.05. Data are presented as mean and standard deviation
Discussion
This prospective crossover trial is the first to demonstrate that HFNC oxygen therapy allows IPF patients to obtain a better exercise performance (i.e. higher Tlim) than SOT, probably to a better recruitment of alveolar spaces, reducing mechanical constraints and/or preventing early deoxygenation at muscle levels. Whatever the mechanisms are, HFNC appears as an excellent option to give oxygen supplementation during exercise to IPF patients.
There is only one previous paper, published by Suzuki et al., that approximates the usefulness of HFNC in ILD patients during exercise [15]. They also performed a randomized crossover study using HFNC versus SOT in a mixed pool of different fibrotic ILD patients who have in common that they reached a very low SpO2 (< 88%) during an incremental CPET. However, they found no differences among both methods in the whole group, probably due to its great heterogeneity [15]. Interestingly, when they performed a post-hoc analysis, they identified a subgroup of patients that were ‘better responders’ to HFNC than to SOT. Although the authors do not discuss the composition of this specific subgroup, the percentage of IPF was slightly higher than in the ‘non-responder group’. This last finding was crucial to lead us to designing the present study including only patients with IPF. Our results strongly support the notion that HFNC is a better option to give supplemental oxygen during exercise in this particular interstitial disease.
Different reasons should be considered to explain the better Tlim obtained with HFNC in the present study. First of all, as previously described and suggested in the Suzuki study, HFNC seems to increase the washout of physiological dead space, thus reducing ineffective ventilation and even improving the work of breathing [13, 15]. Secondly, HFNC can also produce a moderate increase in positive airway pressure that would prevent collapse of critical alveolar units [14]. Both complementary mechanisms are suggested in our study by the bigger improvement in IC with HFNC if compared with SOT, suggesting that the lung parenchyma of our IPF patients was still somewhat distensible and some additional alveolar units might have been recruited for more effective ventilation. The mechanism would be similar—although much less intense—than that previously observed using non-invasive respiratory support (i.e. BiPAP or CPAP) in IPF patients during exercise [25]. However, even with this theoretical improvement in alveolar ventilation, its impact on arterial blood oxygenation was absent since SpO2 during exercise was similar with both supplementation methods. Therefore, although somewhat speculative we hypothesize that one potential reason for the better exercise performance observed with HFNC was mostly related with an improvement in the mechanics of the respiratory system rather than to changes in pulmonary gas exchange. In contrast, the direct relationship observed in the present study between differences in Tlim and in task failure SpO2 between both submaximal exercises would indicate that oxygenation has probably played some role in the better improvement in exercise capacity observed with HFNC.
A complementary conclusion derives from the inverse correlation observed between the mean SpO2 values observed in the 6MWT and the amount of the differential benefits in Tlim obtained with HFNC oxygenation in the submaximal CPET. This might suggest that this last supplementation method would be especially indicated in those patients with greater exertional hypoxemia. A similar relationship between exertional SpO2 and exercise performance in CPET was observed in a previous study where supplemental oxygen was compared with placebo in IPF patients [9].
One novelty of the present study is the complementary assessment of peripheral muscle oxygenation during exercise in IPF patients. Interestingly, although no differences were observed in SpO2 during exercise among both oxygenation methods, our patients showed a significantly higher StO2 with HFNC than with SOT during free-pedaling and a tendency in the same direction during submaximal effort and at task failure (shown in Fig. 3). Moreover, differences in both SpO2 and StO2 among both oxygenation methods showed a trend to correlate with differences in Tlim. We observed no significant correlations between SpO2 and StO2 in any of the situations here analyzed. A finding that is in line with a previous study on ILD patients, where discrepancies were also observed between both variables during two incremental leg exercises [8], being explained by potential changes in muscle ability to extract and use the oxygen delivered by blood [8]. Muscle dysfunction has been suggested as a potential contributor to exercise intolerance in ILD patients [26], and more specifically, quadriceps strength has already been shown to be an independent factor to maximal exercise capacity (represented by VO2 peak) in IPF patients [6]. However, the mechanisms of muscle weakness in interstitial disorders still remain unclear, although as it occurs in other chronic lung diseases, physical deconditioning may play an important role [3]. In the present study we observed no loss in either upper or lower leg muscle strength (a property that mainly depends on muscle mass) but this does not necessarily exclude abnormalities in muscle endurance (more dependent on the aerobic metabolism). In fact, the absence of significant correlations between SpO2 and StO2 suggest this latter possibility.
With regard to symptoms, no differences were found in our study between neither dyspnea or leg fatigue at the end of the test between both oxygen supplementation methods. Previous investigations on ILD, or more specifically on IPF, have demonstrated less dyspnea and fatigue scores on exertion with SOT when compared with placebo [9, 27]. In the unique preceding study comparing HFNC with SOT, the authors were unable to find differences in symptoms [15].
Current guidelines recommend rehabilitation programs in ILD patients, as this has demonstrated clear benefits such as improvement in dyspnea, exercise capacity and quality of life [28, 29]. However, data are lacking on the effects of different methods of oxygen supplementation during training in patients with exertional desaturation. From our present results we could anticipate that HFNC could be a useful oxygen supplementation method to obtain better outcomes from rehabilitation programs and therefore an excellent alternative to improve functional capacity in IPF subjects. Given the limited access to HFNC oxygen devices, its use nowadays would be limited mainly to controlled training programs. However, the ongoing HOPE study, which has the objective of determining the effect of different oxygenation methods during exercise training in IPF patients treated with Nintedanib, will probably clarify this point [30].
One of the strengths of our study is that it focuses on IPF patients, reducing the heterogeneity derived from the inclusion of different fibrotic ILDs employed in previous studies. In fact, patients with IPF seem to present a higher alveolar-arterial O2 gradient (AaPO2) during exercise compared to other ILDs, indicating worse VA/Q relationships and/or O2 diffusion capacity [2]. This factor might explain the absence of clear conclusions on the use of HFNC in previous studies that included mixed ILDs. This the first study that compares both oxygen supplementation methods including the assessment of different components that can contribute to exercise limitation in IPF patients. There are also, however, some limitations that should be recognized. The study population was relatively small, and patients were not totally blind regarding the two supplementation techniques. Although they did not know either the flow or FIO2 supplied, they could easily identify the oxygenation method. However, this limitation is almost impossible to overcome since both devices generate clearly differentiated perceptions.
Conclusions
Exercise intolerance is the predominant symptom in IPF. However, to date there are only few treatment strategies directed to improving exercise capacity in such patients. On the basis of the present results, we suggest that HFNC oxygen therapy is a better option than SOT for improving exercise capacity in those IPF patients with exertional desaturation. Taking all our results together we can speculate that reasons for the improvement observed in Tlim in our IPF patients were related to positive changes in ventilatory mechanics and perhaps in muscle oxygenation. However, further and larger studies are needed to confirm these benefits and to establish its appropriate use in rehabilitation programs.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files. Datasets will be available from the corresponding author on reasonable request.
Abbreviations
- 6MWT:
-
Six-minute walking test
- AaPO2 :
-
Alveolar-arterial O2 gradient
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- CPET:
-
Cardiopulmonary exercise test
- DLCO :
-
Carbon monoxide diffusion capacity
- FEV1 :
-
Expiratory flow in the first second
- FIO2 :
-
Inspiratory fraction of oxygen
- FVC:
-
Forced vital capacity
- GAP:
-
Gender-Age-Physiology
- HFNC:
-
High-flow nasal cannula
- HR:
-
Heart rate
- IC:
-
Inspiratory capacity
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- NIRS:
-
Near-infrared spectroscopy
- RR:
-
Respiratory rate
- SD:
-
Standard deviation
- SOT:
-
Standard oxygen therapy
- SpO2 :
-
Peripheral oxygen saturation
- StO2 :
-
Muscle oxygen saturation
- Tlim:
-
Endurance time
- VE:
-
Pulmonary ventilation
- VT :
-
Tidal volume
- WRmax:
-
Maximum exercise capacity
References
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44-68.
Agusti AGN, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143:219–25.
Holland AE. Exercise limitation in interstitial lung disease—mechanisms, significance and therapeutic options. Chron Respir Dis. 2010;7:101–11.
Bonini M, Fiorenzano G. Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2017;26:1–11.
Mendoza L, Gogali A, Shrikrishna D, Cavada G, Kemp SV, Natanek SA, et al. Quadriceps strength and endurance in fibrotic idiopathic interstitial pneumonia. Respirology. 2014;19:138–43.
Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127:2028–33.
Panagiotou M, Polychronopoulos V, Strange C. Respiratory and lower limb muscle function in interstitial lung disease. Chron Respir Dis. 2016;13:162–72.
Wickerson L, Mathur S, Brooks D, Bonetti LV, Singer LG, Granton J, et al. Skeletal muscle oxygenation and regional blood volume during incremental limb loading in interstitial lung disease. ERJ Open Res. 2020;6:00083–2019.
Arizono S, Furukawa T, Taniguchi H, Sakamoto K, Kimura T, Kataoka K, et al. Supplemental oxygen improves exercise capacity in IPF patients with exertional desaturation. Respirology. 2020;25:1152–9.
Dowman LM, McDonald CF, Bozinovski S, Vlahos R, Gillies R, Pouniotis D, et al. Greater endurance capacity and improved dyspnoea with acute oxygen supplementation in idiopathic pulmonary fibrosis patients without resting hypoxaemia. Respirology. 2017;22:957–64.
Bell EC, Cox NS, Goh N, Glaspole I, Westall GP, Watson A, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev. 2017;26:1–7.
Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61:529–41.
Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85:319–25.
Cirio S, Piran M, Vitacca M, Piaggi G, Ceriana P, Prazzoli M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med. 2016;118:128–32.
Suzuki A, Ando M, Kimura T, Kataoka K, Yokoyama T, Shiroshita E, et al. The impact of high-flow nasal cannula oxygen therapy on exercise capacity in fibrotic interstitial lung disease: a proof-of-concept randomized controlled crossover trial. BMC Pulm Med. 2020;20:1–10.
Barberan-Garcia A, Munoz PA, Gimeno-Santos E, Burgos F, Torralba Y, Gistau C, et al. Training-induced changes on quadriceps muscle oxygenation measured by near-infrared spectroscopy in healthy subjects and in chronic obstructive pulmonary disease patients. Clin Physiol Funct Imaging. 2019;39:284–90.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk Test 2002;166:111–7.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and. J Am Soc Echocardiogr. 2010;23:685–713.
Graham BL, Steenbruggen I, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:E70-88.
Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1–31.
Robles PG, Mathur S, Janaudis-Fereira T, Dolmage TE, Goldstein RS, Brooks D. Measurement of peripheral muscle strength in individuals with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2011;31:11–24.
Weisman IM, Marciniuk D, Martinez FJ, Sciurba F, Sue D, Myers J, et al. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77.
Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, Neder JA, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47:429–60.
Puente-Maestu L, Villar F, De Miguel J, Stringer WW, Sanz P, Sanz ML, et al. Clinical relevance of constant power exercise duration changes in COPD. Eur Respir J. 2008;34:340–5.
Moderno EV, Yamaguti WPS, Schettino GPP, Kairalla RA, Martins MA, Carvalho CRR, et al. Effects of proportional assisted ventilation on exercise performance in idiopathic pulmonary fibrosis patients. Respir Med. 2010;104:134–41.
Watanabe F, Taniguchi H, Sakamoto K, Kondoh Y, Kimura T, Kataoka K, et al. Quadriceps weakness contributes to exercise capacity in nonspecific interstitial pneumonia. Respir Med. 2013;107:622–8.
Visca D, Mori L, Tsipouri V, Fleming S, Firouzi A, Bonini M, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med. 2018;6:759–70.
Kenn K, Gloeckl R, Behr J. Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis—a review. Respiration. 2013;86:89–99.
Dowman L, Hill CJ, May A, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2021. https://doi.org/10.1002/14651858.CD006322.pub4.
Ryerson CJ, Camp PG, Eves ND, Schaeffer M, Syed N, Dhillon S, et al. High oxygen delivery to preserve exercise capacity in patients with idiopathic pulmonary fibrosis treated with nintedanib methodology of the HOPE-IPF study. Ann Am Thorac Soc. 2016;13:1640–7.
Acknowledgements
Not applicable.
Funding
This study has been funded by SEPAR 2017 (Fellowship) and Rio Hortega; ISCIII (Project and fellowship).
Author information
Authors and Affiliations
Contributions
DB-B, MD: Design and methodology, acquisition of funding, visits performance, data collection, data analysis and writing. PC, PhD: Visits performance, data collection and writing. AR-P, Physiotherapist: Visits performance, data collection and writing. CM-O, MD: Visits performance, data collection and writing. RC, PhD: Expertise, writing and feedback. JAR-P (PhD): data collection and feedback. RV-S, Nurse: Visits performance, data collection. JG, PhD: Expertise, feedback, acquisition of funding and writing. XD, Statistic: Data analysis. OAC, MD: Data analysis and feedback. DAR, PhD: Design and methodology, expertise, feedback, acquisition of funding and writing. EB, PhD: Design and methodology, expertise, feedback, acquisition of funding and writing. The last two authors had equal contribution in this paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by the local ethics committee CEIC-PSMar, Barcelona, Spain with approval number 2017/7397/I, carried out according to the principles of the Declaration of Helsinki for human investigations and registered as a clinical trial (NCT04564664). All participants signed the appropriate informed consent prior to their inclusion.
Consent for publication
Not applicable.
Competing interests
DBB has received honoraria for a speaker role from Roche, RCH has received honoraria for a speaker role from GlaxoSmithKline, Teva, Chiesi and Boehringer Ingelheim. DARC has consultant relationship with Janssen, GlaxoSmithKline, Viforpharma, MSD, Ferrer and Chiesi and has received honoraria for a speaker role from Bayer Healthcare. EBV has received honoraria for a speaker role from ROCHE and Boehringer Ingelheim. OAC has received honoraria for a speaker role from Astra Zeneca and Boehringer Ingelheim. All the conflicts of interest are not related to the submitted manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Incremental CPET parameters in the overall population.
Additional file 2: Table S2
. Peripheral muscle oxygen saturation (StO2) measured by NIRS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Badenes-Bonet, D., Cejudo, P., Rodó-Pin, A. et al. Impact of high-flow oxygen therapy during exercise in idiopathic pulmonary fibrosis: a pilot crossover clinical trial. BMC Pulm Med 21, 355 (2021). https://doi.org/10.1186/s12890-021-01727-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01727-9