Abstract
Background
Conventional spirometric parameters have shown poor correlation with symptoms and health status of chronic obstructive pulmonary disease (COPD). While it is well-known that the pattern of the expiratory flow-volume curve (EFVC) represents ventilatory dysfunction, little attempts have been made to derive quantitative parameters by analyzing the curve. In this study, we aimed to derive useful parameters from EFVC via graphic analysis and tried to validate them in patients with COPD.
Methods
Using Graphical Analysis 3.4 Vernier Software, we derived from the EFVC such parameters as area of obstruction (Ao), area of triangle (AT), area of rectangle (AR) and ratio of volume at 75 and 25 % peak expiratory flow (PEF) (0.25/0.75 V). For validation, we reviewed clinical and spirometric data of 61 COPD patients from Seoul National University Airway Registry (SNUAR) and Korean obstructive Lung Disease (KOLD) cohorts.
Results
Of all parameters, only RV/TLC significantly correlated with scores from St. George’s Respiratory Questionnaire (SGRQ) (r = 0.447, p = 0.037). Six-minute walking distance (6MWD) highly correlated with Ao/AR (r = −0.618, p = 0.005) and Ao/PEF (r = −0.581, p = 0.009) whereas neither FEV1 nor FEV1/FVC had significant correlation with 6MWD.
Conclusions
Ao/AR and Ao/PEF are promising parameters which correlate well with the exercising capacity of COPD patients.
Similar content being viewed by others
Background
Chronic obstructive lung disease (COPD) is defined as having airflow limitation, measured as forced expiratory volume in one second (FEV1) divided by forced vital capacity (FVC). These conventional spirometric parameters, such as FEV1 or FVC, are currently accepted standards in grading the severity of airflow limitation; when FEV1/FVC is less than 0.70, diagnosis of COPD is made.
However, these parameters cannot exactly determine the health status of patients with COPD. For example, FEV1 shows weak association with physical activity and subjective symptom scores such as St. George’s Respiratory Questionnaire (SGRQ) [1–3]; little correlation between FEV1 and hospital readmission in such patients is also reported [4]. Therefore, dyspnea scores and objectively measured exercise capacity should also be considered and taken into account.
Exercise capacity, which can be simply measured by 6-min walking distance (6MWD), is reflective of activities of daily living and functional status of patients with COPD but shows poor correlation with FEV1 or FEV1/FVC [5, 6].
While it is well-known that the concave shape of expiratory flow-volume curve (EFVC) is suggestive of the presence of underlying small airway obstruction, few attempts have been made to quantify the concave area and correlate it with clinical indices in patients with COPD [7–9]. In this study, we sought to derive new useful graphic parameters from a commonly used spirometric curve and performed correlation analysis with symptom severity and exercise capacity. We initially hypothesized that the concave area under the flow-volume curve might reflect symptom severity and health status in patients with COPD.
Methods
Study subjects and general methods
The following study was approved by the institutional review board of Seoul National University Bundang Hospital (B-1108/134-004 and B-0508-023-009). Informed written consent for participation in the study was obtained from all participants.
For validation of new graphic parameters, we analyzed the clinical and baseline pre-bronchodilator best spirometric data of 61 COPD patients from the Seoul National University Airway Registry (SNUAR) and the Korean Obstructive Lung Disease (KOLD) cohort [10]. 40 patients from SNUAR and 21 patients from KOLD cohort were included in the analysis. The SNUAR cohort included stable COPD patients who were prospectively recruited from the outpatient pulmonary clinic of Seoul National University Bundang Hospital. The diagnosis of COPD was made according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2003 criteria. The KOLD cohort consisted of patients with stable COPD, who were prospectively recruited from the pulmonary clinics of 11 hospitals in Korea from June 2005 to September 2009.
Spirometry was performed using a Vmax 22 instrument (Sensor-Medics; Yorba Linda, CA, USA). Lung volumes were measured by body plethysmography (V6200; SensorMedics). Diffusing capacity for carbon monoxide (DLco) was measured by the single-breath method using a Vmax229D (Sensor-Medics). All pulmonary function tests were performed as recommended by the American Thoracic Society and European Respiratory Society.
Graphic analysis methods
For graphic analysis of the EFVC, we used Graphical Analysis 3.4 Vernier Software program. Using the program, we first integrated the area under the curve. Figure 1 shows an example of pre-bronchodilator best EFVC and integration of the area under the curve using the software.
Figure 2 shows the lighted area below the imaginary diagonal line and above the EFVC; we defined this area as Au, which reflects concavity of the curve. Our new graphic parameter, Area of obstruction (Ao) and area of triangle (At) were calculated as follows.
Graphic explanation of new parameters. Au was defined as the lighted area below the imaginary diagonal line and above the expiratory flow-volume curve. Area of obstruction (Ao) and area of triangle (At) were calculated as follows. Xp denotes the expirated lung volume at PEFR. The ratio of lung volumes at 75 and 25 % of PEFR (0.25/0.75 V) were also measured. Area of rectangle (AR), not shown here, was defined as follows. Ao = Au/At; At = PEFR × (FVC–Xp)/2; AR = (actual PEFR/predicted PEFR) × (actual FVC/predicted FVC) × 100 %
As Ao approaches to 1, the concavity of EFVC becomes more severe. As Ao gets closer to 0, the EFVC becomes less concave. Xp denotes the lung volume at PEFR.
Because the baseline pulmonary function depends on the patients’ heights and other factors, we needed to define Ao in ratio rather than raw values. In order to calibrate and adjust for such individual differences, we divided Au by At. Using a raw value such as Au may result in wrong direction and inappropriate interpretation of result (see Additional file 1: Table S1).
Another parameter, area of rectangle (AR) were defined as follows; we used the reference equation derived from the Korea National Health and Nutrition Examinations Survey IV to calculate predicted FVC in Korean COPD patients [11, 12].
The ratio of lung volumes at 75 and 25 % of PEFR (0.25/0.75 V) were also calculated.
Statistical analysis
Statistical analysis was performed using the statistics software, PASW Statistics for Windows, Version 18.0, Chicago: SPSS Inc. The association between clinical and spirometric parameters was determined by using Pearson correlation analysis.
Results
The demographic, clinical, and conventional spirometric characteristics are shown in Table 1. Data are presented as mean ± SD. The patients were mostly men (96.7 %) with mean age of 71 years old, 44 pack-years of smoking history and mean body mass index (BMI) of 23.4 kg/m2. Mean FEV1 was 65 % and FEV1/FVC 0.48.
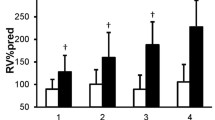
Among 61 subjects, the pre-bronchodilator EFVC data for three subjects were missing, so they were excluded in the correlation analysis. Table 2 displays the correlation result between clinical and known spirometric parameters after adjusting for age, BMI and smoking. Of all conventional parameters, only residual volume (RV) divided by total lung capacity (TLC) significantly correlated with SGRQ (r = 0.447, p = 0.037). Inspiratory capacity (IC), as percent predicted, showed positive correlation with 6MWD.
The results for new parameters derived from EFVC are given in Table 3. The new graphic parameters significantly correlated with RV/TLC were Ao, Ao/FVC, Ao/AR, and Ao/PEF.
Among clinico-physiological parameters, 6MWD highly correlated with Ao/AR (r = −0.618, p = 0.005) and Ao/PEF (r = −0.581, p = 0.009). Neither FEV1 nor FEV1/FVC had significant correlation with 6MWD.
Discussion
We set out to determine whether new parameters could further reflect the clinical status of patients with COPD. In this study, we demonstrated that Ao/AR and Ao/PEF significantly negatively correlated with 6MWD. 6MWD is a simple, objective test of measuring functional capacity targeted at people with at least moderately severe impairment and can be used as a follow-up tool to show response after intervention. In addition, the new parameters, Ao/AR and Ao/PEF, highly correlated with RV/TLC, which is a measure of hyperinflation. On the other hand, conventional parameters, such as FEV1 or FEV1/FVC, did not show correlation with 6MWD in our study.
Most of previous studies sought to derive parameters reflecting concavity of the EFVC, but did not further investigate the correlation with clinical and functional indices. Mead et al. first developed the slop ratio as an index of the curvature of maximal EFVC, but it was regarded impractical to use [13]. Afterwards, studies conducted in asthma patients revealed that the concave shape of the curve became less bowed after the steroid treatment [14]; the relationship between the severity of wheezing and the concavity of EFVC was also suggested [15, 16]. In order to quantify the concavity, Vermaak et al. attempted to measure the area under the curve (Aex) as an imaginary triangle with FVC as the base, PEF the perpendicular axis, and straight descending portion of maximal EFVC as the hypotenuse, that seemed to be the most sensitive index to assess the degree of bronchodilation in patients with COPD [17, 18].
More recently, Nozoe et al. studied spontaneous expiratory (SEFV) curve of 34 stable COPD patients at resting state and found that the area under the curve divided by the surrounding rectangular area, the rectangular area ratio (RAR), is indicative of concavity of SEFV curve; FEV1 was the most powerful predictor of concavity of the curve [19]. Ma et al. also used the same parameter, RAR, in analysis of SEFV curve during exercise [20]. While RAR is an excellent novel parameter to measure concavity of the EFVC, these studies did not provide the data showing the relationship of RAR with symptom severity and exercise capacity. Another interesting pilot study by Williams et al. investigated tidal flow-time and flow-volume centroids of spirograms, which showed shorter time to reach PEF and more left-shifted centroid pattern in patients with COPD [21]. Assessing SEFV curve as in this recent study seems promising and is more applicable to elderly patients with comorbidities who cannot perform the maximal EFVC. Nevertheless, utilization of SEFV curve is not widely accepted yet in practice as much as the maximal EFVC. Future studies are warranted in examining SEFV curve to check the relationship between new parameters and functional indices.
The authors acknowledge that the present study has several limitations. First, the size of study sample was too small to fully validate our new parameters. Second, we analyzed the pre-bronchodilator best expiratory curves rather than the post-bronchodilator curves, which could have affected the curvature. Third, most patients were in moderate to severe stage of COPD, GOLD II-III, and we did not include the patients with mild COPD (FEV1 ≥ 80 %).
Conclusion
In this study, we found that Ao/AR and Ao/PEF are potentially useful parameters which correlate well with exercising capacity in patients with COPD. Yet, further studies are warranted to validate the new parameters in a sample of sufficient size and also in SEFV curves.
Abbreviations
- COPD:
-
chronic obstructive pulmonary disease
- SNUAR:
-
Seoul National University Airway Registry
- KOLD:
-
Korean Obstructive Lung Disease
- EFVC:
-
expiratory flow-volume curve
- Au:
-
area under the curve
- Ao:
-
area of concavity
- AT:
-
area of triangle
- AR:
-
area of rectangle
- PEF:
-
peak expiratory flow
- FEV1 :
-
forced expiratory volume in one second
- FVC:
-
forced vital capacity
- RV:
-
residual volume
- TLC:
-
total lung capacity
- Aex:
-
the area under the curve
- 6MWD:
-
6-min walking distance
- SGRQ:
-
St. George’s Respiratory Questionnaire
- BMI:
-
body mass index
- DLco:
-
diffusing capacity for carbon monoxide
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
References
Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59–63. doi:10.1080/15412550802587943.
Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–7. doi:10.1164/rccm.200407-855OC.
Watz H, Pitta F, Rochester CL, Garcia-Aymerich J, ZuWallack R, Troosters T, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–37. doi:10.1183/09031936.00046814.
Osman IM, Godden DJ, Friend JA, Legge JS, Douglas JG. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax. 1997;52(1):67–71.
Wijkstra PJ, Van Altena R, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J. 1994;7(2):269–73.
Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–70.
Sovijarvi AR. Flow-volume response to inhaled methacholine in asthmatics; comparison of area under the curve (AFV) with conventional parameters. Eur J Respir Dis Suppl. 1986;143:18–21.
Seppala OP. Reproducibility of methacholine induced bronchoconstriction in healthy subjects: the use of area under the expiratory flow-volume curve to express results. Respir Med. 1990;84(5):387–94.
Lapp NL, Hyatt RE. Some factors affecting the relationship of maximal expiratory flow to lung volume in health and disease. Dis Chest. 1967;51(5):475–81.
Park TS, Lee JS, Seo JB, Hong Y, Yoo JW, Kang BJ, et al. Study design and outcomes of Korean Obstructive Lung Disease (KOLD) cohort study. Tuberculosis and Respiratory Diseases. 2014;76(4):169–74. doi:10.4046/trd.2014.76.4.169.
Eom SY, Kim H. Reference values for the pulmonary function of Korean adults using the data of Korea National Health and Nutrition Examination Survey IV (2007–2009). J Korean Med Sci. 2013;28(3):424–30. doi:10.3346/jkms.2013.28.3.424.
Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology (Carlton, Vic). 2011;16(4):659–65. doi:10.1111/j.1440-1843.2011.01951.x.
Mead J. Analysis of the configuration of maximum expiratory flow-volume curves. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(2):156–65.
Kraan J, van der Mark TW, Koeter GH. Changes in maximum expiratory flow-volume curve configuration after treatment with inhaled corticosteroids. Thorax. 1989;44(12):1015–21.
Kapp MC, Schachter EN, Beck GJ, Maunder LR, Witek Jr TJ. The shape of the maximum expiratory flow volume curve. Chest. 1988;94(4):799–806.
Schachter EN, Kapp MC, Maunder LR, Beck G, Witek TJ. Smoking and cotton dust effects in cotton textile workers: an analysis of the shape of the maximum expiratory flow volume curve. Environ Health Perspect. 1986;66:145–8.
Vermaak JC, Bunn AE, de Kock MA. A new lung function index: the area under the maximum expiratory flow-volume curve. Respiration. 1979;37(2):61–5.
Struthers AD, Addis GJ. Respiratory function measurements in clinical pharmacological studies including an assessment of the area under the MEFV curve as a new parameter in chronic bronchitic patients. Eur J Clin Pharmacol. 1988;34(3):277–81.
Nozoe M, Mase K, Murakami S, Okada M, Ogino T, Matsushita K, et al. Relationship between spontaneous expiratory flow-volume curve pattern and air-flow obstruction in elderly COPD patients. Respir Care. 2013;58(10):1643–8. doi:10.4187/respcare.02296.
Ma S, Hecht A, Varga J, Rambod M, Morford S, Goto S, et al. Breath-by-breath quantification of progressive airflow limitation during exercise in COPD: a new method. Respir Med. 2010;104(3):389–96. doi:10.1016/j.rmed.2009.10.014.
Williams EM, Powell T, Eriksen M, Neill P, Colasanti R. A pilot study quantifying the shape of tidal breathing waveforms using centroids in health and COPD. J Clin Monit Comput. 2014;28(1):67–74. doi:10.1007/s10877-013-9497-7.
Acknowledgements
The authors thank Dr. J. Patric Barron for revising the manuscript style.
The also authors thank the members of the Korean Obstructive Lung Disease (KOLD) Cohort Study Group: Prof. Tai Sun Park, Prof. Joon Beom Seo, Prof. Moo Song Lee, Prof. Jin Won Huh, Prof. Seung Won Ra, Prof. Jae Seung Lee, Prof. Sei Won Lee, Prof. Eun Jin Chae, Prof. Nam Kug Kim (Univ. of Ulsan), Prof. Deog Kyeom Kim, Prof. Sang-Min Lee, Prof. Tae-Hyung Kim, Prof. Sang-Heon Kim (Hanyang Univ.), Prof. Young Sam Kim (Yonsei Univ.), Prof. Woo Jin Kim (Kangwon National Univ.), Prof. Hye Kyeong Park, Prof. Sung-Soon Lee (Inje Univ.), Prof. Ji-Hyun Lee, Eun Kyung Kim (Bundang CHA Hospital), Prof. Jin Hwa Lee (Ewha Womans Univ.), Prof. Sang Yeub Lee (Korea Univ.), Prof. Seong Yong Lim (Sungkyunkwan Univ.), Prof. Tae Rim Shin, Yong Il Hwang (Hallym Univ.), Prof. Seung Soo Sheen (Ajou Univ.), Prof. Prof. Kwang Ha Yoo (Konkuk Univ.), Prof. Chin Kook Rhee (Catholic Univ.), and Prof. Young Kyung Lee (Kyung Hee Univ.).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
The authors have no conflicts of interest or financial ties to disclose.
Authors’ contributions
JL and HY contributed to conceiving and designing the study, data collection, interpreting the data, writing the manuscript, and approving the final version of the manuscript. CL, JHL, YC and JP contributed to interpreting the data, providing critical revisions and approving the final version of the manuscript. YO and SL contributed to designing the study, data collection, and approving the final version of the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1: Table S1
Correlation analysis between Au and selected variables. Pearson correlation coefficients are shown in the left column with p-values in the right column. (DOC 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, J., Lee, CT., Lee, J.H. et al. Graphic analysis of flow-volume curves: a pilot study. BMC Pulm Med 16, 18 (2016). https://doi.org/10.1186/s12890-016-0182-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-016-0182-8