Abstract
Background
Secretory leukocyte protease inhibitor (SLPI) is a protein with anti-protease and antimicrobial properties that is constitutively secreted from the airway epithelium. The importance of maintaining a balance between proteases and anti-proteases, and robust innate defence mechanisms in the airways, is exemplified by inflammatory lung conditions such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF). Both conditions present with a high protease burden in the airways which leads to tissue destruction. These patients also have an impaired innate immune system in the lungs with bacterial colonization and frequent airway infections. Moreover, both diseases are associated with airway hypoxia due to inflammation and mucus plugs. The aim of the present study was to investigate the role of hypoxia on SLPI production from the airway epithelium.
Methods
Primary human bronchial epithelial cells were grown in sub-immersed cultures or as differentiated epithelium in air liquid interface cultures. Cells were incubated at 21% O2 (normoxia) or 1% O2 (hypoxia), and the release of SLPI was analysed with ELISA. RT-PCR was used to study the expression of SLPI and transforming growth factor β1 (TGF-β1).
Results
Hypoxia decreased the constitutive production of SLPI by bronchial epithelial cells. The multifunctional cytokine TGF-β1, which is known to affect SLPI expression, showed increased expression in hypoxic bronchial epithelial cells. When bronchial epithelial cells were exposed to exogenous TGF-β1 during normoxia, the SLPI production was down-regulated. Addition of TGF-β1-neutralizing antibodies partially restored SLPI production during hypoxia, showing that TGF-β1 is an important regulator of SLPI during hypoxic conditions.
Conclusions
The mechanism described here adds to our knowledge of the pathogenesis of severe pulmonary diseases associated with hypoxia, e.g. COPD and CF. The hypoxic down-regulation of SLPI may help explain the protease/anti-protease imbalance associated with these conditions and vulnerability to airway infections. Furthermore, it provides an interesting target for the treatment and prevention of exacerbation in these patients.
Similar content being viewed by others
Background
Secretory leukocyte protease inhibitor (SLPI) is a small protein of 11.7 kDa that is secreted by mucosal linings, including the airway epithelium [1]. As the name implies, SLPI has anti-protease activity and plays an important role in neutralizing enzymes such as neutrophil elastase in order to prevent excessive tissue damage during inflammation [2]. Moreover, the protein also possesses antimicrobial activity against gram-positive and gram-negative bacteria, mycobacteria and fungi [3-6], although the mechanisms behind its antimicrobial properties are not fully understood.
Chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) are two conditions affecting the lungs. Although the pathophysiology differs between the two diseases, they have several clinical manifestations in common. COPD is typically caused by long-term exposure to cigarette smoke, resulting in deterioration of lung function. In severe cases, these patients usually require long-term oxygen treatment due to hypoxaemia [7]. CF, on the other hand, is caused by a defect in the CF transmembrane conductance regulator gene, resulting in impaired chloride transport and excess mucus production [8]. Inflammation and infection are hallmarks of both diseases, and also major causes of the deterioration of respiratory function. The pathogenesis of both conditions is believed to involve a protease-antiprotease imbalance, where the protease activity from inflammatory cells is not adequately counter-acted by anti-proteases [9,10]. Moreover, bacteria often colonize the lungs of these patients, and they frequently suffer from airway infections and exacerbation that have profound effects on morbidity and mortality [11-14]. Given that SLPI has both antimicrobial and antiprotease properties, regulation of the protein may have important implications for disease progression.
Transforming growth factor β1 (TGF-β1) is a pleiotropic, multifunctional growth factor with fibrogenic and immunomodulatory properties. The protein has been implicated as an important regulator in the pathogenesis of inflammatory pulmonary conditions such as COPD, and an increase in TGF-β1 signalling has been seen in the lung in several studies on COPD (for a review, see [15]). Interestingly, TGF-β1 has also been shown to regulate SLPI expression [16].
In healthy tissues, the oxygen tension is normally between 2.5 and 9%, corresponding to 20–70 mmHg oxygen. During infections and inflammatory processes, oxygen is consumed by inflammatory cells at the infectious site, resulting in a hypoxic microenvironment with oxygen levels below 1% (<8 mmHg) [17,18]. COPD and CF patients have hypoxic areas in their lungs due to mucus plugs in the bronchi, chronic inflammation and tissue remodelling, resulting in shunting of blood from poorly to well ventilated areas. It is known that hypoxia increases the inflammatory function of inflammatory cells [18], but very little is known about the effects of hypoxia on the production of anti-proteases such as SLPI in the airway epithelium.
The present study was performed to investigate the impact of hypoxia on the secretion of SLPI by the respiratory epithelium. The results show that SLPI is down-regulated in bronchial epithelial cells in response to hypoxia, and that this is mediated via a TGF-β1-dependent mechanism.
Methods
Chemicals and reagents
Recombinant human TGF-β1 was purchased from Millipore (Darmstadt, Germany). Neutralizing monoclonal antibody against TGF-β1 and corresponding isotype IgG were obtained from R&D Systems (Minneapolis, MN, USA).
Cells and culturing conditions
Primary human bronchial epithelial cells from non-smokers (3H Biomedical, Uppsala, Sweden) were grown in BEGM medium supplemented with BEGM bullet kit (Lonza, Basel, Switzerland) in poly-l-lysine-coated flasks (Sigma, St. Louis, MO, USA). The cells were seeded in 24-well plates (Sigma) coated with PurCol® (Advanced BioMatrix, San Diego, CA, USA) (1:100 v/v in H2O). Cells were grown until they were confluent and then incubated for 72 h with or without different stimuli under normoxia (21% O2, 5% CO2) or hypoxia (1% O2, 5% CO2). Hypoxic incubation was performed in a C-Chamber (BioSpherix, Lacona, NY, USA). After 48 h of incubation, the medium was replaced with fresh medium including stimuli. This medium had been pre-incubated in the cell incubator under hypoxic or normoxic conditions. At the end of the incubation period, the cell medium was collected and stored at −80°C until analysis.
For the growth of air liquid interface (ALI) cell cultures, Transwell® inserts in a 12-well plate were coated with 300 μl PurCol (1:100 v/v in H2O) for 20 min. Primary human bronchial epithelial cells were thereafter seeded onto the inserts and maintained in BEGM medium in sub-immersed cultures. When confluence was reached, the apical medium was removed and the baso-lateral medium was replaced by ALI medium, consisting of one part BEGM medium supplemented with BEGM bullet kit and 3 mg/ml BSA (Sigma), and one part D-MEM (Invitrogen, Walthem, MA, USA) supplemented with 1 mM sodium pyruvate MEM, 2 mM L-glutamine and non-essential amino acids (all from Invitrogen). The cell medium was thereafter changed at least 5 times/week. After 3–4 weeks in ALI culture, the cells were incubated at 21% or 1% O2 as above. Hypoxic incubation of the ALI cultures was performed with a glove box, InvivO2 Hypoxia 400 workstation (Ruskinn Technology Ltd, Bridgend, UK), to allow work under hypoxic conditions. After 48 h of incubation, the baso-lateral medium was changed and the apical surfaces were rinsed with PBS to remove excess mucus and pre-formed peptides. Incubation was terminated after 72 h by rinsing the apical surfaces with 100 μl 10 mM Tris buffer with 5 mM glucose. The rinsing fluids were stored at −80°C until analysis.
SLPI detection
SLPI was detected in cell medium supernatants and rinsing fluids with ELISA (R&D Systems) according to the manufacturer’s protocol. As the volumes of the medium and rinsing fluids were known, the total amounts of SLPI released apically and baso-laterally could be compared.
RNA isolation and real-time PCR
Total RNA was isolated from primary human bronchial epithelial cells using the acid guanidinium phenol chloroform method and the RNeasy Mini Kit supplied by Qiagen (Hilden, Germany). The optical density ratio (OD 260 nm/OD 280 nm) of RNA was always greater than 1.95. Reverse transcription was performed according to the manufacturer’s instructions on 1 μg total RNA using an iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). RT-PCR was then used to quantify TGF-β1 and SLPI mRNA expression with primer pairs from OriGene Technologies (Rockville, MD, USA). Data were normalized to succinate dehydrogenase complex subunit A. This was chosen as internal standard based on analyses of the expression of a panel of 7 genes commonly used for normalization under hypoxic conditions (data not shown). Expression was analysed using the iTaq™ Universal SYBR® Green Supermix (Bio-Rad). Amplification was performed at 55°C for 40 cycles in an iCycler Thermal Cycler (Bio-Rad) and data were analysed using iCycler iQ Optical System Software (Bio-Rad).
Neutrophil elastase activity assay
Neutrophil elastase activity was analysed with the chromogenic substrate N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (Sigma). The baso-lateral cell medium from ALI-cultures was diluted 1:4 in HEPES buffer (0.1 M HEPES pH 7.5 + 0.5 M NaCl). Fresh cell medium was used as a control. Equal amounts of diluted cell medium and 0.05 U/ml neutrophil elastase in HEPES buffer (40 μl each) (Sigma, St. Louis, MO, USA) were mixed in a 96-well plate. Samples were incubated at room temperature for 20 min. To each well, 100 μl of 0.2 mM N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide in HEPES buffer was added. The plate was incubated at room temperature, and the absorbance was determined at 405 nm at different time points.
Statistical analysis
Statistical calculations were performed using Student’s T-test or one-way ANOVA. Differences were considered statistically significant at p < 0.05.
Results
SLPI expression in bronchial epithelial cells is reduced in response to hypoxia
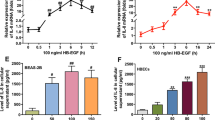
In order to study the effects of hypoxia on the secretion of SLPI in the airways, primary human bronchial epithelial cells were grown in ALI cultures. Under these conditions, cells differentiate to form a ciliated epithelium with mucus production similar to the in vivo airway epithelium. The differentiated cell cultures were incubated at 21% or 1% O2 for 72 h, after which the cell medium was collected, and the apical surfaces were rinsed to collect the periciliary liquid (PCL) including secreted proteins and peptides. Cells grown under hypoxic conditions were adherent and could not be morphologically distinguished from normoxic cells with light microscopy. The SLPI content in medium and rinsing fluids was analysed with ELISA. SLPI was mostly released into the baso-lateral medium (349 ± 25 ng/ml) compared to the rinsing fluid (53 ± 12 ng/ml) (Figure 1). However, if it is assumed that the depth of the PCL was 5 μm [19], the apical SLPI levels in the PCL would have been much higher at approximately 8 μg/ml, which is in good agreement with in vivo data [20]. SLPI concentrations were significantly reduced in both compartments in response to hypoxia (Figure 1).
We next investigated the effects of hypoxia on SLPI expression in non-differentiated primary bronchial epithelial cells grown in sub-immersed cultures. These cells were incubated at 21% or 1% O2, and SLPI concentrations in the cell medium were analysed after 72 h of incubation. SLPI levels were found to be reduced in cells grown under hypoxic conditions (Figure 2A), in line with the data from ALI-cultures. Cells were also harvested and analysed for mRNA expression using RT-PCR. The results showed that SLPI mRNA is down-regulated in response to hypoxia (Figure 2B), indicating that SLPI expression in a hypoxic environment is controlled at the transcriptional level, and is not a consequence of an overall reduction in metabolism.
SLPI expression in bronchial epithelial cells is reduced in response to hypoxia. A) SLPI concentrations in cell medium from primary human bronchial epithelial cells grown under normal oxygenation (21% O2) or under hypoxic conditions (1% O2) (n = 5). B) SLPI mRNA levels from bronchial epithelial cells incubated at 21% or 1% O2. The values of fold change were calculated by normalizing to the data from the 21% O2 samples. The figure shows means and SEM of eight independent experiments.
Since the response to hypoxia was similar in both cell culturing systems, we continued to work with non-differentiated cells to facilitate the studies of underlying mechanisms.
Hypoxia regulates SLPI expression via TGF-β1 signaling
TGF-β1 is a growth factor that has been shown to down-regulate SLPI [16]. To confirm that SLPI can be regulated by TGF-β1 in the airways, bronchial epithelial cells were stimulated for 72 h with different concentrations of TGF-β1. SLPI concentrations in the cell medium were thereafter detected with ELISA. SLPI expression decreased with increasing TGF-β1 concentrations (Figure 3A), and the down-regulation of SLPI expression was more pronounced over time (Figure 3B).
In order to analyse the effect of hypoxia on TGF-β1 expression, RNA was isolated from cells grown in 21% and 1% oxygen, and the mRNA expression of TGF-β1 and SLPI was subsequently quantified using RT-PCR. TGF-β1 mRNA was significantly up-regulated in response to hypoxia (Figure 4A). Cells were then incubated in 21% or 1% O2, in the presence of a neutralizing antibody against TGF-β1. An isotype control antibody was used in parallel. Compared to the control antibody, SLPI expression in hypoxic cells was increased in response to anti-TGF-β1 (Figure 4B). Taken together, these results suggest that hypoxia down-regulates SLPI expression via up-regulation of TGF-β1 in bronchial epithelial cells.
Expression of TGF-β1 and SLPI in response to hypoxia. A) Expression of TGF-β1 mRNA in bronchial epithelial cells cultured in 21% or 1% oxygen. The fold changes were calculated by normalizing to data from the 21% O2 samples (n = 4). B) SLPI concentrations in the cell medium from bronchial epithelial cells incubated at 21% or 1% O2 in the presence of a neutralizing antibody against TGF-β1 (50 μg/ml) or an isotype control antibody (n = 5).
Medium from hypoxic cells is less potent in inhibiting neutrophil elastase activity
To investigate whether hypoxia affects the anti-protease property of the airway epithelium, baso-lateral cell medium from ALI cells stimulated with 21% or 1% oxygen was incubated with neutrophil elastase. Elastase activity was thereafter measured using a chromogenic assay. The results show a small but statistically significant difference between normoxic and hypoxic cell medium, where medium from normoxic cells inhibited elastase activity more efficiently (Additional file 1: Figure S1). These results indicate that hypoxia reduces the anti-protease potential of the airway epithelium.
Discussion
We have demonstrated a mechanism whereby SLPI is down-regulated in the airway epithelium in response to hypoxia (Figure 5). Under normal conditions, SLPI is constitutively expressed in epithelial cells. When oxygen is limited, hypoxia promotes up-regulation of TGF-β1, which in turn down-regulates SLPI.
Schematic model of hypoxia-driven SLPI expression. During normal oxygenation, the bronchial epithelium constitutively secretes SLPI, both apically and baso-laterally. When the oxygen concentrations decrease, the epithelium increases its expression of TGF-β1, which in turn down-regulates SLPI production.
When tissues are exposed to hypoxia, cells respond by up-regulating a number of genes, mainly through the hypoxia-inducible factor (HIF) pathways. HIFs are heterodimeric transcription factors that are rapidly degraded under conditions of normal oxygenation, but are stabilized and accumulate during hypoxia [21]. It has been reported that HIF-1α is up-regulated in the airways of patients with COPD and chronic bronchitis [22,23]. Although HIF-1α has been shown to regulate the TGF-β1 gene in mesenchymal stem cells [24], further studies are needed to investigate whether TGF-β1 and SLPI in the airways are regulated via HIF-1α or other hypoxia-driven pathways under hypoxic conditions. However, the increase in HIF-1α in the airways of COPD patients indicates that they have hypoxic regions in their lungs. Studies using direct oxygen measurements in the lungs of CF patients have demonstrated a hypoxic environment in the airway mucus, with mean oxygen levels as low as 2.5 mmHg [25]. SLPI concentrations in the airways of COPD and CF patients are reduced during bacterial exacerbation, and it has been postulated that the mechanism behind this observation involves proteolytic cleavage of the protein by neutrophil elastase [26-29]. Here, we propose a complementary mechanism whereby aggravated hypoxia during infection contributes to the reduction in SLPI concentration. Interestingly, it has also been reported that down-regulation of SLPI in COPD involves TGF-β1 signalling [30], which supports our model.
Conflicting data have been reported regarding the effects of hypoxia on TGF-β1 secretion. Boussat and co-workers found no change in TGF-β1 protein levels in the cell medium from pulmonary epithelial cells grown under hypoxic conditions [31], whereas others have reported an increase in TGF-β1 protein in response to hypoxia [24]. Our data show that neutralization of TGF-β1 with an antibody increases SLPI levels in hypoxic cells, suggesting that TGF-β1 concentrations are elevated under hypoxic conditions. Interestingly, Ambalavanan and co-workers showed that mice exposed to hypoxia exhibit an increased level of active TGF-β1 in their lungs, whereas total TGF-β1 concentrations were unchanged compared with controls [32]. This observation may explain the conflicting results on TGF-β1 levels in response to hypoxia.
SLPI was originally identified as a physiologically important inhibitor of neutrophil elastase and other proteases. Dysregulation of SLPI levels in the lungs of COPD and CF patients would therefore render them more vulnerable to damage caused by the inflammatory response. It is now known that the protein also has antimicrobial and immunomodulatory functions. SLPI has been shown to be effective against a number of gram-positive and gram-negative bacteria, including species common in the CF and COPD respiratory tract, such as Pseudomonas aeruginosa and Staphylococcus aureus [3,33]. Furthermore, SLPI can inhibit the inflammatory response by binding to lipopolysaccharide, thereby preventing macrophage activation [34]. This has been demonstrated in vivo, where SLPI-deficient mice challenged with lipopolysaccharide showed higher mortality and higher IL-6 expression than wildtype mice [35]. Moreover, clinical studies in which CF patients were given aerosolized recombinant SLPI have demonstrated that SLPI not only neutralizes neutrophil elastase activity, but also has an immunoregulatory effect, reducing IL-8 and neutrophil levels in the epithelial lining fluid [36,37]. This multifunctional role of SLPI makes it interesting from a therapeutic point of view, as altering this system could potentially reduce over-exaggerated proteolytic activity in inflammatory lung diseases such as COPD and CF, modulate the inflammatory response, and protect these patients from bacterial infections [38].
A limitation of the present study is that only epithelial cells were studied. Fibroblasts, endothelial cells and inflammatory cells were not studied, and their contributions to SLPI regulation in an in vivo setting are therefore unknown. Moreover, the experiments were carried out under non-inflammatory conditions. Further studies are thus needed to evaluate how hypoxia effects SLPI expression in the presence of normal airway microbiota, airway pathogens or other inflammatory stimuli.
Conclusion
In conclusion, the present study demonstrates that SLPI is down-regulated in the bronchial epithelium during hypoxia. These findings may help explain the pathogenesis of inflammatory lung diseases, and provide a potential target for the treatment and prevention of exacerbation in these patients.
Abbreviations
- SLPI:
-
Secretory leucocyte protease inhibitor
- COPD:
-
Chronic obstructive pulmonary disease
- CF:
-
Cystic fibrosis
- TGF-β1:
-
Transforming growth factor β1
- ALI:
-
Air liquid interface
- PCL:
-
Periciliary liquid
- HIF-1α:
-
Hypoxia-inducible factor-1α
References
Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond). 2006;110:21–35.
Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986;83:6692–6.
Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4.
Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–7.
Gomez SA, Arguelles CL, Guerrieri D, Tateosian NL, Amiano NO, Slimovich R, et al. Secretory leukocyte protease inhibitor: a secreted pattern recognition receptor for mycobacteria. Am J Respir Crit Care Med. 2009;179:247–53.
Cooper MD, Roberts MH, Barauskas OL, Jarvis GA. Secretory leukocyte protease inhibitor binds to Neisseria gonorrhoeae outer membrane opacity protein and is bactericidal. Am J Reprod Immunol. 2012;68:116–27.
Calverley PM. COPD: what is the unmet need? Br J Pharmacol. 2008;155:487–93.
Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001.
Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–21.
Birrer P, McElvaney NG, Rudeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:207–13.
Bandi V, Apicella MA, Mason E, Murphy TF, Siddiqi A, Atmar RL, et al. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–9.
Rosell A, Monso E, Soler N, Torres F, Angrill J, Riise G, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:891–7.
Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–65.
Zemanick ET, Sagel SD, Harris JK. The airway microbiome in cystic fibrosis and implications for treatment. Curr Opin Pediatr. 2011;23:319–24.
Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy. 2012;67:1193–202.
Jaumann F, Elssner A, Mazur G, Dobmann S, Vogelmeier C. Transforming growth factor-beta1 is a potent inhibitor of secretory leukoprotease inhibitor expression in a bronchial epithelial cell line. Munich Lung Transplant Group. Eur Respir J. 2000;15:1052–7.
Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–9.
Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17.
Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–23.
Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol. 2001;13:89–95.
Metzen E, Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biol Chem. 2004;385:223–30.
Polosukhin VV, Cates JM, Lawson WE, Milstone AP, Matafonov AG, Massion PP, et al. Hypoxia-inducible factor-1 signalling promotes goblet cell hyperplasia in airway epithelium. J Pathol. 2011;224:203–11.
Lee SH, Lee SH, Kim CH, Yang KS, Lee EJ, Min KH, et al. Increased expression of vascular endothelial growth factor and hypoxia inducible factor-1alpha in lung tissue of patients with chronic bronchitis. Clin Biochem. 2014;47:552–9.
Hung SP, Yang MH, Tseng KF, Lee OK. Hypoxia-induced secretion of TGF-beta1 in mesenchymal stem cell promotes breast cancer cell progression. Cell Transplant. 2013;22:1869–82.
Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–25.
Parameswaran GI, Sethi S, Murphy TF. Effects of bacterial infection on airway antimicrobial peptides and proteins in COPD. Chest. 2011;140:611–7.
Parameswaran GI, Wrona CT, Murphy TF, Sethi S. Moraxella catarrhalis acquisition, airway inflammation and protease-antiprotease balance in chronic obstructive pulmonary disease. BMC Infect Dis. 2009;9:178.
Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo MB, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1117–24.
Weldon S, McNally P, McElvaney NG, Elborn JS, McAuley DF, Wartelle J, et al. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J Immunol. 2009;183:8148–56.
Luo BL, Niu RC, Feng JT, Hu CP, Xie XY, Ma LJ. Downregulation of secretory leukocyte proteinase inhibitor in chronic obstructive lung disease: the role of TGF-beta/Smads signaling pathways. Arch Med Res. 2008;39:388–96.
Boussat S, Eddahibi S, Coste A, Fataccioli V, Gouge M, Housset B, et al. Expression and regulation of vascular endothelial growth factor in human pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L371–8.
Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, et al. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L86–95.
Wiedow O, Harder J, Bartels J, Streit V, Christophers E. Antileukoprotease in human skin: an antibiotic peptide constitutively produced by keratinocytes. Biochem Biophys Res Commun. 1998;248:904–9.
Ding A, Thieblemont N, Zhu J, Jin F, Zhang J, Wright S. Secretory leukocyte protease inhibitor interferes with uptake of lipopolysaccharide by macrophages. Infect Immun. 1999;67:4485–9.
Nakamura A, Mori Y, Hagiwara K, Suzuki T, Sakakibara T, Kikuchi T, et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J Exp Med. 2003;197:669–74.
McElvaney NG, Nakamura H, Birrer P, Hebert CA, Wong WL, Alphonso M, et al. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992;90:1296–301.
McElvaney NG, Doujaiji B, Moan MJ, Burnham MR, Wu MC, Crystal RG. Pharmacokinetics of recombinant secretory leukoprotease inhibitor aerosolized to normals and individuals with cystic fibrosis. Am Rev Respir Dis. 1993;148:1056–60.
Zani ML, Tanga A, Saidi A, Serrano H, Dallet-Choisy S, Baranger K, et al. SLPI and trappin-2 as therapeutic agents to target airway serine proteases in inflammatory lung diseases: current and future directions. Biochem Soc Trans. 2011;39:1441–6.
Acknowledgements
The authors wish to thank Pia Andersson and Gisela Håkansson for excellent technical assistance. This study was supported by grants from the Swedish Heart and Lung Foundation, the Swedish Government Funds for Clinical Research (ALF), and the Magnus Bergvall, Tore Nilson, and Skåne University Hospital in Lund foundations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LP and AE conceived of the study, participated in the design and coordination of the study, and drafted the manuscript. LP, AJ, MG and MM carried out the experiments. All authors read and approved the final manuscript.
Additional file
Additional file 1: Figure S1.
Cell medium from hypoxic cells has reduced capacity to neutralize neutrophil elastase. Cell medium from air-liquid interface cultures exposed to normoxia or hypoxia were incubated with neutrophil elastase, and the elastase activity in the samples was thereafter analysed with a chromogenic assay. The figure shows neutrophil elastase (NE) activity in the presence of normoxic or hypoxic cell medium, compared to control medium (n = 6).
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Påhlman, L.I., Jögi, A., Gram, M. et al. Hypoxia down-regulates expression of secretory leukocyte protease inhibitor in bronchial epithelial cells via TGF-β1. BMC Pulm Med 15, 19 (2015). https://doi.org/10.1186/s12890-015-0016-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-015-0016-0