Abstract
Background
Detection of small peripheral lung nodules is constantly increasing with the development of low dose computed tomography lung cancer screening programs. A tissue diagnosis is often required to confirm malignity, with endobronchial biopsies being associated with a lower pneumothorax rate than percutaneous approaches. Endoscopic diagnosis of peripheral small size lung nodules is however often challenging using traditional bronchoscopy and endobronchial ultrasound alone. New virtual bronchoscopic navigation techniques such as electromagnetic navigational bronchoscopy (ENB) have developed to improve peripheral navigation, with diagnostic yield however remaining in the 30–50% range for small lesions. Recent studies have shown the benefits of combining Cone beam computed tomography (CBCT) with ENB to improve diagnostic yield to up to 83%. The use of ENB however remains limited by disposable cost, bronchus sign dependency and inaccuracies due to CT to body divergence.
Case presentation
This case report highlights the feasibility and usefulness of CBCT-guided bronchoscopy for the sampling of lung nodules difficult to reach through traditional bronchoscopy because of nodule size and peripheral position. Procedure was scheduled in a mobile robotic hybrid operating room with patient under general anaesthesia. CBCT acquisition was performed to localize the target lesion and plan the best path to reach it into bronchial tree. A dedicated software was used to segment the lesion and the bronchial path which 3D outlines were automatically fused in real time on the fluoroscopic images to augment live guidance. Navigation to the lesion was guided with bronchoscopy and augmented fluoroscopy alone. Before the sampling, CBCT imaging was repeated to confirm the proper position of the instrument into the lesion. Four transbronchial needle aspirations (TBNA) were performed and the tissue analysis showed a primary lung adenocarcinoma.
Conclusions
CBCT and augmented fluoroscopy technique is a safe and effective and has potential to improve early stage peripheral lesions endobronchial diagnostic yield without ENB. Additional studies are warranted to confirm its safety, efficacy and technical benefits, both for diagnosis of oncological and non-oncological disease and for endobronchial treatment of inoperable patients.
Similar content being viewed by others
Background
Lung cancer is the most common cause of cancer death worldwide with 2.21 million new cases and 1.8 million cancer deaths in 2020 [1]. Early diagnosis is a clinically important challenge to improve prognosis. The development of low-dose chest computed tomography (CT) lung screening programs has resulted in an increased incidence of small nodules suspected of early-stage lung cancer requiring sampling to confirm malignancy [2, 3]. Percutaneous needle aspiration has been associated with a high sensitivity (up to 90%) [4, 5] but is limited by a significant complication rate with up to 25% pneumothorax reported in the literature [6]. Conventional fluoroscopy-guided transbronchial biopsies are associated with significantly lower pneumothorax rates but also lower diagnostic yield, in particular for early stage small peripheral nodules [7]. In the last years, new interventional pulmonology technologies have been to obtain safe and effective tissue collection [8, 9] by improving navigation guidance through the bronchial pathway, device flexibility to reach lesions at tight angulation relative to the airway, and real time assessment of the relationship between the sampling device and the target lesion which can be even smaller than 1 cm. To improve navigation and lesion reach, thin/ultrathin bronchoscopes [10], preoperative CT-based virtual bronchoscopic navigation (VBN) [11, 12] such as electromagnetic navigational bronchoscopy (ENB) [13], bronchoscopic transparenchymal nodule access (BTPNA) [14], transbronchial access tools (TBAT) [15] and robotic-assisted bronchoscopy [16,17,18] are the main advances now available. Radial probe endobronchial ultrasound (R-EBUS) is a useful tool to study a lesion, when correctly reached [19]. ENB has significantly improved peripheral endobronchial navigation, but remains limited by consumable cost, bronchus sign dependency and preoperative CT-to body divergence resulting in a relatively low [30–73%] diagnostic yield in particular in small lesions [20,21,22]. The use of cone beam CT (CBCT) in combination with ENB has been described to confirm proper device position within the lesion before sampling, significantly increasing diagnostic yield to [70–83%] [23,24,25].
CBCT is an intraoperative 3D imaging technique developed in the early 2000s and adopted as standard of care in many endovascular and percutaneous procedures to improve targeting, treatment planning and assessment [26, 27]. In modern interventional radiology and hybrid operating rooms, CBCT-based 3D advanced procedural planning is fused on fluoroscopy to augment live guidance. CBCT and augmented fluoroscopy technical and clinical benefits have been demonstrated and their use is established in interventional radiology, interventional oncology and minimally invasive vascular surgery procedures. These technologies have recently been applied also in the field of interventional pulmonology [27,28,29], with or without VBN [11, 23, 30,31,32], and in thoracic surgery to streamline video-assisted resections [33].
Case presentation
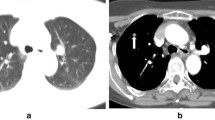
A 78-year-old former smoker (80 p/y) male patient was admitted for a 37 * 28 mm left lower lobe pulmonary nodule detected on follow up CT scan. Patient history included a right upper lobe lung resection for adenocarcinoma in 2008 and a trans-urethral resection for prostate adenocarcinoma in 2019 (Gleason Score 6 3 + 3; Grade Group 1); his medical history was also significant for chronic obstructive pulmonary disease (COPD) with frequent exacerbations and for a hypokinetic cardiomyopathy. Nodule had increased in size compared to a previous CT scan and it presented a significant uptake (SUVmax = 6.4) at 18 FDG-PET/CT (Fig. 1). No suspicious mediastinal lymphadenopathy was detected on both chest CT scan and FDG-PET/CT. The lung lesion was difficult to reach through traditional bronchoscopy combined with standard C-arm fluoroscopy guidance due to the nodule’s position and size. A percutaneous transthoracic needle aspiration was judged as a high-risk procedure based on the nodule position and the risk of pneumothorax in a COPD patient. Considering the diagnostic difficulty, a CBCT-guided bronchoscopy was proposed and accepted by the patient. Procedure was scheduled in a mobile robotic hybrid operating room (Fig. 2; Discovery IGS 740, GE Healthcare, Chicago, IL) providing intraprocedural 3D and superior 2D imaging compared to standard surgical C-arms, along with advanced planning, guidance and assessment with augmented fluoroscopy. The procedure was conducted through a laryngeal mask in general anaesthesia. A standard flexible video bronchoscope with a 2.0 mm working channel was used (BF-H190, Olympus Medical Systems, Tokyo, Japan).
Thorax CBCT imaging was initially acquired to define target position and the easiest path to reach it within the bronchial tree. Images were obtained through a 5 s rotational acquisition around the patient, providing intraprocedural CT-like soft tissue cross-sectional imaging of the lungs. To minimize CBCT reconstruction blurring effects due to breathing, patient was put into apnoea during the acquisition. Using dedicated CBCT augmented planning and guidance software (ASSIST, GE Healthcare), interventional pulmonologist segmented the volumes of interest constituted by the target to be biopsied and the optimal endobronchial path to reach it. Tumor and endobronchial path CBCT-based 3D volumes were then automatically fused on the two-dimensional x-ray fluoroscopy imaging to augment live guidance (Fig. 3A). Fusion remained automatically registered following table movements and C-arm angulations. Fusion opacity and rendering options (outline, volume) were adjustable from table side to maximize live fluoroscopy and augmented guidance visualization along the procedure. Augmented fluoroscopy was used in combination with traditional bronchoscopy to guide the navigation following the planned path (Fig. 3B).
A R-EBUS probe (UM-S20-17S; Olympus Medical Systems) helped to identify the correct fifth generation bronchus leading to the lesion; it was a branch of the segmental LB6 (apical of the left lower bronchus). Then a needle was put in that pathway and a second CBCT acquisition was performed to confirm that the tip was into the target lesion. Tissue sampling was performed under augmented fluoroscopy guidance with four transbronchial needle aspirations (TBNA) in the target site. The angiographic unit was optimised in terms of quality imaging level and dose to the patient [34]. Total fluoroscopy time was 13.4 min. Total kerma area product (KAP) and air kerma at the interventional reference point (IRP) were 59.15 Gy cm2 and 159.3 mGy, respectively. Regarding these data, fluoroscopy accounts for 22.13 Gy cm2 and 69.1 mGy, while CBCT explains for 37.02 Gy cm2 and 90.2 mGy, respectively. The patient’s peak skin dose was 148 mGy. The exposure to ionising radiation for the first operator, the closest to the radiation field, was 2 µSv (body dose equivalent), 1.1 µSv (lens dose equivalent), and 5.7 µSv (hand dose equivalent) [35, 36]. These doses were estimated using the measurements carried out around the angiograph and assuming the worst-case scenario, i.e., without considering the attenuation of the operator protection devices (shielded aprons and collars of 0.5 mm of Pb equivalent). These dose estimates agreed with the operator dosimeters whose readings were not distinguishable from their minimum detection threshold [10 µSv, measurements in Hp (10) and Hp (0.07) [35]]. Rapid on-site evaluation (ROSE) was not performed. The procedure ended without any complication. The tissue sampling showed epithelial cells consistent with adenocarcinoma (TTF1 positive, p40 negative), confirming the pulmonary primitivity of the neoplasia. The patient was referred to the oncologist for appropriate treatment.
Discussion and conclusions
Transbronchial sampling of small peripheral lung lesions is increasingly important [7, 37]. Virtual navigation tools have been developed to improve endobronchial planning and guidance towards the target or the region of interest to be investigated, but they are limited by consumable cost and inaccuracies between the preoperative CT and the live patient, while lacking real-time intraoperative 3D imaging of the device/target spatial relationship before sampling. CBCT and augmented fluoroscopy are not yet a widespread approach for endobronchial biopsy of small peripheral pulmonary nodules but they could be a promising avenue for simplified and more accurate navigation and for real-time assessment. Indeed, especially when used together with R-EBUS, they allow a target confirmation and a precise identification of the relationship between the airways and the lesion. CBCT advanced guidance delayed adoption in interventional pulmonology is probably due to preliminary clinical experiences describing complications (pneumothorax, COPD exacerbation) and high radiation exposure levels [25, 29, 38]. Large clinical trials on the accuracy and safety of this technique are lacking.
We herein report the case of a fragile patient with a difficult to reach lung nodule, successfully sampled through endobronchial approach using CBCT and augmented fluoroscopy as main guidance. First, this case highlights and confirms the safety of bronchoscopy in elderly patients with multiple comorbidities [39]. Then, it provides evidence on the feasibility and the usefulness of CBCT advanced guidance in the sampling of difficult lung nodules. In this case it is also important to highlight the use of TBNA, a tool that has a high diagnostic accuracy for peripheral pulmonary lesions with and without the presence of “bronchus sign” on CT [39, 40]; on the other hand, it allowed us to reach a more peripheral site during the exam. CBCT seamless multimodality integration in the latest interventional suites, allowing to combine information from different imaging modalities (CBCT, CT, PET/CT, MRI) is also of interest and will be assessed in our practice. Additional studies are warranted to confirm the safety and efficacy of this technique, opening the avenue of CBCT advanced imaging to several pulmonary clinical applications such as non-oncological diseases diagnosis [41, 42], endobronchial treatment for inoperable patients (cryotherapy, photodynamic therapy, microwave ablation, radiofrequency ablation or, thermal vapor ablation) [43, 44] and for the placement of fiducial markers [45].
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3D:
-
Three-dimensional space
- CBCT:
-
Cone beam computed tomography
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- ENB:
-
Electromagnetic navigational bronchoscopy
- FDG:
-
Fluorodeoxyglucose
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- R-EBUS:
-
Radial probe endobronchial ultrasound
- SUVmax:
-
Maximum standardized uptake value
- TTF1:
-
Transcription termination factor 1
- TBNA:
-
Transbronchial needle aspiration
- VBN:
-
Virtual bronchoscopic navigation
References
World Health Organization. Cancer. 2021. https://www.who.int/news-room/fact-sheets/detail/cancer.
World Health Organization. Computed Tomography Scanners, per 100 000—European Health Information Gateway. https://gateway.euro.who.int/en/indicators/hlthres_37-computed-tomography-scanners-per-100-000/visualizations.
Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA. 2019;322(9):843–56.
Di Bardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015;7:S304–16.
Wang Y, Jiang F, Tan X, Tian P. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: diagnostic yield and complications in 1484 patients. Medicine. 2016;95(31):e4460.
Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27(1):138–48.
Han Y, Kim HJ, Kong KA, Kim SJ, Lee SH, Ryu YJ, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: a systematic review and meta-analysis. PLoS ONE. 2018;13(1):e0191590.
Katsis JM, Rickman OB, Maldonado F, Lentz RJ. Bronchoscopic biopsy of peripheral pulmonary lesions in 2020: a review of existing technologies. J Thorac Dis. 2020;12(6):3253–62.
Wagh A, Ho E, Murgu S, Hogarth DK. Improving diagnostic yield of navigational bronchoscopy for peripheral pulmonary lesions: a review of advancing technology. J Thorac Dis. 2020;12(12):7683–90.
Tanner NT, Yarmus L, Chen A, Wang Memoli J, Mehta HJ, Pastis NJ, et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: a multicenter, prospective. Randomized Trial Chest. 2018;154(5):1035–43.
Asano F, Eberhardt R, Herth FJF. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration. 2014;88(5):430–40.
Asano F, Shinagawa N, Ishida T, Shindoh J, Anzai M, Tsuzuku A, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy a randomized clinical trial. Am J Respir Crit Care Med. 2013;188(3):327–33.
Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87(2):165–76.
Herth FJF, Eberhardt R, Sterman D, Silvestri GA, Hoffmann H, Shah PL. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax. 2015;70(4):326–32.
Bowling MR, Brown C, Anciano CJ. Feasibility and safety of the transbronchial access tool for peripheral pulmonary nodule and mass. Ann Thorac Surg. 2017;104(2):443–9.
Lin J, Ost DE. Robotic bronchoscopy for peripheral pulmonary lesions: a convergence of technologies. Curr Opin Pulm Med. 2021;27(4):229–39.
Chen AC, Pastis NJ, Mahajan AK, Khandhar SJ, Simoff MJ, Machuzak MS, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845–52.
Chaddha U, Kovacs SP, Manley C, Hogarth DK, Cumbo-Nacheli G, Bhavani SV, Kumar R, Shende M, Egan JP 3rd, Murgu S. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med. 2019;19(1):243.
Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011;37(4):902–10.
Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions: results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193(1):68–77.
Folch EE, Pritchett MA, Nead MA, Bowling MR, Murgu SD, Krimsky WS, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective. Multicenter NAVIGATE study. J Thorac Oncol. 2019;14(3):445–58.
Izquierdo A, García MM, Choukri MM, Velando AG, Sánchez JA, Fernández-NavamuelBasozabal I, et al. Diagnostic yield of electromagnetic navigation bronchoscopy in subcentimetric pulmonary nodules. Chest. 2020;157(6):A345.
Pritchett MA, Schampaert S, De Groot JAH, Schirmer CC, Van Der Bom I. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchol Interv Pulmonol. 2018;25(4):274–82.
Pritchett MA. Prospective analysis of a novel endobronchial augmented fluoroscopic navigation system for diagnosis of peripheral pulmonary lesions. J Bronchol Interv Pulmonol. 2021;28(2):107–15.
Verhoeven R, Fütterer J, Hoefsloot W, Van Der Heijden EHFM. Added value of cone beam CT imaging to electromagnetic navigation bronchoscopy in diagnosing small pulmonary lesions. Eur Respir J. 2019;54(suppl 63):PA3116.
Casal RF, Sarkiss M, Jones AK, Stewart J, Tam A, Grosu HB, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis. 2018;10(12):6950–9.
Setser R, Chintalapani G, Bhadra K, Casal RF. Cone beam CT imaging for bronchoscopy: a technical review. J Thorac Dis. 2020;12(12):7416–28.
Gulias-Soidan D, Crus-Sanchez NM, Fraga-Manteiga D, Cao-González JI, Balboa-Barreiro V, González-Martín C. Cone-beam CT-guided lung biopsies: results in 94 patients. Diagnostics. 2020;10(12):1068.
Sobieszczyk MJ, Yuan Z, Li W, Krimsky W. Biopsy of peripheral lung nodules utilizing cone beam computer tomography with and without trans bronchial access tool: a retrospective analysis. J Thorac Dis. 2018;10(10):5953–8.
Verhoeven RLJ, Fütterer JJ, Hoefsloot W, Van Der Heijden EHFM. Cone-beam CT image guidance with and without electromagnetic navigation bronchoscopy for biopsy of peripheral pulmonary lesions. J Bronchol Interv Pulmonol. 2021;28(1):60–9.
Yang SM, Yu KL, Lin KH, Liu YL, Sun SE, Meng LH, et al. Localization of small pulmonary nodules using augmented fluoroscopic bronchoscopy: experience from 100 consecutive cases. World J Surg. 2020;44(7):2418–25.
Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic navigation bronchoscopy with tomosynthesis-based visualization and positional correction: three-dimensional accuracy as confirmed by cone-beam computed tomography. J Bronchol Interv Pulmonol. 2021;28(1):10–20.
Zhao ZR, Lau RWH, Ng CSH. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis. 2016;8:S319–27.
Bertolini M, Trojani V, Nitrosi A, Iori M, Sassatelli R, Ortenzia O, et al. Characterization of GE discovery IGS 740 angiography system by means of channelized Hotelling observer (CHO). Phys Med Biol. 2019;64(9):095002.
Ferrari P, Becker F, Jovanovic Z, Khan S, Bakhanova E, Principi S, Kristic D, Pierotti L, Mariotti F, Faj D, Turk T, Nikezic D, Bertolini M. Simulation of H (p) (10) and effective dose received by the medical staff in interventional radiology procedures. J Radiol Prot. 2019;39(3):809–24.
Iori M, Isolan L, Piergallini L, Chendi A, Lasagni L, Cucchi G, et al. How direct measurements on worker eyes with Scheimpflug camera can affect lens dose conversion coefficients in interventional radiology. J Radiol Prot. 2021. https://doi.org/10.1088/1361-6498/abf56f.
Facciolongo N, Piro R, Menzella F, Lusuardi M, Salio M, Agli LL, et al. Training and practice in bronchoscopy a national survey in Italy. Monaldi Arch Chest Dis. 2013;79(3–4):128–33.
Ali EAA, Takizawa H, Kawakita N, Sawada T, Tsuboi M, Toba H, et al. Transbronchial biopsy using an ultrathin bronchoscope guided by cone-beam computed tomography and virtual bronchoscopic navigation in the diagnosis of pulmonary nodules. Respiration. 2019;98(4):321–8.
Mondoni M, Radovanovic D, Sotgiu G, Di Marco F, Carlucci P, Centanni S, et al. Interventional pulmonology techniques in elderly patients with comorbidities. Eur J Intern Med. 2019;59:14–20.
Chao TY, Chien MT, Lie CH, Chung YH, Wang JL, Lin MC. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial. Chest. 2009;136(1):229–36.
Ashraf SF, Lau KKW. Navigation bronchoscopy: a new tool for pulmonary infections. Med Mycol. 2019;57:S287–93.
Zhou G, Ren Y, Li J, Yang T, Su N, Zhao L, et al. Safety and diagnostic efficacy of cone beam computed tomography-guided transbronchial cryobiopsy for interstitial lung disease: a cohort study. Eur Respir J. 2020;55(5):2000724.
Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211(1):68–72.
Scott Ferguson J, Henne E. Bronchoscopically delivered thermal vapor ablation of human lung lesions. J Bronchol Interv Pulmonol. 2019;26(2):108–13.
Bowling MR, Folch EE, Khandhar SJ, Kazakov J, Krimsky WS, LeMense GP, et al. Fiducial marker placement with electromagnetic navigation bronchoscopy: a subgroup analysis of the prospective, multicenter NAVIGATE study. Ther Adv Respir Dis. 2019;13:1–11.
Acknowledgements
The authors acknowledge Aya Rebet (for her support in the writing of the manuscript), Pierluigi Aragosti, Paola Conti, Victor Hugo Cornejo, Simona Devoti, Gabriele Li Pizzi, Loretta Melioli, Fiorella Noci, Romano Sassatelli, Annunziata Scialò and Gianluca Volta (for their work in the performing of the bronchoscopy) and Eleonora Viviani (for her support in the analysis of the images).
Funding
None.
Author information
Authors and Affiliations
Contributions
RP, ST, EC, MF, MB, NF and MI wrote a draft of the manuscript which was critically reviewed by all authors and finally approved. RP and NF performed the bronchoscopy here described. ST, MI and MB extracted data from the clinical database and medical records. EC and RP reviewed and choose the images. All authors participated in the literature review. All authors have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The written consent for publication of clinical details and his images was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Piro, R., Fontana, M., Casalini, E. et al. Cone beam CT augmented fluoroscopy allows safe and efficient diagnosis of a difficult lung nodule. BMC Pulm Med 21, 327 (2021). https://doi.org/10.1186/s12890-021-01697-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01697-y