Abstract
Background
A cross-sectional population-based survey in the Beichen district of Tianjin was conducted to estimate the prevalence of dry eye disease (DED) among the population over 50 years old with Dry Eye Workshop (DEWS) II and Chinese expert consensus (CEC) criteria.
Methods
A total of 5791 subjects over 50 years old were enrolled. Ocular surface disease index (OSDI) questionnaire, lipid layer thickness (LLT), partial blink ratio (PBR), fluorescein tear film breakup time (FBUT), Schirmer I test (SIT), fluorescein staining, meibomian gland dropout, meibomian gland expression scores (MES) and quantity scores (MQS) was assessed. Additionally, basic information, weight, disease history, living habits, anxiety, and depression condition were collected.
Results
According to the CEC, the prevalence of DED was 39.0%, whereas 44.0% based on DEWS II. The prevalence of DED increased with age and was substantially greater among women (41.1%, 95% CI, 39.5-42.6%) than males (35.1%, 95% CI, 33.1 -37.2%) (p < 0.001). Furthermore, the severity of DED was more severe in women (p = 0.006). The associated risk factors were age, female, depression, smoking, insomnia, and glaucoma. On the other hand, 53.6% of these populations were diagnosed as asymptomatic DED, and the morbidity was higher in males (p = 0.007).
Conclusions
The prevalence of DED in China was relatively high, which was associated with aging, female(sex), depression, smoking and sleep problems. Hence, it is crucial for clinicians and caregivers to be aware of the existence of asymptomatic DED within the susceptible population.
Similar content being viewed by others
Introduction
Dry eye disease (DED) is a chronic ocular surface disease caused by multiple factors. The unstable tear film or imbalance of the ocular surface microenvironment is caused by abnormal tear quality, quantity, and dynamics. It can be accompanied by ocular surface inflammatory reaction, tissue damage, and nerve abnormality, resulting in various ocular discomfort symptoms and/or visual dysfunction [1]. DED can cause a variety of eye symptoms, such as pain, itching, photophobia, fatigue, burning, foreign body sensation, and visual impairment [2, 3], therefore, has a significant impact on the mental state, quality of life, and work efficiency of patients. In China, the average annual economic loss of each dry eye patient due to treatment was estimated to be 465.54 ± 303.08 USD [4].
In recent years, factors such as environmental pollution and the popularity of smartphones and computers has been reported to worsen the prevalence of DED [5]. However, it has also been suggested that due to differences in demographic characteristics and the DED diagnostic criteria or tests used in the study, the prevalence of DED can be biased [6]. According to the 2017 Dry Eye Workshop (DEWS) II, the prevalence of DED worldwide was 5 − 50%. In the United States, Dana et al. [7] analyzed the United States Health Care System and found that prevalence of DED in the United States was 5.28%. Caffery et al. [8] investigated the population of Ontario and found that the prevalence of DED was 22.0%. However, the prevalence of DED in Asia was the highest and had become an independent risk factor for DED [9]. Sherry et al. [10]. found that the prevalence of DED in people over 18 years old was 36.4% in Lebanon. In India, the prevalence of DED in rural areas was 34.98%, while in urban areas, 65.02% [11]. A survey in Japan showed that the prevalence of DED in people aged 40–74 was 48.2% [12]. Meta-analysis showed the prevalence of DED in China was 13.55% according to the literature published from 1990 to 2016 [3], especially the prevalence reached 52.4% in Tibet [13].
DED is a multifactorial disease, and senility is one of the most important contributing factor. Bakkar et al. [14]. and Wang et al. [15]. found that the prevalence of DED increased with age. Dana R et al. [7] reported that the prevalence of DED increased from 0.02% at the age of 2–17 to 11.66% of the population over 50 years old through the analysis of the data from the United States Health Care System. In addition to the region and age, sex is also an important factor. Bikbov et al. [16]. Reported that the prevalence of DED in females was higher in Russia. Moreover, DED has been related to video terminal usage, diabetes, sleep disorders, anxiety and depression, abnormal lipid metabolism, the use of contact lenses, eye surgery and other factors [12, 17,18,19,20,21,22]. However, there is a paucity of epidemiological studies on DED from China and the study data usually consists of outpatients. Hence, the objective of this investigation was to examine the frequency and determinants of DED among individuals aged 50 and above, not receiving clinical treatment.
Methods
Study population
The data was from the Beichen Eye Study. Beichen Eye Disease was an epidemiological survey of eye diseases based on community population by Tianjin Medical University Eye Hospital from December 2019 to February 2022. Beichen District, located in the north of the central of Tianjin, is in the transition stage from rural to urban, with small population flow, which meets the basic requirements of epidemiological investigation. This study obtained the ethical approval from the Ethics Committee of the Tianjin Medical University Eye Hospital (Ethics approval number: 2019ky-22). All subjects signed the informed consent form. The Beichen District has 16 streets (towns) and a multi-stage random sampling method was adopted. Four streets (towns) were randomly selected at the first stage and then 12 villages (neighborhood communities) with 8,218 residents were randomly selected. The list of sampling frame was obtained from the Beichen District Health Commission and neighborhood committees. Recruitment information was released to selected villages through leaflets and promotional videos and residents contacted by phones to confirm whether they qualified for enrolment. Individuals had to be aged 50 years of age or older, and to have been a resident at least 6 months in the target neighborhood community to be eligible for inclusion in the study. Exclusion Criteria: (1)Those who had moved from their residing address, had not lived there for more than 6 months, life expectancy less than 3 months or had severe mental illness, not contactable (after three unsuccessful phone calls); (2) Any eye disease diagnosed as affecting tear quality, tear volume or tear production, such as eyelid entropion, ectropion, ptosis, eyelid dyskinesia and conjunctivitis; (3) History of eye surgery or retinal laser photocoagulation within the past 6 months; (4) Systemic diseases outside the study; (5) Long-term oral or topical use of antibiotics or dry eye disease related drugs. A total of 5791 subjects were included in the study. Written informed consent was obtained from each participant. The present research adhered to the tenets of the Declaration of Helsinki.
Questionnaire
The information was collected by three trained investigators. The questionnaire include: (1) Basic information: name, sex, age, education level and occupation; (2) Disease history: hypertension, diabetes, abnormal lipid metabolism, cardiovascular and cerebrovascular diseases, and eye diseases; (3) Life habits: The daily behaviors such as using video terminals, smoking, drinking and sleeping. (4) Anxiety and depression questionnaire. (5) Ocular Surface Disease Index (OSDI) questionnaire. The OSDI score was defined as normal (0–12 points), mild (13–22 points), moderate (23–32 points), or severe (33–100 points) [23, 24].
Comparison between the diagnostic criteria
According to the Chinese expert consensus (CEC) on DED [1, 25], the diagnostic criteria for DED are: (1) The patient complained one of the subjective symptoms, such as dryness sensation, foreign body sensation, burning sensation, fatigue, discomfort, redness of eyes, visual fluctuation, etc., the China Dry Eye Questionnaire ≥ 7 points or the OSDI ≥ 13 points; At the same time, fluorescein tear film breakup time (FBUT) ≤ 5s or noninvasive breakup time (NIBUT) < 10s, or Schirmer I test (without anesthesia) ≤ 5 mm/5min can be diagnosed DED. (2) The patient has symptoms related to DED, the China Dry Eye Questionnaire ≥ 7 points or the OSDI questionnaire ≥ 13 points; At the same time, FBUT > 5s and ≤ 10s or NIBUT = 10 ~ 12s, Schirmer I test (without anesthesia) > 5 mm/5min and ≤ 10 mm/5min, need to use fluorescein sodium staining to check the corneal and conjunctiva. Positive staining (≥ 5 points) can diagnose DED. Dry Eye Workshop (DEWS) II states that, symptoms and at least one positive result (NIBUT or FBUT < 10s, Osmolarity ≥ 308mOsm/L in either eye or interocular different > 8mOsm/L, ocular surface staining > 5 corneal spots or > 9 conjunctival spots or lid margin ≥ 2 mm length & ≥ 25% width) should also constitute the diagnosis of DED [26]. Participants were assessed using both DED diagnosis criteria and compared (CEC and DEWS II). In addition, we defined asymptomatic DED as a population with the OSDI score < 13 but clinical signs that meet the Chinese DED criteria [27].
Examinations
Systemic examinations
The examination of height, weight, waistline, hipline and blood pressure of the study populations was performed. In addition, routine blood test, blood sugar, glycosylated hemoglobin (HbA1c), lipid metabolism and hepatic and renal functions of each participant were tested by venous blood collection.
Ocular examinations
Lipid layer thickness (LLT) of tear film and partial blink ratio (PBR) were measured by LipiView® II Ocular Surface Interferometer (TearScience, Inc. NC, USA). In addition, we used LipiView Interferometer to take photos of the meibomian gland (separate measurement over the upper lid and lower lid) of each participant, and scored the meibomian gland dropout according to the photos: 0 point, no meibomian gland dropout; 1 point, meibomian gland dropout ratio < 1/3; 2 points, the meibomian gland dropout ratio is 1/3 − 2/3; 3 points, meibomian gland dropout ratio > 2/3. Subsequently, FBUT and corneal were examined using fluorescein staining with slit lamp examination. In a nutshell, the Fluorescein Sodium Ophthalmic Strips (Tianjin Jingming New Technological Development Co.,Ltd ) was soaked with normal saline and then gently coated on the conjunctival sac. It is evenly distributed in the cornea by multiple blinks. Cobalt blue light of slit-lamp biological microscope was used for observation [28]. The expressibility of meibum was observed with the Meibomian Gland Evaluator (separate measurement over the upper lid and lower lid). There are 5 glands at each position, and a total of 15 glands were observed. Three positions were detected on each eyelid of the participant: Nasal, middle and temporal. 0 point, all glands expressible; 1 point, 3–4 glands expressible; 2 points, 1–2 glands expressible; 3 points, no glands expressible. At the same time, the characteristics of its secretion were also scored. The scoring standard is: 0, clear liquid; 1 point, cloudy liquid; 2 points, cloudy particulate fluid; 3 points, inspissated, like toothpaste. The upper and lower lids of each eye were also detected and recorded. Finally, Schirmer I test was used to detect the basic tear secretion (Fig. 1).
Data analysis
All data were analyzed using the Statistical Package for Social Sciences (SPSS) software (SPSS for windows version 24.0; SPSS Inc, USA). The values conforming to the normal distribution are expressed by the mean ± standard deviation, and the data not conforming to the normal distribution are expressed by the median (interquartile interval). Chi-square test was used to test the difference of prevalence among groups. Wilcoxon (Mann-Whitney) rank sum test was used for data that did not conform to the normal distribution. Chi-square test and logistic regression model were used to analyze the related factors of DED. P values < 0.05 were considered statistically significant.
Results
A total of 5791 volunteers were assessed in this study, with median age of 63 (range: 58, 67). Of these, 2056 comprised of males (35.5%), with a median age of 64 (range: 59, 68) and 3735 females (64.5%), with a median age of 62 (range: 57, 67). The population of individuals between the ages of 60 and 69 was 2896, accounting for 50.0% of the total, 1959 individuals (33.8%) were aged 50–59 and 936 individuals (16.2%) were aged 70 and above. Junior high school degree held the highest proportion of subjects, 2518 (43.5%), in relation to their educational attainment. With regard to weight distribution, the study population comprised of 4229 individuals (74.2%) who were classified as overweight or obese. (Table 1).
Based on the CEC for DED, the study found that 2256 individuals had DED, resulting in a prevalence rate of 39.0%. When applying the DEWS II diagnostic criterion, we observed that 2547 patients had DED, with a prevalence rate of 44.0%. This prevalence rate was significantly greater than that determined by the CEC (p < 0.001) (Table 2).
The following data analysis was based on the CEC for DED. Prevalence was highest among aged 70 and above (42.4%; 95% CI, 39.2-45.6%) and lowest among those aged 50–59 years (35.8%; 95% CI, 33.7-37.9%). Prevalence among those aged 60–69 years was 40.0% (95% CI, 38.2–41.8%). The prevalence of dry eye increased with age, and the prevalence of DED among those aged 50–59 years was significantly lower than among those aged 60–69 years (p = 0.01) and aged 70 and above (p = 0.002), while the prevalence of DED among those aged 60–69 years and aged 70 and above had no significant statistical difference (p = 0.556) (Table 3).
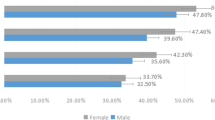
In terms of sex, the number of male patients with dry eye was 722, accounting for 35.1% (95% CI, 33.1-37.2%) of the male subjects (2056) in this study, whereas the number of female dry eye patients was 1534, reaching 41.1% (95%CI, 39.5-42.6%) of the female subjects (3735). The prevalence of female was significantly higher than that of male (p < 0.001) (Fig. 2). After comparing the prevalence of DED in different sexes of each age group, it was found that the prevalence of DED in female of all age was higher than that in male, but there was no statistically significant difference among those aged 50–59 years (p = 0.077), and the difference was the largest among those aged 60–69 years (p < 0.001) (Fig. 3).
According to the OSDI scores, study population with DED were divided into mild, moderate and severe. After comparing the prevalence of DED severity among sexs and ages (Table 4), it was found that the prevalence of severe DED in females were significantly higher than that in males (p = 0.006), however, there was no statistically significant difference in other groups (P > 0.05).
Ocular surface symptoms and sign
Compared with non-DED populations, FBUT, Schirmer I test, PBR and meibomian gland expressibility of DED population were significantly worse (p < 0.05). The mean LLT, meibomian gland morphology and secretion characteristics scores were not significant difference (Table 5).
Risk factors for DED
Logistic regression analysis was performed on the possible influencing factors of DED, and the analysis results are shown in Table 6. Increasing age, female, depression, smoking, insomnia, and glaucoma were strongly associated with DED, while mobile phone use was negatively associated with DED. Compared to non- depressed populations, mild and moderately severe depressed populations were more prone to DED (p < 0.05). The number of volunteers with sleep disorder suffering from DED was found to be 1188, accounting for 52.7% of the total number of DED participants. There was a statistically significant association between sleep disorder and DED (p < 0.001). The proportion of smokers in DED populations (20.5%) was higher than that in non-DED populations (19.5%), and the difference was obvious (p = 0.002). In the blood test results of participants in this study, the prevalence of DED with high cholesterol was lower than that of normal cholesterol (Table 6).
Asymptomatic DED
Furthermore, the study identified 3105 of the participants had asymptomatic DED, resulting in the prevalence of 53.6%. Among them, the number of asymptomatic males with DED were found to be 1151, accounting for 56.0% (95% CI, 53.8 -58.1%) of males in this study; 1954 female, accounting for 52.3% of the females in this study (95%CI, 50.7-53.9%). The incidence of asymptomatic DED in male was found to be higher than that in female, with statistically significant difference (p = 0.007) (Fig. 4).
Comparison of the prevalence of DED among sexs. Compare the prevalence of asymptomatic DED in different sexs. The number of asymptomatic DED in male was 1151(56.0%, 95% CI 53.8% -58.1%); in female was 1954 ( 52.3%, 95%CI 50.7-53.9%) The prevalence of DED in male was significantly higher than that in female (p = 0.007)
In terms of age, the number of asymptomatic DED patients in 50–59 years old was 1106, accounting for 56.5% (95% CI, 54.3 -58.7%) of the participants in this age group. On the contrary, the lowest morbidity of asymptomatic DED was 48.6% (95% CI, 45.4 -51.8%) among those aged 70 and above. There were statistically significant differences in the prevalence rates among different age groups (Table 7).
Discussion
This study was the community-based epidemiological survey of DED in Beichen District, Northern China. According to the CEC diagnostic criteria for DED, the analysis found that the prevalence of DED was 39.0%, coinciding to the worldwide prevalence of DED (5 -50%) according to the DEWS II in 2017 [9]. When we used DEWS II diagnostic criteria, the prevalence of DED was 44.0% in our study, which was significantly higher than CEC criteria. A major challenge in the epidemiological study of DED is that there are no specific or universal diagnostic criteria that is utilized by various regions and populations [9]. Compared to the DEWS II criteria, CEC diagnoses fewer cases. Research has showed that there is a good concordance between the CEC and the Asian Dry Eye Society diagnostic criteria was (97.2%) [29]. A large amount of evidence suggests that the probability of DED in Asian people was 1.5–2.2 times that of western population [9]. This disparity in the prevalence could be related to the diagnostic criteria utilized by publications as most Asian publications tends to follow the criteria laid out by DEWS However, research also suggests that the prevalence of DED could be regional as the prevalence of DED in India was found to be 32%28 64% in Palestine [30] and Inner Mongolia, China, was found to have a prevalence rate of 34.5% [31]. Nevertheless, while the effect of diagnostic criteria and regions on the prevalence of DED can be further explored, it is important monitor and address the high prevalence of DED among communities.
Another possible explanation for the high occurrence seen in this research might be the advanced age of the participants. Previous studies have consistently shown a strong correlation between dry eye disease (DED) and older age. DEWS II, a comprehensive report on DED, also noted a progressive rise in DED prevalence among those aged 50 and above [9] which is consistent with the results of this study. In addition, a large DED survey conducted by Dana et al. [7] found that the prevalence of DED increased from 0.20% aged 2–17 to 11.66% aged over 50, which also confirmed that the prevalence of DED aged over 50 was higher. Our research revealed substantial variations in the occurrence of DED among individuals over the age of 50 at various age intervals. Furthermore, there was a notable correlation between age and the likelihood of developing DED, with the risk increasing as age advanced. However, there was no notable disparity in the intensity of DED across different age groups.
We observed that the incidence and intensity of DED were greater in females compared to males. The DEWS II confirms that there is a significant difference in the occurrence of DED across sexs in individuals aged 50 years and older. Furthermore, sex was found to be an independent risk factor for DED in our study [9]. In addition, studies by Wang et al. [15]. Dana et al. [7]. and Bikbov et al. [32]. have reported the higher risk of DED in elderly female population due to the decline in estrogen and progestogen secretion [3, 33]. Furthermore, females have been reported to have a higher susceptibility to immunological illnesses compared to males, including Sjogren’s syndrome, systemic lupus erythematosus, thyroid disease, and rheumatoid arthritis, all of which may also contribute to DED [34].
Upon examination of the risk factors, DED correlated with sleep disorders, smoking, depression, glaucoma, mobile phone use, and television viewing or video game playing on a television screen. Increasingly, researchers are focusing on the connection between sleep disturbances and DED. A large, community-based study in Hangzhou, China showed a strong positive association between sleep disorders and higher severity of DED [35]. Similar to previous studies, our research also found that 1188 people with DED had sleep disorders, accounting for 52.7% of the total number of DED, which has obvious statistical significance in logistic regression analysis. Depression can also affect DED, which is similar to sleep disorders. It was reported that patients with depression often have sleep disorders [36, 37]. He et al. [19] conducted a study on 321 DED patients from the Tianjin Medical University Eye Hospital and found that there was a significant correlation between DED and depression, and sleep disorders was the mediator between DED and depression; Coincidentally, the study by Liu et al. [38]also found that depression is closely related to DED, and sleep disorders was associated with anxiety and depression in patients aged 41 to 65. Therefore, according to previous studies, the impact of depression on DED is likely to be achieved through sleep disorders. Conversely, the quality of life decreased with DED can lead to anxiety, depression, sleep disorders and other conditions. Compared to other irritant eye diseases (such as chronic conjunctivitis, allergic conjunctivitis, etc.), DED can cause more severe sleep disorders [39]. In summary, DED, sleep disorders, and depression interact with each other. The occurrence of one disease may lead to the occurrence or exacerbation of the other two diseases. In a large registry study, 52.6% (10,338/19,665) of glaucoma patients were diagnosed with DED [40]. Similar to the findings in the general population, DED in patients with glaucoma is more prevalent in the elderly population. And it was more common in women (56.9%) than men (45.7%) [41]. Similar to these studies, our study also found that glaucoma is a significant risk factor for DED. The increased prevalence of DED in patients with glaucoma is often associated with the use of anti-glaucoma drugs. In a large study of glaucoma patients (N = 10,325), the odds of DED were found to increase with the number of drugs used (OR = 1.23,2 drugs; OR = 1.63,3 drugs; OR = 2.60, containing 4 drugs) [42]. Together, these findings suggest that it is particularly important to evaluate DED in patients with glaucoma.
Interestingly, we found a decrease in the prevalence of DED among elderly people who use smartphone and watch or play game on TV. In contrast, extensive cross-sectional analysis found that the use of video terminals can lead to an increase in the prevalence of DED. Nguyen et al.‘s research even suggested that using visual display terminal (VDT) and other sedentary activities can increase the incidence of DED [43]. We speculate that this may be related to the age of the study population. Most of the study population is students or staff, with a younger age, this article focused on middle-aged and elderly people over 50 years old. This may be due to the fact that older people spend less time and frequency using electronic devices than younger people. In China and other low- and middle-income countries, especially in rural areas, a digital divide between older and younger people is still prevalent [44]. Older people’s inability to afford Internet access or related devices, or their lack of knowledge and skills to use these technologies make a significant difference in the frequency of their use of electronic devices compared with younger generations [45]. In addition, having severe DED significantly affects visual quality, and this population may be less likely to choose to use VDT [46], resulting in a lower prevalence of DED among older adults who use VDT. We need more mechanistic research to determine the impact of VDT on the population aged 50 and above.
Research had found that dyslipidemia, especially hypercholesterolemia, could increase the melting point of meibomian gland lipid, which could not be squeezed from the meibomian gland, leading to DED [20]. However, there were different results in different cross-sectional studies: Vehof et al. [47]. found no significant correlation between DED and dyslipidemia; Rathnakumar et al. [20]. found that the increased prevalence of DED was related to hypercholesterolemia in female; Through a survey of 2272 middle-aged people in South Korea, Choi et al. [48]found that dyslipidemia was more likely to lead to an increase in the prevalence of DED in male. This study differs from previous studies in that the increase in total cholesterol leads to a decrease in the prevalence of DED, while the rest of the blood results have no significant correlation with DED.
Patients exhibiting signs of ocular surface disease but reporting no symptoms of discomfort was called asymptomatic DED, which was mentioned in DEWS II [26]. We observed that the prevalence of asymptomatic DED was 53.6%. Surprisingly, the condition of asymptomatic DED is opposite to that of DED. The prevalence of asymptomatic DED in male was higher than that in female, and the younger the age, the higher the incidence of asymptomatic DED. The mismatch between symptoms and signs is a common situation and a dilemma in DED research [49,50,51]. For example, it is quite common to encounter a patient with severe symptoms but lacking evidence of staining. Similarly, a patient may be asymptomatic but present with obstructed meibomian glands, short breakup time, and high osmolality [52]. There are several studies that show that people with chronic pain syndromes, atopic disorders, known allergies, antihistamine use, osteoarthritis, depression, and antidepressant use will have more symptoms of DED than signs [50]. In our study, there are possible reasons why the signs of DED outweigh the symptoms in men. First, there are sex differences in sensitivity of the ocular surface. Several studies conducted in different settings have shown increased corneal or conjunctival sensitivity in women [53, 54]. Second, sex differences in general pain sensitivity may play a role. Compared to women, men show less negative pain responses (i.e., increased threshold and tolerance to pain) [55]. Ocular pain was the common complaint among DED patients in outpatient [56, 57], but longstanding DED were likely to cause corneal nerve damage [50]. Corneal sensitivity reduced can mask discomfort symptoms [50]. These asymptomatic DED study populations were at risk of developing manifest DED with time or provocation, for example following ophthalmic surgery [22] should attract our more attention.
In conclusion, the prevalence of DED can vary with the diagnostic assessment criteria used. The prevalence of DED among individuals aged 50 and above was found to be 39.0%. It increased with age and was overall higher in female. Elderly women had the highest prevalence of DED and impacted their visual quality. In addition, depression, smoking and sleep disorders also increased the risk of DED. On the other hand, the prevalence of asymptomatic DED was 53.6% and male were more susceptible, this should prompt clinicians and caregivers to be vigilant in order to minimize the likelihood of developing clinical DED.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- DED:
-
Dry eye disease
- DEWS:
-
Dry Eye Workshop
- CEC:
-
Chinese expert consensus
- OSDI:
-
Ocular Surface Disease Index questionnaire
- LLT:
-
Lipid layer thickness
- FBUT:
-
Fluorescein tear film breakup time
- SIT:
-
Schirmer I test
- MES:
-
Meibomian gland expression scores
- MQS:
-
Meibomian quantity scores
- FBUT:
-
Fluorescein tear film breakup time
- NIBUT:
-
Noninvasive breakup time
- PBR:
-
Partial blink ratio
- VDT:
-
Visual display terminal
References
Wang Y, Tang XJ, Liu Q, Chen L. The incidence and risk factors for Dry Eye after Pediatric Strabismus surgery. Ophthalmol Ther. 2023;12(1):87–98.
Hyon JY, Yang HK, Han SB. Association between Dry Eye Disease and psychological stress among Paramedical Workers in Korea. Sci Rep. 2019;9(1):3783.
Song P, Xia W, Wang M, Chang X, Wang J, Jin S et al. Variations of dry eye disease prevalence by age, sex and geographic characteristics in China: a systematic review and meta-analysis. J Glob Health. 2018;8(2).
Luo Y, Yang W, Qi M, Wang Y, Li S, Wang M, et al. Annual Direct Economic Burden and influencing factors of Dry Eye diseases in Central China. Ophthalmic Epidemiol. 2023;30(2):121–8.
Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The relationship between Dry Eye Disease and Digital screen use. Clin Ophthalmol. 2021;15:3811–20.
Chatterjee S, Agrawal D, Sanowar G, Kandoi R. Prevalence of symptoms of dry eye disease in an urban Indian population. Indian J Ophthalmol. 2021;69(5):1061.
Dana R, Bradley JL, Guerin A, Pivneva I, Stillman IÖ, Evans AM, et al. Estimated prevalence and incidence of Dry Eye Disease based on coding analysis of a large, all-age United States Health Care System. Am J Ophthalmol. 2019;202:47–54.
Caffery B, Srinivasan S, Reaume CJ, Fischer A, Cappadocia D, Siffel C, et al. Prevalence of dry eye disease in Ontario, Canada: a population-based survey. Ocul Surf. 2019;17(3):526–31.
Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–65.
Sherry A, Aridi M, Ghach W. Prevalence and risk factors of symptomatic dry eye disease in Lebanon. Contact Lens Anterior Eye. 2020;43(4):355–8.
Titiyal J, Falera R, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India: ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol. 2018;66(2):207.
Hanyuda A, Sawada N, Uchino M, Kawashima M, Yuki K, Tsubota K, et al. Physical inactivity, prolonged sedentary behaviors, and use of visual display terminals as potential risk factors for dry eye disease: JPHC-NEXT study. Ocul Surf. 2020;18(1):56–63.
Lu P, Chen X, Liu X, Yu L, Kang Y, Xie Q, et al. Dry Eye Syndrome in Elderly tibetans at High Altitude. Cornea. 2008;27(5):545–51.
Bakkar MM, Shihadeh WA, Haddad MF, Khader YS. Epidemiology of symptoms of dry eye disease (DED) in Jordan: a cross-sectional non-clinical population-based study. Contact Lens Anterior Eye. 2016;39(3):197–202.
Wang MTM, Muntz A, Mamidi B, Wolffsohn JS, Craig JP. Modifiable lifestyle risk factors for dry eye disease. Contact Lens Anterior Eye. 2021;44(6):101409.
Bikbov MM, Gilmanshin TR, Zainullin RM, Kazakbaeva GM, Iakupova EM, Fakhretdinova AA, et al. Prevalence and associations of dry eye disease and meibomian gland dysfunction in the ural eye and medical study. Sci Rep. 2022;12(1):18849.
Olaniyan SI, Fasina O, Bekibele CO, Ogundipe AO. Relationship between dry eye and glycosylated haemoglobin among diabetics in Ibadan, Nigeria. Pan Afr Med J. 2019;33.
Ayaki M, Kawashima M, Negishi K, Tsubota K. High prevalence of sleep and mood disorders in dry eye patients: survey of 1,000 eye clinic visitors. Neuropsychiatr Dis Treat. 2015;889.
He Q, Chen Z, Xie C, Liu L, Wei R. The Association between Dry Eye Disease with Depression, anxiety and sleep disturbance during COVID-19. Front Psychiatry. 2022;12.
Rathnakumar K, Ramachandran K, Baba D, Ramesh V, Anebaracy V, Vidhya R, et al. Prevalence of dry eye disease and its association with dyslipidemia. J Basic Clin Physiol Pharmacol. 2018;29(2):195–9.
Almutairi AH, Alalawi BS, Badr GH, Alawaz RA, Albarry M, Elbadawy HM. Prevalence of dry eye syndrome in association with the use of contact lenses in Saudi Arabia. BMC Ophthalmol. 2021;21(1):147.
Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–38.
Zhang X, Yang L, Zhang Q, Fan Q, Zhang C, You Y, et al. Reliability of Chinese web-based Ocular Surface Disease Index (C-OSDI) Questionnaire in Dry Eye patients: a randomized, crossover study. Int J Ophthalmol Int J Ophthalmol. 2021;14(6):834–43.
Zhou Y, Murrough J, Yu Y, Roy N, Sayegh R, Asbell P, DREAM Study Research Group, et al. Association between Depression and Severity of Dry Eye symptoms, signs, and inflammatory markers in the DREAM Study. JAMA Ophthalmol. 2022;140(4):392–9.
Liu ZG. [Paying attention to the expert consensus on dry eye to standardize and promote the clinical diagnosis and treatment of dry eye]. Zhonghua Yan Ke Za Zhi. 2020;56(10):726–9.
Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539–74.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83.
Eom Y, Lee JS, Keun Lee H, Myung Kim H, Suk Song J. Comparison of conjunctival staining between lissamine green and yellow filtered fluorescein sodium. Can J Ophthalmol. 2015;50(4):273–7.
Ou Yang W, Zuguo L, Xuguang S. Concordance between Chinese dry eye diagnostic criteria and Asian dry eye diagnostic criteria. Chin J Experimental Ophthalmol. 2022;40(11):1038–45.
Shanti Y, Shehada R, Bakkar MM, Qaddumi J. Prevalence and associated risk factors of dry eye disease in 16 northern West Bank towns in Palestine: a cross-sectional study. BMC Ophthalmol. 2020;20(1):26.
Wu J, Wu X, Zhang H, Zhang X, Zhang J, Liu Y et al. Dry Eye Disease among Mongolian and Han older adults in grasslands of Northern China: Prevalence, Associated Factors, and Vision-Related Quality of Life. Front Med (Lausanne). 2021;8.
Bikbov MM, Kazakbaeva GM, Rakhimova EM, Rusakova IA, Fakhretdinova AA, Tuliakova AM, et al. The prevalence of dry eye in a very old population. Acta Ophthalmol. 2022;100(3):262–8.
Sriprasert I, Warren DW, Mircheff AK, Stanczyk FZ. Dry eye in postmenopausal women. Menopause. 2016;23(3):343–51.
Matossian C, McDonald M, Donaldson KE, Nichols KK, MacIver S, Gupta PK. Dry Eye Disease: consideration for women’s Health. J Womens Health. 2019;28(4):502–14.
Yu X, Guo H, Liu X, Wang G, Min Y, Chen SHS et al. Dry eye and sleep quality: a large community-based study in Hangzhou. Sleep. 2019;42(11).
Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cogn Emot. 2013;27(3):567–76.
Ayaki M, Kawashima M, Negishi K, Kishimoto T, Mimura M, Tsubota K. Sleep and mood disorders in women with dry eye disease. Sci Rep. 2016;6(1):35276.
Liu Z, Sun S, Sun X, Wu Y, Huang Y. Differences of anxiety and depression in Dry Eye Disease patients according to Age groups. Front Psychiatry. 2022;13.
Ayaki M, Tsubota K, Kawashima M, Kishimoto T, Mimura M, Negishi K. Sleep disorders are a prevalent and serious comorbidity in Dry Eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES143–50.
Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1593–601.
Kobia-Acquah E, Gyekye GA, Antwi-Adjei EK, Koomson NY, Cobbina F, Donkor R, et al. Assessment of Ocular Surface Disease in Glaucoma patients in Ghana. J Glaucoma. 2021;30(2):180–6.
Chen HY, Lin CL, Tsai YY, Kao CH. Association between glaucoma medication usage and dry eye in Taiwan. Optom Vis Sci. 2015;92(9):e227–232.
Nguyen L, Magno MS, Utheim TP, Hammond CJ, Vehof J. The relationship between sedentary behavior and dry eye disease. Ocular Surf. 2023;28:11–7.
Yao Y, Zhang H, Liu X, Liu X, Chu T, Zeng Y. Bridging the digital divide between old and young people in China: challenges and opportunities. Lancet Healthy Longev. 2021;2(3):e125–6.
Gibson A, Bardach SH, Pope ND. COVID-19 and the Digital divide: will Social workers Help Bridge the gap? J Gerontol Soc Work. 2020 Aug-Oct;63(6–7):671–3.
Thomas J, Almidani L, Swenor BK, Varadaraj V. Digital Technology Use among older adults with Vision Impairment. JAMA Ophthalmol. 2024;142(5):445–52.
Vehof J, Snieder H, Jansonius N, Hammond CJ. Prevalence and risk factors of dry eye in 79,866 participants of the population-based lifelines cohort study in the Netherlands. Ocul Surf. 2021;19:83–93.
Choi HR, Lee JH, Lee HK, Song JS, Kim HC. Association between Dyslipidemia and Dry Eye Syndrome among the Korean middle-aged Population. Cornea. 2020;39(2):161–7.
Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161–6.
Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in Dry Eye Disease. Ophthalmology. 2017;124(3):280–6.
Nichols KK, Nichols JJ, MPH M, Mitchell GL. The Lack of Association between Signs and symptoms in patients with Dry Eye Disease. Cornea. 2004;23(8):762–70.
Ong ES, Felix ER, Levitt RC, Feuer WJ, Sarantopoulos CD, Galor A. Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol. 2018;102(5):674–9.
Golebiowski B, Papas EB, Stapleton F. Factors affecting corneal and conjunctival sensitivity measurement. Optom Vis Sci. 2008;85(4):241–6.
Golebiowski B, Papas EB, Stapleton F. Corneal and conjunctival sensory function: the impact on ocular surface sensitivity of change from low to high oxygen transmissibility contact lenses. Invest Ophthalmol Vis Sci. 2012;53(3):1177–81.
Tesón M, Calonge M, Fernández I, Stern ME, González-García MJ. Characterization by Belmonte’s gas esthesiometer of mechanical, chemical, and thermal corneal sensitivity thresholds in a normal population. Invest Ophthalmol Vis Sci. 2012;53(6):3154–60.
Tsubota K, Pflugfelder SC, Liu Z, Baudouin C, Kim HM, Messmer EM, et al. Defining Dry Eye from a clinical perspective. Int J Mol Sci. 2020;21(23):9271.
McMonnies CW. Why the symptoms and objective signs of dry eye disease may not correlate. J Optom. 2021;14(1):3–10.
Acknowledgements
Not applicable.
Funding
This study was supported by Tianjin Health Commission (TJWJ2022MS013), Tianjin Science and Technology Bureau (21JCZDJC01010), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-037A).
Author information
Authors and Affiliations
Contributions
L.C: data collection, written first draft of the manuscript.Q.G: analyze the data and assemble it, assist in writing and revising manuscripts.E.E.P: performed formal analysis of data, reviewed and edit manuscript.F.L, Z.Z, Z.F: assist in the collection of some clinical data.Y.H, R.Y, H.L, X.L: made intellectual contributions to conceptualization of the work.C.Z: contribute to conceptualization of work, designed experiments, supervised work, funding, reviewed and edited manuscript; S.Z: conceptualization, critical revision of manuscript, funding, management, and supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study obtained the ethical approval from the Ethics Committee of the Tianjin Medical University Eye Hospital (Ethics approval number: 2019ky-22). All subjects signed the informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chang, L., Guo, Q., Pazo, E.E. et al. Prevalence of dry eye in people over 50 years old in Beichen district, Tianjin city: a cross-sectional population-based survey. BMC Public Health 24, 2111 (2024). https://doi.org/10.1186/s12889-024-19616-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19616-1