Abstract
Background
India grapples with an alarming burden of tuberculosis (TB), reporting 2.6 million incident cases in 2023, necessitating intensified efforts toward TB elimination. The prevalence of catastrophic costs, defined as expenses exceeding 20% of annual household income, varies widely. Our objective was to determine the association between catastrophic costs from TB-HIV and TB-diabetes care and unfavorable TB treatment outcomes.
Methods
We conducted a cohort study in Bhavnagar, India, from July 2019 to January 2021, involving 234 TB-HIV and 304 TB-diabetes patients. Catastrophic costs were assessed using the World Health Organization’s tool. Unfavorable TB treatment outcomes included positive results from sputum smear, nucleic acid amplification, or culture tests at treatment completion, death during treatment, or treatment cessation for a month (for drug-sensitive TB) or two months (for drug-resistant TB). Firth regression was employed to address quasi-separation issues and identify predictors.
Results
Among TB-HIV patients, 12% faced catastrophic costs, with 20% experiencing unfavorable TB outcomes. In this group, significant predictors included weight (OR: 0.93, 95% CI: 0.89–0.98), family type (OR: 2.5, 95% CI: 1.2–5.5), and initial hospitalization (OR: 2.6, 95% CI: 1.1–6.3). For TB-diabetes patients, 5% faced catastrophic costs, and 14% had unfavorable outcomes, with significant predictors being below the poverty line (BPL) (OR: 2.9, 95% CI: 1.5–5.9) and initial hospitalization (OR: 3.4, 95% CI: 1.1–11.1). Catastrophic cost incidence was higher in TB-HIV (12% vs. 4% in TB only) and TB-diabetes (5% vs. 4% in TB only) patients. However, catastrophic costs did not show a direct association with unfavorable outcomes in either group.
Conclusions
Our study found no direct association between catastrophic costs and unfavorable TB outcomes among TB-HIV/TB-diabetes patients. Instead, factors such as weight, family type, BPL status, and initial hospitalization were significant predictors. These findings underscore the importance of socio-economic conditions and initial hospitalization, advocate for enhanced support mechanisms including nutritional and financial aid, especially for BPL families.
Similar content being viewed by others
Background
India has long held the unenviable distinction of having the highest tuberculosis (TB) burden worldwide, reporting a staggering 2.6 million cases in 2023 alone [1]. In 2022, India accounted for nearly 27% of the global TB cases, out of a total of 10.6 million cases worldwide [2]. This persistent challenge has prompted India to intensify its efforts towards TB elimination, a disease that has plagued populations worldwide for many years [3].
Apart from reducing the incidence and deaths due to TB, one of the targets of elimination is reaching to zero catastrophic costs due to TB by 2020 [4]. Catastrophic costs, defined as costs exceeding 20% of annual household income, can push families below the poverty line [5, 6]. Globally, recent estimates indicate that 43% of households affected by TB face these catastrophic costs [7]. In India, the prevalence of such costs varies widely, ranging from 4 to 68%, contingent upon factors such as study location, site of disease, and drug resistance patterns [6, 8].
Various studies conducted in different countries—Peru, Indonesia, Brazil, and the Republic of Moldova—have delved into the relationship between catastrophic costs and TB treatment outcomes [9,10,11,12]. While the former three countries revealed an association between catastrophic costs and unfavorable TB treatment outcomes, the Moldova study found no significant link for patients with drug-resistant TB. A recent study in China found some evidence of association of catastrophic costs with loss to follow up among patients with TB [13].
The presence of two primary comorbidities associated with TB—human immunodeficiency virus (HIV) and diabetes—has been consistently linked to adverse impacts on TB treatment outcomes [9, 14]. Furthermore, the financial burden of managing HIV and diabetes alongside TB care has proven substantial, effectively doubling the costs incurred when dealing solely with TB among patients grappling with these concurrent health challenges [15,16,17]. Specifically, the additional complication of HIV co-infection has been demonstrated to elevate the occurrence of catastrophic costs arising from TB care by 8%, underscoring the heightened economic toll placed on individuals with overlapping health conditions [16]. In a parallel vein, the presence of diabetes comorbidity has been associated with a modest 1% increase in the occurrence of catastrophic costs attributed to TB care [15].
In Bhavnagar, India, our previous research has shed light on the prevalence, predictors, coping strategies, and enablers of catastrophic costs stemming from TB-HIV co-infection and TB-diabetes comorbidity [15, 16, 18, 19]. However, the potential correlation of these catastrophic costs with unfavorable TB treatment outcomes remains unexplored. In light of this, our study aims to fill this knowledge gap by investigating the possible connection between catastrophic costs resulting from combined TB-HIV and TB-diabetes care and unfavorable TB treatment outcomes. Through this effort, we aim to uncover insights that might highlight the importance of improved financial support for individuals with both TB and other health conditions.
Methods
Study design, duration, and setting
We conducted a cohort study among patients with TB-HIV and TB-diabetes co-prevalence from July 2019 to January 2021 in Bhavnagar region in the state of Gujarat in western part of India. This paper is a part of a larger study and the median costs incurred and percentage of catastrophic costs incurred due to TB-HIV co-infection and TB-diabetes comorbidity are reported in other published studies [15, 16]. The Bhavnagar region comprises both a district, which is predominantly rural, and a semi-urban conglomerate city. With a population of 2.8 million, the Bhavnagar district is primarily rural, while the Bhavnagar city, under the municipal corporation, houses around 0.6 million people [20]. This region witnesses an annual occurrence of approximately 2500–3000 incident cases of TB [21].
Study population, tool, and procedures
The study involved 234 patients with TB-HIV co-infection and 304 patients with TB-diabetes comorbidity, all of whom were notified in the public sector between January 2017 and December 2020 under the National TB Elimination Program (NTEP) in the Bhavnagar region [15, 16]. Under the NTEP, all TB patients are systematically tested for HIV and diabetes, and conversely, all patients diagnosed with HIV and diabetes are screened for TB as part of a bidirectional screening initiative [22, 23]. The diagnosis of HIV and diabetes was confirmed through standard laboratory procedures using blood samples, as reported by the NTEP. TB diagnosis followed NTEP guidelines, involving symptom screening, sputum testing, and chest X-ray findings. It is important to note that we included only those TB-HIV/TB-diabetes patients who were notified in the NTEP program; therefore, the investigators did not perform any diagnostic tests themselves but obtained the list of comorbid patients from the district TB officer.
The cost assessment employed a tool derived from the World Health Organization’s validated questionnaire designed to estimate costs incurred by TB patients [5, 6]. This tool facilitated the calculation of catastrophic costs—defined as instances where combined TB-HIV or TB-diabetes costs exceeded 20% of the annual household income [5, 6, 15, 16]. The study’s methodologies and cost calculations have been previously outlined in our earlier research [15, 16].
Cost estimation duration
The costs incurred by patients due to TB were calculated from the onset of symptoms until the completion of treatment [15, 16]. For patients with HIV or diabetes, the costs were estimated from the time of diagnosis and initiation of treatment until the completion of TB treatment [15, 16]. Thus, the combined costs for TB-HIV or TB-diabetes represented the sum of TB-related costs and the costs associated with managing HIV or diabetes over the same treatment period [15, 16].
Follow up
The follow up for the TB treatment outcomes was passive, that is, the treatment outcomes were extracted from the Nikshay online portal (https://nikshay.in/), a registry of patients notified under the national TB program in India.
Variables
The study’s outcome variable pertained to unfavorable TB treatment outcomes, classified as either positive sputum smear, nucleic acid amplification, or culture test results at TB treatment completion, patient death during TB treatment (regardless of the underlying cause of death), or continuous treatment cessation for a month (for drug-sensitive TB) or two months (for drug-resistant TB) [24]. Conversely, treatment success was defined by negative sputum or culture tests post-treatment, or successful completion of treatment without clinical deterioration as determined by the treating physician [24]. Exposure variables encompassed the catastrophic costs incurred due to TB-HIV co-infection or TB-diabetes comorbidity, measured against the threshold of 20% of annual household income [15, 16].
Various confounding variables were considered, including age, gender, marital status, weight, education, family structure, below poverty line (BPL) status, standard of living (SLI) index, residence type, and health-related factors [15, 16, 18, 19]. Additionally, the site of TB and drug resistance were included as confounding variables. The site of TB was identified through the Nikshay notification register as reported by the NTEP program, but information on combined pulmonary-extrapulmonary TB was not available. Drug-resistant TB was defined as resistance to any anti-TB drug, including MDR/RR, mono-H, and other types of drug resistance [25]. Patients not on a drug-sensitive TB regimen were classified as having drug-resistant TB according to NTEP program definitions [25].
Families possessing the below poverty line (BPL) ration card were categorized as belonging to a BPL family, while those having the above poverty line (APL) ration card were categorized as belonging to a non-BPL family. The standard of living index (SLI) was derived from asset ownership data, including details such as the type of housing, the number of rooms, and possession of vehicles like cars or trucks (see Additional file 1) [6, 15, 16, 26, 27]. The SLI scores ranged from 1 to 23, with scores from 1 to 7 categorized as low SLI, and scores from 8 to 23 categorized as middle/high SLI [15, 16].
Statistical analysis
We conducted logistic regression analysis using logit function in the R software, given that our outcome variable was binary in nature. However, we encountered a significant challenge during this analysis due to the quasi-separation of the fitted logistic model. This situation led to difficulties in accurately estimating coefficients and generating reliable confidence intervals. The issue of quasi-separation resulted in inflated standard errors of the regression coefficients, rendering the model inadequate for our purposes.
To address this challenge, we turned to the Firth regression approach, a specific form of penalized likelihood regression. This technique proved robust in overcoming the issues posed by quasi-separation. It effectively dealt with data separation, which can hinder the convergence of standard logistic regression estimates. Our application of the Firth regression method demonstrated its effectiveness in this analysis.
Both the TB-HIV and TB-diabetes fitted models yielded similar log-likelihood values and achieved statistical significance, underscoring the strength of the Firth regression technique. To implement Firth logistic regression, we employed the logistf() function from Heinze’s logistf package within the R programming environment. The fitting process employed the Iteratively Reweighted Least Squares (IRLS) method, with a maximum of 100 iterations.
We conducted univariate Firth logistic regression, including variables with a p-value ≤ 0.2 in subsequent multivariable Firth logistic regression models. To ensure the integrity of the analysis, we examined potential co-linearity among measures of impoverishment such as below poverty line status, standardized living index (SLI), and catastrophic spending. Variables exhibiting multicollinearity, indicated by a variance inflation factor (VIF) > 10 or tolerance < 0.1, were excluded from the multivariable model.
In addition, for sensitivity analysis, we explored the association of catastrophic costs due to TB-HIV and TB-diabetes at various lower annual household income thresholds, utilizing univariable Firth logistic regression. Throughout our analyses, a p-value threshold of 0.05 was maintained to determine the statistical significance of predictor variables, ensuring the robustness and reliability of our findings.
Results
Characteristics of patients
We enrolled 234 patients with TB-HIV co-infection and 304 patients with TB-diabetes comorbidity from a total of 10,278 TB cases recorded in the notification register between 2017–2020 [15, 16]. For TB-HIV, exclusions included 2,651 without HIV, 5,653 from the private sector, 1,028 under 18 years of age, 304 with diabetes, and 408 previously treated cases [16]. For TB-diabetes, exclusions included 2,651 non-diabetic patients, 5,653 from the private sector, 1,028 under 18 years of age, 234 with HIV, and 408 previously treated cases [15].
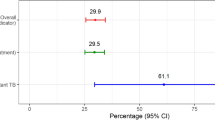
The median age was 37 years (IQR: 29–48) for TB-HIV patients and 52 years (IQR: 42–61) for TB-diabetes patients (Table 1). The majority were male, comprising 78% of TB-HIV and 72% of TB-diabetes patients. Most participants were married (79% TB-HIV, 92% TB-diabetes) and lived in extended families (80% TB-HIV, 87% TB-diabetes). Economic characteristics showed that 70% of TB-HIV and 80% of TB-diabetes patients received cash assistance for TB, with a median amount of INR 3000 (~ US$ 44). Below poverty line (BPL) cardholders were 50% of TB-HIV and 34% of TB-diabetes patients. Initial hospitalization due to TB was observed in 13% of TB-HIV and 8% of TB-diabetes patients. Within the cohort of 234 patients with TB-HIV co-infection, 20% experienced unfavorable TB treatment outcomes, while among the 304 patients with TB-diabetes comorbidity, this figure stood at 14% [15, 16].
Percentage of catastrophic costs
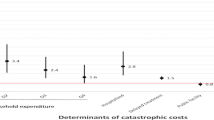
When considering the 20% threshold of annual household income, 4% of patients with TB-HIV co-infection and TB-diabetes comorbidity faced catastrophic costs specifically related to TB alone, excluding additional costs from HIV or diabetes comorbidity (Fig. 1) [15, 16]. In contrast, the percentage of patients facing catastrophic costs due to combined TB-HIV co-infection was 12%, while those with TB-diabetes comorbidity faced a 5% occurrence of such combined costs.[15, 16] This indicates that while TB-related costs alone impacted a smaller proportion of patients, the inclusion of comorbidity-related costs significantly increased the percentage of catastrophic costs.
Combined TB-HIV catastrophic costs and unfavorable TB treatment outcomes
On Firth penalized logistic regression analysis, factors including weight, family type, and initial hospitalization for TB emerged as significant predictors of unfavorable TB treatment outcomes among TB-HIV co-infected patients (Table 2). Patients who were initially hospitalized for TB had 2.6 (95% CI: 1.1–6.3) times higher odds of experiencing unfavorable treatment outcomes. Each additional kilogram of weight corresponded to a 7% reduction in the likelihood of unfavorable TB treatment outcomes (adjusted OR = 0.93, 95% CI: 0.89–0.98, p-value = 0.002). This relationship reflects an exponential decline in the likelihood of unfavorable outcomes. Specifically, the reduction percentage compounds with each additional kilogram, rather than being simply additive. To illustrate this, we provide a line chart (Fig. 2) showing the percentage reduction in the likelihood of unfavorable TB treatment outcomes for different weight increases. Notably, individuals residing in nuclear families exhibited 2.5 (95% CI 1.2–5.5) times higher odds of unfavorable TB treatment outcomes compared to those in extended families. However, it’s important to highlight that catastrophic costs due to TB-HIV did not prove to be a statistically significant predictor of unfavorable TB treatment outcomes in our study.
Even when considering lower thresholds of annual household income, the association between catastrophic costs attributed to TB-HIV and unfavorable TB treatment outcomes remained statistically non-significant (Table 3).
Combined TB-diabetes catastrophic costs and unfavorable TB treatment outcomes
On Firth penalized logistic regression analysis, factors such as living below the poverty line (BPL) and undergoing hospitalization during the initial TB clinic visit emerged as significant predictors of unfavorable TB treatment outcomes among patients with TB-diabetes comorbidity (Table 4). Individuals from BPL families exhibited 3 (95% CI 1.5–5.9) times higher odds of unfavorable TB treatment outcomes, while those who were hospitalized during their first TB clinic visit faced 3.4 (95% CI 1.1–11.1) times higher odds of unfavorable TB treatment outcomes. Notably, there was no observed association between catastrophic costs due to TB-diabetes and unfavorable TB treatment outcomes.
Even when considering lower thresholds of annual household income, the impact of catastrophic costs due to TB-diabetes on unfavorable TB treatment outcomes remained statistically insignificant (Table 5).
Discussion
TB remains a disease of poverty, influenced by various social and structural determinants such as unemployment and socioeconomic status [28, 29]. Families affected by TB often find themselves tangled in a cycle of high TB-related costs, impoverishment, malnutrition, and complicated forms of TB, collectively affecting treatment outcomes [10]. Notably, the coexistences of HIV and diabetes with TB hold the potential to directly impact TB treatment outcomes [9, 14]. However, the repercussions of costs associated with managing HIV and diabetes on TB treatment outcomes have remained unexplored until now—highlighting the focal point of our investigation.
Summarizing our findings, the combined catastrophic costs due to TB-HIV and TB-diabetes comorbidity did not show an association with unfavorable TB treatment outcomes in our study setting, possibly due to the study’s small sample size. Nonetheless, various factors, including weight, family type, living below the poverty line, and initial hospitalization, emerged as significant predictors of unfavorable TB treatment outcomes among these patients. In our study setting, patients with TB experienced lower catastrophic costs, attributed partly to the decentralized model of TB care and the semi-urban, rural environment [6, 15, 16]. Despite the nearly doubled economic burden incurred by HIV and diabetes care alongside TB [15,16,17], the overall prevalence of combined catastrophic costs remained below the global and Indian averages [7, 8]. The exclusion of patients treated in the private sector may have contributed to underestimated cost estimations. Furthermore, the notable achievement of high TB treatment completion rates in India, as evidenced by an 85% rate reported in a recent annual report [30], might clarify the absence of an apparent link between catastrophic costs and unfavorable TB treatment outcomes in our study setting.
We wish to highlight the intriguing and thought-provoking nature of our findings regarding the statistically insignificant yet protective association between catastrophic costs and unfavorable treatment outcomes among patients with TB-diabetes comorbidity. This phenomenon mirrors a similar discovery in our previous study involving patients with silico-tuberculosis [31], where TB-diabetes bidirectional screening appeared to safeguard against unfavorable TB treatment outcomes. Patients with diabetes often opt for care in the private sector, potentially enhancing their adherence to treatment regimens and, consequently, improving their outcomes [15]. Paradoxically, the financial burden might act as a driving force, compelling strict adherence to the prescribed treatments. This observation underscores the need for further investigation and collaborative development of targeted support interventions, shedding light on the complex interplay between financial strain, motivation, and treatment adherence.
In both the TB-HIV and TB-diabetes groups, the catastrophic costs due to TB-HIV co-infection or TB-diabetes comorbidity did not show a statistically significant association with unfavorable TB treatment outcomes in our study. It’s important to note that there is a dearth of studies examining this particular association among TB comorbid patients, making our research novel in this context. While the majority of existing studies investigating the linkage between catastrophic costs and treatment outcomes primarily focus on TB patients without comorbidities, our findings align with those from a Moldovan study, which also reported no significant link between catastrophic costs and unfavorable TB treatment outcomes [9]. However, contrasting findings have emerged from studies conducted in other countries, revealing a notable relationship between catastrophic costs and unfavorable treatment outcomes [10,11,12]. Notably, some of these studies focused on drug-resistant TB patients, a factor in itself that can elevate the risk of unfavorable treatment outcomes [10]. Unfavorable TB treatment outcomes can be influenced by various factors, including lower income, malnutrition, alcohol, and tobacco abuse [32]. While increased costs have been associated with delayed TB diagnosis [32], poverty has also been linked to unfavorable treatment outcomes [9]. Irrespective of the factors at play, the reduction of catastrophic costs attributed to TB has been proposed as a crucial step towards achieving TB elimination [11].
While catastrophic costs due to TB-HIV co-infection did not exhibit an association with unfavorable treatment outcomes, our study revealed several other significant predictors. A decrease in weight, membership in a nuclear family, and initial hospitalization were all factors that significantly predicted unfavorable TB treatment outcomes. In the context of TB management, absolute body weight (measured in kilograms) is often considered an indicator of nutritional status [33]. Improved nutritional status, reflected by increased body weight, has been shown to positively impact TB outcomes [33, 34]. This aligns with our findings, where being underweight was similarly identified as a predictor of unsuccessful treatment outcomes, a conclusion shared by other researchers [32, 35, 36]. The relationship between weight and TB outcomes highlights the importance of addressing malnutrition in TB management [33]. Likewise, our study’s correlation between smaller family size and poor TB treatment outcomes finds resonance in research conducted in Somalia, which also observed an association between smaller family units and poor treatment outcomes for TB [37]. These insights underscore the importance of addressing these multifaceted factors to improve TB treatment effectiveness and outcomes.
Among patients with TB-diabetes, belonging to a family below the poverty line (BPL) and initial hospitalization during the TB visit emerged as significant predictors of unfavorable treatment outcomes in our study. The intricacies of multiple socioeconomic determinants interact to impact treatment interruption and mortality among TB patients. Essential to the elimination of TB from India is the eradication of poverty, a pivotal social determinant [38]. Lower income levels inherently place families at risk of malnutrition, overcrowding, poor ventilation, and infections—some of these factors are also implicated in unfavorable TB treatment outcomes [32, 39].
An important consideration arising from our findings is the potential benefit of using BPL status as an early indicator for identifying patients at risk of poor treatment outcomes. Rather than relying solely on catastrophic cost assessments, which can be time-consuming and labor-intensive, assessing BPL status at the beginning of TB treatment could serve as a more efficient strategy for targeting interventions. This approach aligns with the differentiated TB care strategy, which already considers several risk factors such as HIV, diabetes, smoking, harmful use of alcohol, and undernourishment for early TB deaths [40,41,42,43]. Therefore, we recommend incorporating BPL status assessment into the differentiated TB care model in India to improve patient outcomes.
Evidence underscores improved TB treatment outcomes with financial interventions [44,45,46]. A systematic review and meta-analysis reported lowered treatment interruptions and enhanced cure rates through social protection mechanisms, however, the same analysis did not find notable impacts on failure or death rates [47]. Notably, targeted cash transfer schemes cover a smaller population, potentially excluding those in need [48, 49]. In contrast, the implementation of universal, unconditional cash transfer schemes could offer poverty reduction, diminish under-nutrition, and foster better TB treatment outcomes [28, 38]. In recent years, the National TB Elimination Program (NTEP) has implemented various measures to mitigate costs for TB patients. The Nikshay Poshan Yojana provides INR 500 (~ US$6) per month for nutritional support to all TB patients [28, 45, 50, 51]. Additionally, collaborative TB-HIV and TB-diabetes activities, along with their successful implementation, have improved outcomes through early detection and prompt management [22, 23]. Moreover, expensive drugs such as Bedaquiline, Pretomanid, and Delamanid have been introduced under the programmatic management of drug-resistant TB in India, further enhancing patient support and treatment outcomes [25].
Our study also revealed a significant association between hospitalization during the initial visit to a TB clinic and unfavorable treatment outcomes among patients both with TB-HIV co-infection as well as TB-diabetes comorbidity. Notably, similar findings have been reported in other studies, identifying initial hospitalization as a risk factor for early treatment interruption and other adverse treatment outcomes [52, 53]. In India, the Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (PM-JAY) program, launched in 2018, stands as a significant healthcare initiative [54]. This program aims to extend financial protection of Indian Rupee (INR) 0.5 million to more than 12 crore vulnerable and economically disadvantaged families by providing comprehensive health insurance coverage for a wide array of medical treatments [54]. The integration of the Ayushman Bharat PM-JAY program with the National Tuberculosis Elimination Program (NTEP) could offer a transformative approach, allowing TB patients to access costly medical services free of charge at empaneled hospitals [55, 56].
Strengths and limitations
This is the first study determining the association between catastrophic costs and unfavorable TB treatment outcomes among TB-HIV co-infected and TB-diabetes comorbid patients. The absence of a significant association may be attributed to the study’s limited sample size, which could have hindered the detection of established predictors, including drug-resistant TB. It is essential to emphasize that the absence of an association in a small study does not negate the possibility of a relationship; instead, it underscores the necessity for larger-scale investigations to establish definitive conclusions. The study’s longitudinal duration of four years led to the enrollment of participants at various points in time, resulting in the receipt of TB treatment outcomes at differing intervals. Additionally, cost calculations were conducted over the entire TB treatment period, while outcomes were reported immediately after treatment completion. This temporal proximity limited the time gap between catastrophic cost exposure and TB treatment outcomes. As a result, we opted for adjusted odds ratios over adjusted relative risks.
We acknowledge the potential for misclassification in our study due to the nature of data entry into the Nikshay online portal. The treatment outcomes, crucial for our analysis, were extracted from the Nikshay online portal, where data is entered based on the information from TB treatment cards maintained by the diligent TB health staff. It’s essential to note that treatment outcomes, particularly the classification of ‘treatment completed’, rely significantly on clinical assessment rather than exclusive dependence on sputum testing [57]. Furthermore, existing studies have highlighted challenges related to staff familiarity with the software and the increased workload associated with managing both manual treatment registers and the online portal [58]. These challenges can inadvertently lead to data entry errors and misclassification. The misclassification of treatment outcomes is likely to lead to an underestimation of the rate of unfavorable outcomes. This underestimation may have contributed to our study not identifying a significant association between catastrophic costs and poor treatment outcomes. Additionally, because the data was extracted from the Nikshay portal, we lack information on whether the HIV-positive patients had progressed to AIDS, which is a significant limitation in evaluating TB treatment outcomes. The complexities of the Nikshay portal, coupled with issues of missing data, necessitate targeted interventions [59, 60]. Comprehensive training programs and systematic quality improvement initiatives are imperative to mitigate misclassification risks and enhance the accuracy of data recorded in the Nikshay portal [59, 60].
Another limitation of this study is that the NTEP program records any death during TB treatment as a TB-related death, regardless of the actual cause, which may include unrelated causes such as accidents [61]. The Nikshay portal does not specify the underlying cause of death [61]. Additional limitations of our study have been comprehensively outlined in previously published works [15, 16]. Nonetheless, we believe that our findings hold generalizability for analogous semi-urban and rural Indian settings. The decentralized structure of TB care, a cornerstone of the National TB Elimination Program (NTEP) in India, ensures uniformity in healthcare delivery across diverse regions. Importantly, our study region mirrors the socioeconomic and healthcare characteristics prevalent in a significant portion of India, where approximately 65% of the population resides in rural areas [62]. The presence of a standardized three-tier healthcare system, comprising primary, secondary, and tertiary centers at the village, town, and district levels, respectively, further enhances the applicability of our findings to similar semi-urban and rural Indian settings. The study also follows the STROBE reporting guidelines for observational studies [63].
Conclusions
In conclusion, our study’s findings highlight that while catastrophic costs due to TB-HIV/TB-diabetes do not exhibit a significant association to unfavorable TB treatment outcomes, several factors including weight loss, TB-related hospitalization, and membership in a below poverty line (BPL) family emerge as notable predictors of adverse treatment outcomes. These findings reinforce our recommendation for the implementation of a comprehensive approach, including the provision of nutritional support and increased financial assistance to TB patients with co-infections/comorbidities to counter under-nutrition and improve treatment outcomes. The potential integration of NTEP with the world’s largest health insurance initiative, the Ayushman Bharat PM-JAY, also warrants exploration for its potential to augment the effectiveness of TB treatment strategies.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available in the Mendeley repository, https://data.mendeley.com/datasets/fch8cvvnhx/2.
Abbreviations
- BPL:
-
Below Poverty Line
- CI:
-
Confidence Intervals
- HIV:
-
Human Immunodeficiency Virus
- INR:
-
Indian Rupees
- IRLS:
-
Iteratively Reweighted Least Squares
- IQR:
-
Interquartile Range
- MDR:
-
Multi-drug Resistant
- NTEP:
-
National Tuberculosis Elimination Program
- PM-JAY:
-
Pradhan Mantri Jan Arogya Yojana
- SLI:
-
Standard of Living Index
- TB:
-
Tuberculosis
- VIF:
-
Variance Inflation Factor
- WHO:
-
World Health Organization
References
Central TB Division (Ministry of Health & Family Welfare). India TB Report 2024. New Delhi: Government of India; 2024.
World Health Organization. Global Tuberculosis Report 2023. Geneva: World Health Organization; 2023.
Central TB Division (Ministry of Health & Family Welfare). National Strategic Plan for Tuberculosis Elimination 2017–2025. New Delhi: Government of India; 2017.
World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO Press, Geneva, Switzerland; 2015.
World Health Organization. Tuberculosis patient cost surveys: a handbook. Geneva, Switzerland: WHO Press, Geneva, Switzerland; 2017.
Rupani MP, Cattamanchi A, Shete PB, Vollmer WM, Basu S, Dave JD. Costs incurred by patients with drug-susceptible pulmonary tuberculosis in semi-urban and rural settings of Western India. Infect Dis Poverty. 2020;9:144.
Ghazy RM, El Saeh HM, Abdulaziz S, Hammouda EA, Elzorkany AM, Khidr H, et al. A systematic review and meta-analysis of the catastrophic costs incurred by tuberculosis patients. Sci Rep. 2022;12:558.
Chandra A, Kumar R, Kant S, Parthasarathy R, Krishnan A. Direct and indirect patient costs of tuberculosis care in India. Tropical Med Int Health. 2020;25:803–12.
Plesca V, Ciobanu A, Sereda Y, Dadu A. Do catastrophic costs impact treatment outcomes in people with rifampicin-resistant tuberculosis in the Republic of Moldova? Monaldi Arch Chest Dis. 2021;91:1650.
Wingfield T, Boccia D, Tovar M, Gavino A, Zevallos K, Montoya R, et al. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study. Peru PLoS Med. 2014;11:e1001675.
Fuady A, Houweling TAJ, Mansyur M, Burhan E, Richardus JH. Catastrophic costs due to tuberculosis worsen treatment outcomes: A prospective cohort study in Indonesia. Trans R Soc Trop Med Hyg. 2020;114:666–73.
Guidoni LM, Zandonade E, Fregona G, Negri LDSA, Oliveira SMDVL, Prado TND, et al. Catastrophic costs and social sequels due to tuberculosis diagnosis and treatment in Brazil. Epidemiol Serv Saude. 2021;30:e2020810.
Wang Y, Huang Z, Chen H, Yuan Y, McNeil EB, Zhang A, et al. The association between household financial burden and patient mobility and their impact on loss to follow-up among multidrug-resistant tuberculosis patients in Guizhou. China Risk Manag Healthc Policy. 2023;16:909.
Sahakyan S, Petrosyan V, Abrahamyan L. Diabetes mellitus and treatment outcomes of pulmonary tuberculosis: a cohort study. Int J Public Health. 2020;65:37–43.
Rupani MP, Vyas S. A sequential explanatory mixed-methods study on costs incurred by patients with tuberculosis comorbid with diabetes in Bhavnagar, Western India. Sci Rep. 2023;13:150.
Rupani MP, Vyas S. Costs incurred by patients with tuberculosis co-infected with human immunodeficiency virus in Bhavnagar, western India: a sequential explanatory mixed-methods research. BMC Health Serv Res. 2022;22:1268.
Ghosh S. Studies say costs double as TB patients with HIV or diabetes opt for private care. Ahmedabad: The Indian Express; 2023.
Rupani MP, Vyas S. Predictors Of catastrophic costs of tuberculosis (TB) among patients co-affected with TB-HIV and TB-diabetes in Bhavnagar Region, Western India. National J Community Med. 2022;13:497–502.
Rupani MP, Vyas S. Dissaving in the Era of “Free” care for tuberculosis (TB): a qualitative exploration of financial coping and enablers among patients with co-prevalent TB-HIV/ TB- diabetes in Bhavnagar Region, Western India. National J Community Med. 2022;13:629–35.
Registrar General of India, Ministry of home affairs, Government of India. Census of India 2011 - district census handbook Bhavnagar: primary census abstract. New Delhi: Government of India; 2011.
State TB Cell (Government of Gujarat). NTEP Performance Report, Gujarat. Gandhinagar: India: Commissionerate of Health; 2020.
Central TB Division and Department of AIDS Control (Government of India). National Framework for Joint HIV/TB Collaborative Activities. New Delhi: India: Ministry of Health & Family Welfare, Government of India; 2013.
WHO Country Office for India. National framework for joint TB-Diabetes collaborative activities. New Delhi: Ministry of Health & Family Welfare, Government of India; 2017.
Central TB Division (Ministry of Health and Family Welfare). Training Modules for Programme Managers and Medical Officers (Modules 1–4). India: Government of India; 2020.
Central TB Division (Ministry of Health & Family Welfare). Guidelines for programmatic management of drug resistant tuberculosis in India. New Delhi: Government of India; 2021.
International Institute for Population Sciences. National Family Health Survey (NFHS-2) 1998–99. India: Mumbai; 2000.
Muniyandi M, Ramachandran R, Gopi PG, Chandrasekaran V, Subramani R, Sadacharam K, et al. The prevalence of tuberculosis in different economic strata: a community survey from South India. Int J Tuberc Lung Dis. 2007;11:1042–5.
Dave JD, Rupani MP. Advancing social protection and tuberculosis elimination in India – beyond cash transfers and towards addressing social and structural determinants for a healthier future; a response to the recent commentaries. Int J Health Policy Manag. 2023;12:8130.
Bhargava A, Bhargava M, Pai M. Tuberculosis: a biosocial problem that requires biosocial solutions. The Lancet. 2024;403:2467–9.
Central TB Division (Ministry of Health & Family Welfare). India TB Report 2023. New Delhi: Government of India; 2023.
Rupani MP. Silicosis as a predictor of tuberculosis mortality and treatment failure and need for incorporation in differentiated TB care models in India. Archives of Public Health. 2023;81:173.
Duarte R, Lönnroth K, Carvalho C, Lima F, Carvalho ACC, Muñoz-Torrico M, et al. Tuberculosis, social determinants and co-morbidities (including HIV). Pulmonology. 2018;24:115–9.
Bhargava A, Bhargava M, Meher A, Teja GS, Velayutham B, Watson B, et al. Nutritional support for adult patients with microbiologically confirmed pulmonary tuberculosis: outcomes in a programmatic cohort nested within the RATIONS trial in Jharkhand. India Lancet Glob Health. 2023;11:e1402–11.
Bhargava A, Chatterjee M, Jain Y, Chatterjee B, Kataria A, Bhargava M, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS ONE. 2013;8:77979.
Sahile Z, Tezera R, Mariam DH, Collins J, Ali JH. Nutritional status and TB treatment outcomes in Addis Ababa, Ethiopia: an ambi-directional cohort study. PLoS ONE. 2021;16:e0247945.
Choi H, Lee M, Chen RY, Kim Y, Yoon S, Joh JS, et al. Predictors of pulmonary tuberculosis treatment outcomes in South Korea: a prospective cohort study, 2005–2012. BMC Infect Dis. 2014;14:360.
Kassim SA, Cote A, Kassim SM, Abbas M, Baig MMFA, Ahmed AM, et al. Factors influencing treatment outcomes of tuberculosis patients attending health facilities in Galkayo Puntland, Somalia. J Public Health (Bangkok). 2021;43:887–95.
Rupani MP. Is it the right time for india to move from targeted cash transfers to universal cash transfers for patients with tuberculosis? National J Community Med. 2022;13:494–5.
Belo MTCT, Luiz RR, Teixeira EG, Hanson C, Trajman A. Tuberculosis treatment outcomes and socio-economic status: a prospective study in Duque de Caxias, Brazil. Int J Tuberc Lung Dis. 2011;15:978–81.
Central TB Division (Ministry of Health and Family Welfare). Technical Guidance For Comprehensive Package for Differentiated Care of TB patients. New Delhi: Government of India; 2021.
Washington R, Potty RS, Rajesham A, Seenappa T, Singarajipura A, Swamickan R, et al. Is a differentiated care model needed for patients with TB? A cohort analysis of risk factors contributing to unfavourable outcomes among TB patients in two states in South India. BMC Public Health. 2020;20:1–12.
Shewade HD, Nagaraja SB, Vanitha B, Murthy HJD, Bhargava M, Singarajipura A, et al. Screening for Severe Illness at Diagnosis Has the Potential to Prevent Early TB Deaths: Programmatic Experience From Karnataka, India. Glob Health Sci Pract. 2022;10:e2100736.
Shewade HD, Frederick A, Kiruthika G, Kalyanasundaram M, Chadwick J, Rajasekar TD, et al. The first differentiated tb care model from india: delays and predictors of losses in the care cascade. Glob Health Sci Pract. 2023;11:e2200505.
Wingfield T, Tovar MA, Huff D, Boccia D, Montoya R, Ramos E, et al. The economic effects of supporting tuberculosis-affected households in Peru. Eur Respir J. 2016;48:1396–410.
Dave JD, Rupani MP. Does Direct Benefit Transfer Improve Outcomes Among People With Tuberculosis? – A Mixed-Methods Study on the Need for a Review of the Cash Transfer Policy in India. Int J Health Policy Manag. 2022;11:2552–62.
Ukwaja KN, Alobu I, Gidado M, Onazi O, Oshi DC. Economic support intervention improves tuberculosis treatment outcomes in rural Nigeria. International Journal of Tuberculosis and Lung Disease. 2017;21:564–70.
de Andrade KVF, Nery JS, de Souza RA, Pereira SM. Effects of social protection on tuberculosis treatment outcomes in low or middle-income and in high-burden countries: systematic review and meta-analysis. Cad Saude Publica. 2018;34:e00153116.
Majoka Z, Palacios R. Targeting versus Universality: Is There a Middle Ground? Washington D.C.: World Bank; 2019.
Hanna R, Olken BA. Universal Basic Incomes versus Targeted Transfers: Anti-Poverty Programs in Developing Countries. https://doi.org/10.1257/jep.32.4.201.
Central TB Division (Ministry of Health & Family Welfare). Nutritional Support to TB patients (Nikshay Poshan Yojana). New Delhi: Government of India; 2018.
Central TB Division (Ministry of Health & Family Welfare). Guidance document: Nutritional care and support for patients with tuberculosis in India. New Delhi: Government of India; 2017.
Suliman Q, Lim PY, Md Said S, Tan K-A, Mohd Zulkefli NA. Risk factors for early TB treatment interruption among newly diagnosed patients in Malaysia. Sci Rep. 2022;12:745.
Bogale L, Tsegaye T, Abdulkadir M, Akalu TY. Unfavorable treatment outcome and its predictors among patients with multidrug-resistance tuberculosis in Southern Ethiopia in 2014 to 2019: a multi-center retrospective follow-up study. Infect Drug Resist. 2021;14:1343.
National Health Authority (Government of India). Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB PM-JAY). 2019. https://nha.gov.in/PM-JAY. Accessed 29 Aug 2023.
Central TB Division (Ministry of Health & Family Welfare). Operational guidelines for TB services at Ayushman Bharat health and wellness centres. New Delhi: Government of India; 2020.
Bhargava A, Bhargava M, Meher A. Universal health coverage and tuberculosis care in India in the times of Covid-19: Aligning Ayushman Bharat (National Health Assurance Scheme) to improve case detection, reduce deaths and catastrophic health expenditure. Natl Med J India. 2020;33:298.
Central TB Division (Ministry of Health and Family Welfare. Government of India). Training Modules for Programme Managers and Medical Officers (Modules 1–4). New Delhi: Government of India; 2020.
Dey S, Rao AP, Kumar A, Narayanan P. Awareness & utilization of NIKSHAY and perceived barriers for tuberculosis case notification among the private practitioners in Udupi district. Karnataka Indian J Tuberc. 2020;67:15–9.
Arora R, Khanna A, Sharma N, Khanna V, Shringarpure K, Kathirvel S. Early implementation challenges in electronic referral and feedback mechanism for patients with tuberculosis using Nikshay – A mixed-methods study from a medical college TB referral unit of Delhi. India J Family Med Prim Care. 2021;10:1678.
Siddaiah A, Ahmed MN, Kumar AMV, D’Souza G, Wilkinson E, Maung TM, et al. Tuberculosis notification in a private tertiary care teaching hospital in South India: a mixed-methods study. BMJ Open. 2019;9:e023910.
Shah HD, Yasobant S, Narkhede KM, Patel J, Bhavsar P, Saha S, et al. A step up to end tuberculosis: lessons from a community-based death review of patients with tuberculosis from western India. Clin Epidemiol Glob Health. 2023;19:101205.
Department of Economic Affairs (Ministry of Finance. Government of India). Economic Survey 2022-23. New Delhi: Government of India; 2023.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24.
Acknowledgements
This research constitutes the final manuscript of the primary author's Ph.D. in Community Medicine at Gujarat University, with the second author serving as the mentor. We extend our heartfelt appreciation to the study participants for their invaluable contributions. Permission for the study was graciously granted by the State Tuberculosis Cell of Gujarat. Our gratitude extends to Ms. Rushita Radadiya, Mr. Vijay Parmar, Mr. Mohit Makwana, Mr. Sanjay Kotadiya, and Mr. Praveen Kumar for their invaluable assistance in data collection and transcript preparation. We are also grateful to the ICMR-National Institute of Occupational Health (ICMR-NIOH) for providing the necessary time and support to complete this study and write the manuscript. We extend special appreciation to the American Thoracic Society (ATS, New York, USA) for their partial funding of this research.
Funding
This work did not receive specific funding for its execution. However, funds remaining from another project funded by the American Thoracic Society (ATS, New York, USA) were utilized to partially support the current research, mainly for data collection purposes. This was done with written permission from the ATS.
Author information
Authors and Affiliations
Contributions
M.R. and S.Y. played vital roles in conceiving, designing, defining intellectual content, conducting literature searches, performing initial data analysis, editing the manuscript, and reviewing it. I.S. executed the final statistical analysis, edited the manuscript, and provided a crucial manuscript review. M.R. gathered the data and initiated the initial manuscript draft. All authors have endorsed the final article version. M.R. will assume the role of research guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study adhered to ethical principles, obtaining approval from the Institutional Review Board (IRB) of Government Medical College Bhavnagar (no. 868/2019, dated 08–07-2019). Necessary permissions were secured from the State TB Cell (Government of Gujarat) and the Gujarat State AIDS Control Society (GSACS) to enroll TB and HIV patients, respectively. Prior informed written consent, in Gujarati language, was obtained from all study participants, ensuring the utmost confidentiality of collected data. The study was conducted in accordance with the Delcaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rupani, M.P., Vyas, S. & Shah, I.A. Cohort study on association between catastrophic costs and unfavorable tuberculosis treatment outcomes among TB-HIV and TB-diabetes comorbid patients in India. BMC Public Health 24, 2028 (2024). https://doi.org/10.1186/s12889-024-19609-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19609-0