Abstract
Background
This prospective cohort study aimed to investigate the relationship between sleep duration and cancer incidence among 9996 participants over a median follow-up period of 9 years.
Methods
Participants without cancer at baseline were followed for over 88,790 person-years. The incidence of cancer and sleep duration was self-reported. The relationship between sleep duration and cancer incidence was analyzed using Cox proportional hazards models adjusted for various confounding factors, including age, gender, lifestyle factors, and comorbidities.
Results
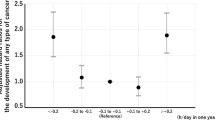
During the follow-up, 325 participants were diagnosed with incident cancer, resulting in an incidence rate of 20.49 per 1000 person-years. After adjusting for confounders, a total sleep duration of less than 6 h was significantly associated with an increased risk of cancer (HR: 1.27; 95% CI: 1.01–1.61). This association was particularly strong for cancers in the digestive and respiratory systems (HR: 1.41; 95% CI: 1.03–1.93). Longer sleep durations (> 9 h) showed a potential increase in cancer risk, but results were not consistently significant. Age-stratified analyses revealed a similar significant increase in cancer incidence among individuals aged 60 years or younger with less than 6 h of sleep per day, showing a 35% increase in overall cancer risk and an 83% increase in digestive and respiratory system cancer. No significant association was found between nocturnal sleep durations or daytime naps and cancer incidence. However, a significant interaction was observed between daytime naps longer than 30 min and cancer incidence in women (p = 0.041).
Conclusions
We observed that short sleep duration may increase the risk of cancer, particularly cancers in the digestive and respiratory systems. Additionally, while longer sleep durations might also increase cancer risk, this finding requires validation with larger sample sizes.
Similar content being viewed by others
Background
Cancer is one of the leading causes of death and a main economic burden in China, with an increasing prevalence and significant health hazards [1]. In 2020, there were an estimated 19.3 million new cancer cases worldwide (18.1 million excluding nonmelanoma skin cancer) and nearly 10.0 million cancer-related deaths (9.9 million excluding nonmelanoma skin cancer) [2].Numerous factors contribute to the development and progression of cancer, including genetic predisposition, environmental exposures, lifestyle choices, and comorbid conditions [3,4,5,6].
Among the various lifestyle factors that have been implicated in cancer risk, sleep has emerged as a critical yet understudied determinant [7,8,9]. Sleep is essential for maintaining physiological homeostasis and overall well-being, playing a crucial role in various biological processes, including immune function, hormonal regulation, and cellular repair [10, 11]. Regrettably, inadequate sleep is a common issue in today’s society, with over one-third of adults in the Americas, Europe, and Asia getting less than the recommended 7 h of sleep per night as advised by public health authorities for maintaining good health [12,13,14,15]. Disruptions in sleep patterns have been associated with an increased risk of several chronic diseases [16], including cancer [17]. Consequently, researchers have carried out comprehensive investigations on sleep disorders, with a major emphasis on rapid eye movement (REM) sleep behavior disorders or those induced by conditions like obstructive sleep apnea (OSA) [18]. The length of sleep is linked to the development and outcome of different illnesses [19, 20].
A systematic review summarized 21 published studies on the relationship between sleep and cancer risk incidence, noting the absence of cohort studies from China [21]. However, recent research from the Kailuan cohort conducted in Tangshan, China, reported that sleep duration trajectories and quality are closely associated with cancer risk [8].Despite growing recognition of the importance of sleep in cancer etiology, current research on the relationship between sleep duration and cancer risk remains limited and inconclusive in China. There have been relatively few studies [22,23,24] on the relationship between sleep duration and cancer risk in previous research, especially lacking long-term cohort studies with large nationally representative samples. Existing studies have predominantly focused on specific cancer types or have been retrospective in nature [25, 26], hindering our understanding of the broader impact of sleep on cancer incidence.
In this study, we aim to address this gap by conducting a cohort study using data from the China Health and Retirement Longitudinal Study (CHARLS) database covering the period from 2011 to 2020. By analyzing the sleep patterns and cancer incidence risk among a representative sample of Chinese middle-age and older adults, we seek to examine the hypothesis that sleep duration plays a significant role in influencing the onset of cancer. This research has the potential to provide valuable insights into the relationship between sleep behavior and cancer risk, contributing to the development of preventive strategies and interventions in cancer management.
Methods
Study design
This study utilized a longitudinal cohort study design to investigate the relationship between sleep duration and cancer incidence. Data collected from the CHARLS database from the years 2011 to 2020. CHARLS is a nationally representative survey of the Chinese population aged 45 and older [27]. It collects data on a wide range of topics including health, retirement, income, and social support. The database has been collected every two years since 2011, and involves home interviews, medical examinations, and other data collection activities. Information on health, demographics, socioeconomic status, and physical and physiological measurements was gathered from 28 provinces and 150 counties across China. More information about CHARLS can be found on the official website (https://charls.pku.edu.cn/) or in publications [28].
Inclusion and exclusion criteria
A total of 17,705 participants were enrolled in the 2011 baseline, with 10,257 eligible participants retained after excluding missing information on cancer diagnosis and participants diagnosed with cancer at baseline, and excluding those with missing data on sleep duration and aged under 45. Considering that cancer diagnoses in this study were self-reported by participants, potential underreporting may exist. Given evidence suggesting an association between cancer and renal function impairment [29], and considering the relationship between sleep and renal function [29], patients with renal function impairment were also excluded. Further details can be seen in Figure S1.
Variable definition
Exposure
Subjective sleep duration was measured retrospectively for the month before the survey was conducted. Participants were asked by trained stuff by the question” During the past month, how many hours of actual sleep did you get at night (average hours for one night)?”and “During the past month, how long did you take a nap after lunch?”. Daytime sleep duration was categorized as 0 min/day, ≤ 30 min/day and over 30 min/day. Nocturnal sleep duration was categorized as extremely short (≤ 6 h/night), short (6–9 h/night) and long (> 9 h/night) [30]. The average total daily sleep duration (the sum of daytime and nocturnal sleep duration) was divided into short (≤ 6 h per day), moderate (6–9 h per day) and long (> 9 h per day).
Main outcome measures
The outcome of the study is cancer (excluding non-melanoma skin cancer). Participants reported their cancer incidences. Based on the location of cancer occurrence, cancers were categorized into corresponding systems. Due to limitations in the number of cancer cases, we ultimately grouped cancer types into the digestive and respiratory systems (digestive system, including cancers occurring in the oral cavity, oesophagus, stomach, liver, pancreas, colon or rectum; respiratory system, including cancers occurring in the larynx, other pharynx, and lung) and other systems (including cancers of the brain, thyroid, breast, kidney, ovary, cervix, endometrium, bladder, skin, non-Hodgkin lymphoma, leukemia, and other organs). Participants were followed from baseline until cancer was detected during the survey or until the last available survey prior to 2020, whichever came first. We calculated person-years for each individual as the number of years from baseline to the first diagnosis of cancer, death, censoring, or the end of follow-up.
Covariates
Age was calculated by 2011 deducted their birth year. Height and weight measurements of individuals were taken without shoes and in light clothing using a calibrated weight scale and stadiometer equipment. Height was accurately measured to the nearest 0.1 cm, and weight was measured to the nearest 0.1 kg. [31]. According to the WHO guidelines for Chinese individuals, overweight and obesity were defined as having a body mass index (BMI) of 23.0 and 27.5 or higher, respectively [32]. Participants’ waist circumference was measured after exhalation using a soft measuring tape around their waist at the navel level. Abdominal obesity was defined as WC ≥ 90 cm for males and ≥ 80 cm for females, based on the International Diabetes Federation cut-offs for the Chinese population. [33].
The highest education levels were categorized as illiterate (no formal education), primary (elementary school), secondary (middle or high school), and college or higher (two-/three-year college and higher degrees). Participants were classified as smokers if they had smoked at least 100 cigarettes in their lifetime, with smoking status categorized as never, current, or former smokers. Drinking was defined as consuming alcohol at least once a month, with drinking history categorized as current, never, or former drinkers. Type 2 diabetes was defined as either having a fasting plasma glucose level above 126 mg/dL or currently using anti-diabetic medications. [34]. Participants’ blood pressure (BP) was determined by averaging three measurements. Hypertension was defined as a diastolic BP ≥ 90 and/or systolic BP ≥ 140 mmHg, or self-reported use of medications for hypertension treatment. We divided the participants into 2 groups (rural and Urban Community). Dyslipidemia is characterized by elevated levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C), as well as decreased levels of high-density lipoprotein cholesterol (HDL-C). Dyslipidemia can be identified by phenotypes such as TC > 6.2 mmol/L, TG ≥ 2.3 mmol/L, LDL-C ≥ 4.1 mmol/L, and HDL-C < 1.0 mmol/L in males or < 1.3 mmol/L in females, or self-reported dyslipidemia [35, 36].
According to the Chronic Kidney Disease-Epidemiology Collaboration equation, glomerular filtration rate (GFR) was calculated [37]: In men, ① serum creatinine ≤ 0.9 mg/dl: GFR = 141 * (serum creatinine (mg/dl)/0.7)-0.441 * (0.993)*age; ② serum creatinine > 0.9 mg/dl; GFR = 144* (serum creatinine (mg/dl)/0.7)-0.441 * (0.993)*age. In women, ① serum creatinine ≤ 0.7 mg/dl: GFR = 144 * (serum creatinine (mg/dl)/0.7)-0.329 *(0.993)*age; ② serum creatinine > 0.7 mg/dl: GFR = 144 /* (serum creatinine (mg/dl)/0.7)-1.209* (0.993)*age. An eGFR of less than 80 mL/min/1.73 m² was considered indicative of renal impairment [37].
Data analysis
Missing data distribution plots were used to evaluate the distribution of missing data. The Multiple Imputation by Chained Equations (MICE) method was applied to impute the missing data. The percentage of missing cases is detailed in Table S1.
Baseline characteristics were summarized by subgroups based on categories of total sleep duration and evaluated using the Mann–Whitney U or Chi-square tests. The relationship between sleep duration and cancer was analyzed using Cox proportional hazards regression models with competing risk analysis, ensuring that the proportional hazard assumption was met. Death was treated as a competing event, and the date of death was obtained from the reports of participants’ relatives or friends. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for the incidence of cancer [38]. The models were adjusted to account for the competing risk of death.
We fitted a crude univariable model (model 1) and two multivariable models (model 2 and model 3). Model 2 accounted for confounding factors such as sex, age (continuous), education, smoking, drinking, and residence. Model 3 further included adjustments for hypertension, diabetes, dyslipidemia, waist circumference, and BMI.
Subgroup analyses by gender (male vs. female) and age (≤ 60 vs. >60 years) were conducted. Additionally, interaction analyses for the subgroups were performed. Sensitivity analyses included handling missing data and excluding cancer cases that occurred within the first two years of follow-up.
All reported p-values are two-tailed, with significance defined as p < 0.05. The analyses were conducted using R 4.2.3 (R-project, Vienna, Austria).
Ethical considerations
All participants signed informed consent. The data obtained in this study were approved by the Peking University/Institutional Review Board (household survey: IRB00001052-11015; biomarker: IRB00001052-11014).
Results
The study included 9996 participants without cancer at baseline. Participants were followed for over 88,790 person-years (median, 9 years) in total. We observed that 325 participants developed incident cancer (incidence rate: 200.49 per 10,000 person-years). Totally, 98 participants lost to follow-up and 31 were died for unknown reason, the follow-up rate was 99.0%.
Table 1 displays the baseline characteristics of participants based on their total sleep duration. Those who slept shorter tended to be older, female, and have higher rates of rural residency and normal weight. They also had a lower prevalence of current smoking and drinking, and a lower level of advanced education. In contrast, those who slept longer had higher rates of current smoking and drinking, advanced education, cardiovascular disease, and slightly higher median values for waist circumference, height, weight, and BMI.
The relationship between sleep duration and cancer incidence is demonstrated in Table 2. After adjusting for age, gender, smoking, drinking, education, residence, hypertension, diabetes, dyslipidemia, waist circumference, BMI, and eGFR, it was found that a total sleep duration of less than 6 h significantly increased the risk of cancer, with HR and 95% CI of 1.27 (1.01, 1.61), and this hazard effect also found for digestive & respiratory system cancers (HR:1.41, 95%CI:1.03–1.93). Longer sleep durations (> 9 h) also show a potential increase in risk, but the results are not consistently significant across models and cancer types. In subgroup analyses stratified by age, a similar significant increase in cancer incidence was observed among individuals aged 60 years or younger, with those sleeping less than 6 h per day exhibiting a 35% increase in overall cancer risk and 83% increase of digestive and respiratory system cancer (Table 3). However, there was no significant association between nocturnal sleep durations and cancer incidence. Additionally, there was no significant association between sleeping more than 30 min per day and cancer risk compared to sleeping ≤ 30 min per day, with an HR (95%CI) of 1.13 (0.90, 1.42) (Table 2). Gender-stratified analyses did not reveal any significant associations between sleep duration and cancer incidence. However, there was a significant interaction between daytime naps longer than 30 min and cancer incidence in women (p = 0.041) (Table 4).
Reanalysis of the study results using missing data, as shown in Table S2, yielded outcomes that were nearly consistent with those obtained using imputed missing values. Additionally, after excluding patients who developed cancer within the first two years of follow-up, the results remained largely consistent, as shown in Table S3. This consistency indicates the robustness of the findings.
Discussion
The study followed nearly 10,000 participants for an average of 9 years and found that short sleep duration was associated with an increased cancer risk, particularly for cancers in the digestive and respiratory systems, compared to those with a moderate sleep duration (6–9 h per day). However, there was no significant association between longer sleep durations or napping and cancer risk.
Self-reported typical sleep characteristics and shift work were not found to be associated with pancreatic cancer risk among UK residents [25]. Similarly, no significant association was observed between sleep duration and breast cancer risk in US residents [7]. However, in Chinese residents, sleep duration trajectories and quality were found to be closely linked to cancer risk and cancer-specific mortality [8]. The relationships between sleep duration and cancer risk have been inconsistent, possibly due to variations in population and study methods. Our prospective cohort study suggests that both inadequate and excessive sleep elevate the risk of cancer. According to the recommended healthy sleep duration outlined in the Thoracic Society Statement, which suggests 6–9 h/day [39], our findings align. We found that compared to individuals who slept 6–9 h per day, those who slept less had an increased risk of cancer. Additionally, those who slept more than 9 h per day showed a nearly statistically significant increase in cancer risk. Notably, our findings align with previous research indicating a U-shaped association between sleep duration and malignant neoplasms of digestive organs [40]. Additionally, meta-analysis study indicated a J-shaped association between sleep duration and all-cause mortality, where both shortened and prolonged sleep durations were linked to increased mortality risk compared to 7 h of sleep [41]. However, the number of participants sleeping more than 9 h was very small, which might explain the lack of statistical significance in this study. This result needs to be validated by studies with larger sample sizes. While the individual hazard ratios for daytime naps longer than 30 min were not significant for either gender, the significant interaction term suggests that gender modifies the effect of daytime nap duration on cancer incidence. Further research is needed to understand the underlying mechanisms and to explore how gender-specific factors contribute to this interaction.
Moving forward, further research is warranted to elucidate the underlying mechanisms linking sleep duration to cancer risk. Investigating the biological pathways involved in sleep regulation and their potential impact on carcinogenesis could provide valuable insights. Additionally, exploring the interplay between sleep duration, lifestyle factors, and genetic predispositions may help refine risk assessment models and inform preventive strategies. In general, poor sleep quality may contribute to cancer development through various mechanisms, including reduced production of melatonin, sleep disruption, and disturbances in lifestyle [42]. Melatonin, a hormone produced by the pineal gland, plays a crucial role in regulating the circadian rhythm. Studies have suggested that shift workers, who are often exposed to light at night, may exhibit lower levels of melatonin or its metabolites, potentially influencing cancer-related processes such as proliferation, metastasis, and angiogenesis [43, 44]. Additionally, well-established risk factors for cancer, such as tobacco smoking, physical inactivity, overweight, and obesity, should be considered [45].
The strengths of our study include a large sample size and nationally representative participants. However, several limitations need to be addressed. Firstly, cancer status was self-reported, and the specific types of cancer could not be distinguished. According to estimates from the National Cancer Center (NCC) of China, approximately 4,064,000 new cancer cases were reported in 2016. This resulted in a crude incidence rate of 293.9 per 100,000 for the whole population and 1,057.56 per 100,000 for individuals aged 60 and above [46, 47]. In our study, the overall cancer incidence rate was found to be 3,251.626 per 100,000. The discrepancy in cancer incidence rates may stem from differences in the study population. Our research includes individuals aged 45 and above, while other studies might focus on different age groups. Additionally, the increasing aging population in China could contribute to variations in incidence rates. Secondly, information on sleep habits, such as the frequency of daytime naps and daytime activities, was lacking. Although older respondents consistently reported poor sleep quality, there was no standardized sleep quality questionnaire available, and sleep quality or patterns were not accounted for. Thirdly, self-reported sleep duration may have been overestimated by approximately 60 min compared to objective measurements for Chinese adults [48].
Conclusion
Our study investigated the relationship between sleep duration and cancer incidence among 9,996 participants over a median follow-up period of 9 years. We observed a significant association between short sleep duration and the incidence of cancer, particularly cancers occurring in the digestive and respiratory systems. Although no significant associations were found between napping or nocturnal sleep and overall cancer incidence, there was an interaction between daytime napping and gender in relation to cancer risk, which warrants further investigation.
Data availability
The data is freely accessible on the website https://charls.pku.edu.cn/.
Abbreviations
- CHARLS:
-
China Health and Retirement Longitudinal Study
- HR:
-
Hazard Ratio
- CI:
-
Confidence Interval
- BMI:
-
Body Mass Index
- WC:
-
Waist Circumference
- BP:
-
Blood Pressure
- TC:
-
Total Cholesterol
- TG:
-
Triglycerides
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- GFR:
-
Glomerular Filtration Rate
- MICE:
-
Multiple Imputation by Chained Equations
- RCS:
-
Restricted Cubic Splines
- REM:
-
Rapid Eye Movement
- OSA:
-
Obstructive Sleep Apnea
- IRB:
-
Institutional Review Board
References
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (London England). 2021;41(10):1037–48.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Ca-a cancer J Clin. 2021;71(3):209–49.
Shreves AH, Small SR, Travis RC, Matthews CE, Doherty A. Dose-response of accelerometer-measured physical activity, step count, and cancer risk in the UK Biobank: a prospective cohort analysis. Lancet. 2023;402(Suppl 1null):S83.
Tan N, Yap D, Tan B, Teo Y, Tan V, See A, et al. 158P obstructive sleep apnea and breast cancer incidence: a systematic review and meta-analysis. Ann Oncol. 2021;32(null):S89.
Wang L, Du M, Wang K, Khandpur N, Rossato S, Drouin-Chartier J et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. BMJ (Clinical Res ed). 2022;null(null):e068921.
Sun D, Liu C, Zhu Y, Yu C, Guo Y, Sun D, et al. Long-term exposure to fine particulate matter and incidence of Esophageal Cancer: a prospective study of 0.5 million Chinese adults. Gastroenterology. 2023;165(1):61–e705.
Cai Y, Zhaoxiong Y, Zhu W, Wang H. Association between sleep duration, depression and breast cancer in the United States: a national health and nutrition examination survey analysis 2009–2018. Ann Med. 2024;56(1):2314235.
Liu C, Zhang Q, Liu C, Liu T, Song M, Zhang Q, et al. Age differences in the Association of Sleep Duration Trajectory with Cancer Risk and Cancer-Specific Mortality: prospective cohort study. JMIR Public Health Surveillance. 2024;10:e50836.
Miyata J, Muraki I, Iso H, Yamagishi K, Yasuda N, Sawada N, et al. Sleep duration, its change, and risk of dementia among Japanese: the Japan Public Health Center-based prospective study. Prev Med. 2024;180:107884.
Feriante J, Singh SREM, Rebound Effect. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Shantanu Singh declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024. StatPearls Publishing LLC.; 2024.
Tang L, Zhang H, Liao Y, Zhou S, Yang Y, Zhang M, et al. Chronic sleep deprivation impairs visual functions via oxidative damage in mice. Am J Pathol. 2024;194(2):307–20.
Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults–United States, 2014. MMWR Morbidity Mortal Wkly Rep. 2016;65(6):137–41.
Chaput JP, Wong SL, Michaud I. Duration and quality of sleep among canadians aged 18 to 79. Health Rep. 2017;28(9):28–33.
Zhu G, Catt M, Cassidy S, Birch-Machin M, Trenell M, Hiden H, et al. Objective sleep assessment in > 80,000 UK mid-life adults: associations with sociodemographic characteristics, physical activity and caffeine. PLoS ONE. 2019;14(12):e0226220.
Chan CMH, Siau CS, Wong JE, Wee LH, Jamil NA, Hoe VCW. Prevalence of Insufficient Sleep and its Associated factors among working adults in Malaysia. Nat Sci Sleep. 2021;13:1109–16.
Belloir J, Makarem N, Shechter A. Sleep and Circadian Disturbance in Cardiovascular Risk. Curr Cardiol Rep. 2022;24(12):2097–107.
Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–61.
Chen PY, Chen TY, Chao PZ, Liu WT, Bai CH, Tsao ST, et al. REM-related obstructive sleep apnea and vertigo: a retrospective case-control study. PLoS ONE. 2021;16(6):e0252844.
Dong L, Xie Y, Zou X. Association between sleep duration and depression in US adults: a cross-sectional study. J Affect Disord. 2022;296:183–8.
Smiley A, King D, Bidulescu A. The Association between Sleep Duration and metabolic syndrome: the NHANES 2013/2014. Nutrients. 2019;11(11).
Chen Y, Tan F, Wei L, Li X, Lyu Z, Feng X, et al. Sleep duration and the risk of cancer: a systematic review and meta-analysis including dose-response relationship. BMC Cancer. 2018;18(1):1149.
Ren Z. Abstract 2181: Association of sleep duration, daytime napping, and night shift work with breast cancer risk. Cancer Res. 2014;74(19Supple):2181.
Svensson T, Saito E, Svensson AK, Melander O, Orho-Melander M, Mimura M, et al. Association of Sleep Duration with All- and major-cause mortality among adults in Japan, China, Singapore, and Korea. JAMA Netw open. 2021;4(9):e2122837.
Wong JY, Bassig BA, Vermeulen R, Hu W, Ning B, Seow WJ, et al. Sleep duration across the adult lifecourse and risk of Lung Cancer Mortality: a Cohort Study in Xuanwei, China. Cancer Prev Res. 2017;10(6):327–36.
Freeman JR, Saint-Maurice PF, Zhang T, Matthews CE, Stolzenberg-Solomon RZ. Sleep and risk of pancreatic cancer in the UK Biobank. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2024.
Elamin N, Althebity N, Alkhamisi TA, Al-Foheidi M. Sleep quality and psychological disorders in breast cancer female patients receiving radiotherapy at a tertiary oncology center in West Saudi Arabia. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2024;32(3):163.
China Health and Retirement Longitudinal Study. http://charls.pku.edu.cn/pages/data/111/zh-cn.html. Accessed on March 2, 2024.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, et al. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrology: CJASN. 2017;12(6):885–92.
Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, et al. An official American thoracic Society Statement: the importance of healthy sleep. Recommendations and future priorities. Am J Respir Crit Care Med. 2015;191(12):1450–8.
Luo H, Li J, Zhang Q, Cao P, Ren X, Fang A, et al. Obesity and the onset of depressive symptoms among middle-aged and older adults in China: evidence from the CHARLS. BMC Public Health. 2018;18(1):909.
consultation We. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Federation ID. The IDF consensus worldwide defi nition of the metabolic syndrome. Brussels: International Diabetes Federation; 2006. https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html. Accessed on March 2, 2024.
American Diabetes A. 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–31.
Jiang CH, Zhu F, Qin TT. Relationships between Chronic diseases and Depression among Middle-aged and Elderly people in China: a prospective study from CHARLS. Curr Med Sci. 2020;40(5):858–70.
Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New Creatinine- and cystatin C-Based equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–49.
Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transpl. 2010;45(9):1388–95.
Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, et al. An official American thoracic Society Statement: the importance of healthy sleep. Recommendations and future priorities. Am j resp crit care. 2015;191(12):1450–8.
Kim Y, Wilkens LR, Schembre SM, Henderson BE, Kolonel LN, Goodman MT. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: the multiethnic cohort study. Prev Med. 2013;57(4):377–85.
Liu TZ, Xu C, Rota M, Cai H, Zhang C, Shi MJ, et al. Sleep duration and risk of all-cause mortality: a flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. 2017;32(null):28–36.
Fritschi L, Glass DC, Heyworth JS, Aronson K, Girschik J, Boyle T, et al. Hypotheses for mechanisms linking shiftwork and cancer. Med Hypotheses. 2011;77(3):430–6.
Hunter CM, Figueiro MG. Measuring light at night and Melatonin Levels in Shift Workers: a review of the literature. Biol Res Nurs. 2017;19(4):365–74.
Talib WH. Melatonin and Cancer Hallmarks. Molecules. 2018;23(3).
Boniol M, Autier P. Prevalence of main cancer lifestyle risk factors in Europe in 2000. Eur J cancer (Oxford England: 1990). 2010;46(14):2534–44.
Guo H, Lin K, Yang K, Ma Z, Cao M, Hu Y, et al. Trends of cancer incidence among Chinese older adults from 2005 to 2016: a log-linear regression and age-period-cohort analysis. Front Public Health. 2022;10null:1023276.
Wang Y, Yan Q, Fan C, Mo Y, Wang Y, Li X, et al. Overview and countermeasures of cancer burden in China. Sci China-Life Sci. 2023;66(11):2515–26.
Jackson CL, Patel SR, Jackson WB 2nd, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, hispanic, and Chinese adults in the United States: multi-ethnic study of atherosclerosis. Sleep. 2018;41(6):1–12.
Acknowledgements
We would like to thank Charls for providing the data.
Funding
This research received no specific funding.
Author information
Authors and Affiliations
Contributions
Yang Jiang and Xinyue Gu contributed equally to this work. Xiao Yang, Aidi Sun, and Huixin Sun provided significant contributions to the research and manuscript preparation. Specifically, the contributions of each author are as follows: Yang Jiang: Conceptualization, Methodology, Writing - Original Draft; Xinyue Gu: Data Curation, Formal Analysis, Writing - Review & Editing; Xiao Yang: Investigation, Resources, Validation; Aidi Sun: Software, Visualization, Supervision; Huixin Sun: Project Administration, Funding Acquisition, Correspondence.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data obtained in this study were approved by the Peking University/Institutional Review Board (household survey: IRB00001052-11015; biomarker: IRB00001052-11014). All participants signed informed consent. The writing and reporting of this study adhere to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, and all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Y., Gu, X., Yang, X. et al. Exploring the association between sleep duration and cancer risk in middle-aged and older Chinese adults: observations from a representative cohort study (2011–2020). BMC Public Health 24, 1819 (2024). https://doi.org/10.1186/s12889-024-19313-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19313-z