Abstract
Background
Since the Coronavirus disease 2019 (COVID-19) pandemic began, the number of individuals recovering from COVID-19 infection have increased. Post-COVID Syndrome, or PCS, which is defined as signs and symptoms that develop during or after infection in line with COVID-19, continue beyond 12 weeks, and are not explained by an alternative diagnosis, has also gained attention. We systematically reviewed and determined the pooled prevalence estimate of PCS worldwide based on published literature.
Methods
Relevant articles from the Web of Science, Scopus, PubMed, Cochrane Library, and Ovid MEDLINE databases were screened using a Preferred Reporting Items for Systematic Reviews and Meta-Analyses-guided systematic search process. The included studies were in English, published from January 2020 to April 2024, had overall PCS prevalence as one of the outcomes studied, involved a human population with confirmed COVID-19 diagnosis and undergone assessment at 12 weeks post-COVID infection or beyond. As the primary outcome measured, the pooled prevalence of PCS was estimated from a meta-analysis of the PCS prevalence data extracted from individual studies, which was conducted via the random-effects model. This study has been registered on PROSPERO (CRD42023435280).
Results
Forty eight studies met the eligibility criteria and were included in this review. 16 were accepted for meta-analysis to estimate the pooled prevalence for PCS worldwide, which was 41.79% (95% confidence interval [CI] 39.70–43.88%, I2 = 51%, p = 0.03). Based on different assessment or follow-up timepoints after acute COVID-19 infection, PCS prevalence estimated at ≥ 3rd, ≥ 6th, and ≥ 12th months timepoints were each 45.06% (95% CI: 41.25–48.87%), 41.30% (95% CI: 34.37–48.24%), and 41.32% (95% CI: 39.27–43.37%), respectively. Sex-stratified PCS prevalence was estimated at 47.23% (95% CI: 44.03–50.42%) in male and 52.77% (95% CI: 49.58–55.97%) in female. Based on continental regions, pooled PCS prevalence was estimated at 46.28% (95% CI: 39.53%-53.03%) in Europe, 46.29% (95% CI: 35.82%-56.77%) in America, 49.79% (95% CI: 30.05%-69.54%) in Asia, and 42.41% (95% CI: 0.00%-90.06%) in Australia.
Conclusion
The prevalence estimates in this meta-analysis could be used in further comprehensive studies on PCS, which might enable the development of better PCS management plans to reduce the effect of PCS on population health and the related economic burden.
Similar content being viewed by others
Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that first emerged in December 31st 2019 in Wuhan, China, causes the infectious coronavirus disease 2019 (COVID-19) [1, 2]. The World Health Organization (WHO) declared COVID-19 a Public Health Emergency of International Concern (PHEIC) on 30 January 2020, then a pandemic on 11 March 2020 [3, 4]. The COVID-19 pandemic has resulted in an increasing number of people recovering from SARS-CoV-2 acute infection [5]. COVID-19 patients might typically recover within a few weeks after symptom onset. However, some patients might experience health-related effects in the longer-term. Widely known as long COVID and post-COVID-19 condition, the conditions that occur post-COVID infection are also referred to with other terms, namely PCS, post-COVID-19 syndrome, long-haul COVID, post-acute COVID-19, long-term effects of COVID, or chronic COVID [6,7,8,9,10,11,12]. The WHO defined the post-COVID-19 condition as symptoms that occur at least 3 months after probable or confirmed SARS-CoV-2 infection that persist for at least 2 months and cannot be explained by an alternative diagnosis [13]. The symptoms might fluctuate, relapse, persist from the initial infection, or might also be new-onset after recovery from the acute illness [13]. In a COVID-19 rapid guideline, the National Institute for Health and Care Excellence (NICE), the Royal College of General Practitioners (RCGP), and the Scottish Intercollegiate Guidelines Network (SIGN) classified long COVID as “ongoing symptomatic COVID-19” and “post-COVID-19 syndrome”. Ongoing symptomatic COVID-19 is defined as signs and symptoms that persist 4–12 weeks after acute COVID-19, while post-COVID-19 syndrome is defined as signs and symptoms that develop during or after an infection in line with COVID-19 that continue for > 12 weeks and are not explained by an alternate diagnosis [14]. Given the increasing number of COVID-19 survivors, the above terms have gained widespread recognition in the scientific and medical communities [10, 11].
Post-recovery symptoms have become of increasing concern to more COVID-19 survivors [6]. Several studies have determined that COVID-19 exerts a wide range of long-term effects on virtually all body systems, including the respiratory, cardiovascular, neurological, gastrointestinal, psychiatric, and dermatological systems [6]. Cough and fatigue are among the multiorgan symptoms described following COVID-19 infection, as are shortness of breath, headache, palpitations, chest discomfort, joint pain, physical limits, depression, and insomnia [7]. A published review revealed that hepatic and gastrointestinal (n = 6), cardiovascular (n = 9), musculoskeletal and rheumatologic (n = 22), respiratory (n = 27), and neurologic and psychiatric (n = 41) issues were the most prevalent late complications which might occur post COVID-19 infection [15]. Certain risk factors such as older age and biological sex cannot be changed, thus management of other preventable and manageable risk factors like chronic comorbidities, may benefit the high-risk people from developing the persistent COVID-19 symptoms, even after few months post-acute COVID-19 infection. Epidemiological studies and related clinical trials addressing leading hypotheses may aid in the development of good management practices, including effective prevention and early intervention strategies to control the risk factors and manage the complications [16]. Regular disease surveillance and monitoring, implementation of related health promotion strategies, plus continuous efforts in researching for the best vaccines and treatment options may help in lowering the prevalence of PCS [17, 18].
An increasing number of published studies have focused on PCS. However, robust studies on this dynamic post-COVID condition are still required to identify the risk factors; explore the underlying aetiology; and plan strategies for preventative, rehabilitation, clinical, and public health management to enhance COVID-19 recovery and long-term outcomes [12]. Such studies should be conducted using the most recent data on PCS prevalence. Therefore, the present study systematically reviewed and determined the pooled prevalence of PCS worldwide based on current published literature.
Methods
Study design
Articles related to PCS and the prevalence data available worldwide were obtained using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework. The review protocol was registered with PROSPERO (CRD42023435280). All authors have a background in the related field and contributed collectively to meeting the study objective. The research question was developed, then a systematic search was conducted to identify and screen eligible studies based on the inclusion and exclusion criteria. Articles were identified from five primary databases. Relevant information and data were extracted from available full-text primary articles to answer the research question. The methodological quality of the included articles was assessed with the Joanna Briggs Institute (JBI) critical appraisal checklist. Subsequently, a meta-analysis was conducted to estimate the pooled prevalence of PCS worldwide.
Outcomes and measures
The overall prevalence estimates of any persistent health conditions and symptoms at ≥ 12 weeks after the index date were set as the primary outcome. The 12-week timeframe was adopted to conform with the clinical definition of PCS, which is symptoms and signs that develop after or during infection consistent with COVID-19, not clarified by different diagnosis, and continue beyond 12 weeks.
Inclusion and exclusion criteria
A set of inclusion and exclusion criteria was utilized as a basis for the identification and selection of relevant articles for this systematic review and meta-analysis study. The inclusion criteria were: 1) availability of full text; 2) article was written in English language; 3) article was published within 1 January 2020 to 27 April 2024; 4) study was related to prolonged post-COVID-19 conditions, and used human populations with COVID-19 diagnosis confirmed using PCR, antibody testing, or a clinical diagnosis; 5) study had an index date using the COVID-19 onset date, first test or diagnosis, hospitalisation date, or discharge date; and 6) study had adequate data on the estimates of overall PCS prevalence in a community, i.e. studies which not only focused on the prevalence of a specific PCS symptom as their only outcome. This was to ensure that the primary outcome in this meta-analysis, which is the pooled overall prevalence of PCS is derived only from studies with identical outcomes, besides limiting the probabilities of any bias resulting from including studies which only published symptom-specific PCS prevalence data estimates. Another inclusion criteria used was 7) assessment date, or follow-up or clinical check-up date at least 12 weeks after the index date. Meanwhile, the exclusion criteria were non-accessible articles and publications with content unrelated to the research question. Non-primary publications such as book chapters or letters to editor, and case reports were also excluded.
Search strategy
The search terms used in the article identification stage were derived from medical subject heading (MeSH) terms and synonyms related to the review topic. Then, two authors (RR and NIS) conducted a systematic search of the abovementioned databases using the search strings developed from combining the identified search terms and Boolean operators. The search string used was (("PCS" OR "post COVID syndrome" OR "post COVID-19 syndrome" OR "post COVID condition*" OR "post COVID-19 condition*" OR "post COVID" OR "post-COVID" OR "post COVID-19" OR "post-COVID-19" OR "post COVID sequela" OR "post-COVID sequela" OR "post COVID sequelae" OR "post-COVID sequelae" OR "long COVID" OR "long-COVID" OR "long haul*" OR "long-haul*" OR "long COVID-19" OR "long-COVID-19" OR "covid syndrome" OR "covid-19 syndrome" OR "post-acute COVID-19 syndrome" OR "post acute COVID-19" OR "post acute COVID" OR "chronic COVID" OR "chronic COVID-19" OR "persistent COVID" OR "persistent post-COVID" OR "persistent post COVID" OR "prolonged COVID" OR "prolonged post-COVID" OR "prolonged post COVID") AND ("prevalence*")). Available filters based on the inclusion and exclusion criteria were applied during the database search.
Data sources
Relevant articles searched and identified from five databases (Web of Science [WOS], Scopus, PubMed, Cochrane Library, Ovid MEDLINE) on 29 April 2024, were downloaded by author RR and collected in Mendeley Desktop version 1.19.8. Subsequently, duplicates were identified and removed by author NIS, and the shortlisted articles were transferred to Microsoft Excel for further screening.
Study selection
Relevant studies were selected via a screening process conducted by two authors, who independently screened the article titles and abstracts, then retrieved the full text of shortlisted articles. Efforts to include all available studies were made and included accessing publications via institutional accounts. Subsequently, two authors (RR and NIS) examined the full texts of potential eligible papers separately, followed by discussions and re-evaluation among them to resolve any contradictory decisions. A third author (AI) was also employed in this process, when there are uncertainties in the decision-making process.
Data extraction
Two authors (RR and NIS) then extracted and tabulated the relevant data elements (article title, authors, publication year, study design, country, study population, study setting, sample size and number of cases identified, duration from index to assessment date, PCS prevalence estimates).
Methodological quality assessment
The methodological quality of the studies was evaluated with the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Studies Reporting Prevalence Data to ascertain how well the article addressed the possibility of bias. All articles screened and selected for inclusion in this systematic review were appraised by two critical appraisers (RR and NIS). The JBI checklist contains 9 items which comprised of 1 question each; (Item 1: Was the sample frame appropriate to address the target population?), (Item 2: Were study participants sampled in an appropriate way?), (Item 3: Was the sample size adequate?), (Item 4: Were the study subjects and the setting described in detail?), (Item 5: Was the data analysis conducted with sufficient coverage of the identified sample?), (Item 6: Were valid methods used for the identification of the condition?), (Item 7: Was the condition measured in a standard, reliable way for all participants?), (Item 8: Was there appropriate statistical analysis?), (Item 9: Was the response rate adequate, and if not, was the low response rate managed appropriately?). Each item is coded as “yes/no/unclear/not applicable”. Each of these items is assessed by scoring (yes = 1), (no = 0), and (unclear or not applicable = 0). The total score of each included study was presented as percentages, which then categorized into 3 levels of risk of bias: (20–50% items scored yes = high risk of bias), (50–80% items scored yes = moderate risk of bias), and (80–100% items scored yes = low risk of bias). Based on the assessment result, both appraisers discussed and finalised the decision on the overall appraisal, i.e., whether to include the assessed study in the review.
Statistical analysis
The meta-analysis was conducted using the metaprop function in the R 4.3.1 meta package. Due to the heterogeneity of the included studies as resulted from differences in studied populations’ factors, varied geographical regions and PCS assessment timepoints, a random-effects model was considered as the better choice for assigning weights to each study in the meta-analysis. The pooled prevalence and effect sizes for each study were included in a forest plot, where the size of each study was proportional to its weight. Statistical heterogeneity was measured with I2 statistics versus p-values, where a p-value of 0.05 and an I2 of ≥ 50% indicated high heterogeneity. Visual inspection of the generated funnel plot’s symmetricity was conducted to determine any influence of publication bias on the findings. Egger’s test and Begg rank correlation test were also conducted for further identification of the presence of any asymmetricities.
Results
Study selection
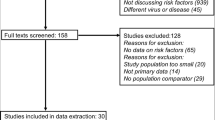
Overall, a total of 3321 records were identified from the main literature search conducted in end of April 2024, of which 907 duplicate articles were removed. Screening of the article titles and abstracts resulted in 2325 articles unrelated to the research question being excluded. All remaining articles were retrieved to determine their accessibility, of which 89 successfully retrieved full-text articles were reviewed and assessed for eligibility. Articles with contents not relevant to this study were excluded. Studies with sample populations with mean or median prolonged signs or symptoms, or health care utilisation, or follow-up time < 12 weeks from acute COVID-19 symptom onset were excluded to ensure that the samples with persistent COVID-19 symptoms in the finalised studies met the definition of PCS. A total of 41 articles were excluded, as these studies and their contents did not align with the review topic or the other inclusion and exclusion criteria. Finally, 48 articles were included in this review. The PRISMA flow diagram in Fig. 1 depicts the literature selection process and search criteria, and the number of articles involved for each process.
Study characteristics and PCS prevalence
Table 1 presents the study characteristics of the 48 included studies, including the overall PCS prevalence data from each study. Among those studies, 21 were from European countries, 14 studies were from American region, 10 were from Asia, two were from Australia, and one study from African continent. Forty one included studies were cohort studies, 5 were cross-sectional and 2 were case–control studies. The studies involved sample sizes of 106–124313 individuals diagnosed with COVID-19 at least 12 weeks prior to the assessment date. The index date-to-assessment date duration ranged from 12 weeks to 25.5 months. Among the included studies, 10 studies focused mainly on the previously hospitalized COVID-19 patients and 1 study researched on PCS among the non-hospitalized COVID-19 patients. Majority of the included studies studied both previously hospitalized and non-hospitalized COVID-19 patients, as shown in 35 studies in Table 1. Most of the examined populations in the 48 included studies were adult-aged, while the percentage of female participants varied from 26.5% to 77.5%.
Methodological quality assessment
Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Studies Reporting Prevalence Data was used to assess the methodological quality of the included studies. The assessment results reflect the methodological quality and risk of bias levels of the individual studies, which were categorized into low (80%-100% scores), moderate (50%–80% scores), and high (20%–50% scores) risk of bias levels. The assessment result aids in finalizing the decision on the overall review of the individual studies, i.e., whether to include the assessed study in the review. Based on the checklist, majority of the 48 included articles in this review were of high methodological quality, with low risk of bias. The risk of bias levels for each study were listed under the last column titled ‘Risk of Bias’ in Table 1 (Summary of characteristics of the 48 included studies table). All 48 assessed studies were accepted to be included in this review.
Pooled prevalence estimate of post-COVID syndrome
As shown by the forest plot in Fig. 2, the prevalence estimates of PCS reported in the 48 included individual studies ranged from 3.4% to 90.41%. Due to the significant high heterogeneity (I2 = 100%, p = 0) and presence of funnel plot asymmetry indicated by Egger’ test observed if meta-analysis was to be conducted using the prevalence data from all 48 included studies, only 16 studies were accepted for meta-analysis of the overall PCS prevalence, after excluding potential influential outliers based on the influence analyses done, including leave-one out analyses, risk of bias assessment for studies, and influential outliers.
In the meta-analysis conducted using the 16 allowed studies, the pooled prevalence of PCS estimated by random-effects model using data from the 16 studies was 41.79% (95% CI: 39.70%-43.88%). The forest plot shown in Figure 3 depicts the results derived from the random effects model, while Fig. 4 shows the funnel plot for the publication bias assessment of the 16 studies.
Assessment of heterogeneity
Generally, heterogeneity is to be expected in a meta-analysis [67]. I2 was used to measure heterogeneity, with limits of ≥ 25%, ≥ 50%, and ≥ 75% each denoting low, moderate, and high heterogeneity. The meta-analysis conducted using random-effects model to calculate the pooled-prevalence of PCS in this study revealed significant mild to moderate heterogeneity across the included studies (I2 = 52%, p < 0.01). The variance in the underlying distribution of true effect sizes, or the between-study heterogeneity, was estimated at τ2 = 0.0009. In meta-analyses, heterogeneity is frequently unavoidable due to variations in study quality, methodology, sample size, and participant inclusion criteria [49, 68].
Assessment of publication bias
Publication bias might occur when journals and authors only publish articles with the outcome of interest and can be detected by visual inspection of funnel plots. As shown in Fig. 4, publication bias was visually implied from the asymmetrical funnel plot. However, further analysis using Egger’s test did not indicate the presence of funnel plot asymmetry, although it was not statistically significant (p = 0.4661). Begg rank correlation test results was also not significant with p-value of 0.7871. These formal tests findings suggested that the results were not influenced by publication bias. Nevertheless, any visual asymmetry in the funnel plot might also be caused by true heterogeneity other than publication bias [69].
PCS prevalence at different Post-COVID assessment timepoints
To assess if the pooled prevalence of PCS was increasing over time after the acute COVID-19 infection, we stratified the included studies based on different assessment or follow-up timepoints. A subgroup analysis was performed to get the PCS pooled prevalence at ≥ 3rd, ≥ 6th, and ≥ 12th months post-COVID-19 infection. As shown in Fig. 5, the estimated Post-COVID Syndrome pooled prevalences at ≥ 3rd, ≥ 6th, and ≥ 12th months timepoints were each 45.06% (95% CI: 41.25%-48.87%, I2 = 59%, p = 0.02), 41.30% (95% CI: 34.37%-48.24%, I2 = 87%, p < 0.01), and 41.32% (95% CI: 39.27%-43.37%, I2 = 21%, p < 0.27), respectively.
Post-COVID syndrome prevalence in male and female
Further subgroup analysis was conducted to examine the PCS prevalences among male and female. For this purpose, data from 10 articles out of all 48 included articles were allowed for the subgroup analysis, after the exclusion of influential outliers to estimate the pooled prevalences with less amount of heterogeneity. As shown in Fig. 6, the estimated Post-COVID Syndrome prevalence were 47.23% in male (95% CI: 44.03%–50.42%), and 52.77% in female (95% CI: 49.58%-55.97%). The studies had significant moderate heterogeneity with I2 = 51%, p = 0.03.
Post-COVID syndrome prevalence in different continental regions
Another subgroup analysis based on stratification of PCS prevalence by continental regions was also performed. For this purpose, data from all 48 articles were included in the analysis.
The estimated Post-COVID Syndrome prevalences according to the continental regions were shown in Fig. 7. The pooled prevalence was 46.28% (95% CI: 39.53%-53.03%) in Europe, 46.29% (95% CI: 35.82%-56.77%) in America, 49.79% (95% CI: 30.05%-69.54%) in Asia, and 42.41% (95% CI: 0.00%-90.06%) in Australia. Only one study from African continent was included in this review, with PCS prevalence reported at 50.33% (95% CI: 44.55%-56.11%). Most of the subgroups showed a significant heterogeneity level with I2 = 100%, p < 0.01.
Discussion
Post-COVID syndrome (PCS)
In this systematic review and meta-analysis, the term described by NICE; which defined PCS as signs and symptoms that develop during or after an infection in line with COVID-19 that continue for > 12 weeks and are not explained by an alternate diagnosis, was used as a basis to identify the overall PCS prevalence data [14] from published studies worldwide. The cut-off point of 12 weeks was strictly used to extract and analyse the relevant data during the systematic review process.
Overall prevalence estimates of PCS worldwide
In this review, a total of 2414 published articles were screened from 3321 articles identified from 5 databases using a PRISMA-guided systematic search. The meta-analysis of 48 included studies that individually reported PCS prevalence data determined that the estimated pooled prevalence of PCS worldwide was 41.79% (95% CI: 39.70%-43.88%). Besides the articles included in this meta-analysis, other notable published studies reporting PCS prevalence data might have been missed due to some limitations in our study, including the suitability of the articles for meta-analysis and the strict inclusion criteria.
The local prevalences reported globally varied, contributing to the high level of possibility for true heterogeneity when meta-analysed. Among the factors causing the variation of the reported prevalence data was the differences of post-COVID-19 assessment timepoints used in each individual studies. Generally, most related published studies reported the prevalence of persisting COVID-19 symptoms at 3, 6, 9, 12, 18 and even 24 months after the onset of acute COVID-19 infection. In this meta-analysis, the follow-up or assessment timepoints were categorized into ≥ 3rd, ≥ 6th, and ≥ 12th months after the index date, whereby the pooled prevalence estimates were 45.06%, 41.30% and 41.32% each, respectively. A cross-sectional study in Malaysia reported that 21.1% or approximately 1 in 5 COVID-19 survivors reported persistent ill health > 3 months post-COVID infection [70]. A study in India reported that 9.4% of people had long-term symptoms after COVID-19 [71]. Two studies in Saudi Arabia by Jabali et al. and Alkwai et al. reported approximately 49% and 51.2% overall PCS prevalence, respectively, while two studies in Turkey by Baris et al. and Kayaaslan et al. reported approximately 27.1% and 47.5% prevalence, respectively [6, 72,73,74]. In the Republic of South Korea, Kim et al. reported 52.7% prevalence for post-acute COVID-19 syndrome 12 months after COVID-19 infection [75]. A study in Japan reported 56.14% prevalence [76], while a study in Mexico reported high prevalence of 68% at approximately 90 days post-COVID infection [77]. In Canada, Estrada et al. reported 28.5% prevalence of persistent post-COVID-19 symptoms 90 days after infection [78]. A large retrospective cohort study in the UK reported an overall prevalence of 36.55% [8], while another UK study reported that 2.3% of COVID-19 survivors reported symptoms persisting for ≥ 12 weeks [79]. Three different post-COVID studies in Germany reported an overall prevalence of 6.5%, 8.3%, and 49.3%, respectively [80,81,82]. Boscolo-Rizzo et al. reported that 53% of Italians reported chronic COVID-related symptoms 12 months following the onset of mild to moderate COVID [83], while 59.5% of people in Luxembourg reported at least one symptom 12 months after COVID infection [84]. Two different post-COVID studies in Spain reported 14.34% and 48% prevalence of persistent symptoms at 6 months post-COVID, respectively [85, 86]. In the Netherlands, 12.7% of COVID-19 patients experienced persistent somatic symptoms that could be attributed to COVID-19 after a median 101 days after infection [87]. A cohort study in Switzerland stated that 26% of people with PCR-confirmed SARS-CoV-2 infection reported not having fully recovered after 6–8 months [88]. A prospective cohort study in Russia stated that 47.1% of previously hospitalised patients with COVID-19 reported persistent symptoms at a median 218 days post-discharge [89]. A prospective cohort study in France reported a higher prevalence at 60% [90]. A meta-analysis by Lopez-Leon et al. determined that 80% (95% CI: 65%–92%) of people diagnosed with COVID-19 developed at least one long-term symptom beyond 2 weeks and up to 110 days following acute COVID-19 infection [91]. A review by Chen et al. that meta-analysed post-COVID-19 condition prevalence at 120 days after COVID-19 infection revealed that the estimated global pooled prevalence was 49% (95% CI: 40%–59%) [92]. The review also estimated that the prevalence at 30, 60, 90, and 120 days after COVID-19 infection was 37% (95% CI: 26%–49%), 25% (95% CI: 15%–38%), 32% (95% CI: 14%–57%), and 49% (95% CI: 40%–59%), respectively [92]. Rahmati M. et al. also reported that a total of 41.7% of COVID-19 survivors experienced at least 1 unresolved symptom at 2-year after SARS-CoV-2 infection, and still suffer from either neurological, physical, and psychological sequela [93]. In another meta-analysis by O'Mahoney L. L. et al., which included studies with mean follow-up 126 days post-COVID-19 infection, at least 45% of those survived, went on to experience at least one unresolved symptom, regardless of hospitalisation status [94]. The 41.79% pooled prevalence of PCS worldwide estimated in this review is quite in line with most of the reported pooled-prevalences in other meta-analyses.
Symptom-specific PCS prevalence
This review mainly focused on determining the pooled prevalence estimate of PCS in general, hence the strict inclusion criteria. In view of the higher bias expectation due to the criteria and keywords set for obtaining the primary outcome of this study, we did not conduct subgroup analyses for symptom-specific pooled prevalence estimates. Compared to the limited number of studies focusing mainly on the overall community-based PCS prevalence, numerous studies have focused on the symptom-specific prevalence estimates related to the conditions occurring post-COVID infection, although the varied terms used based on the initial infection-to-assessment date interval.
Regarding symptom-specific prevalence, the WHO study on the clinical case definition by a Delphi consensus noted that shortness of breath, tiredness, and cognitive impairment are among the typical symptoms of PCS, which might affect daily functioning [95]. A review of the sequelae of other coronavirus infections determined that fatigue, psychological symptoms, and respiratory symptoms were common among SARS and Middle East respiratory syndrome (MERS) survivors [96]. A comprehensive systematic review and meta-analysis reported that the most common symptoms at the 3- to < 6-month assessment were fatigue (32%), shortness of breath (25%), sleep disorder (24%), and difficulty focusing (22%) [97]. Moy et al. stated that the most frequently reported symptoms were fatigue, brain fog, anxiety, insomnia, and depression, with female patients presenting 58% higher probability (95% CI: 1.02, 2.45) of experiencing persistent symptoms [70].
Sociodemographic-specific PCS prevalence
For sociodemographic-specific prevalence, PCS prevalence was generally higher in the female population. Female patients were less likely to have recovered [88] and were more susceptible to prolonged symptoms compared to male patients [98]. However, some research suggested that there might be a referral bias due to the higher participation in follow-up care by female patients compared to male patients [99]. A cohort study in Moscow reported that women were associated with post-COVID conditions at the 6- and 12-month assessments (OR: 2.04, 95% CI: 1.57–2.65 and OR: 2.04, 95% CI: 1.54–2.69, respectively) [100]. Furthermore, women experienced moderate or severe dyspnoea more often than men (53.8% vs. 21.1%) [101]. Martin-Loeches et al. stated that women were 69% more likely to develop persistent post-COVID-19 symptoms than men [102]. Moreover, most patients with persistent symptoms post-COVID infection were female (63.8%) [22]. In China, women were more likely to experience fatigue and anxiety or depression at the 6-month follow-up after COVID-19 infection [103]. A prospective cohort study in Milan, Italy, reported that women had a threefold higher risk of having persistent COVID-19 symptoms [104]. A few studies suggested that hormones might be involved in perpetuating the hyperinflammatory status of the acute COVID-19 phase in female patients even after recovery [30, 31]. While stronger immunoglobulin G (IgG) antibody production in female patients in the early phase of the illness might contribute to a more favourable outcome therein, it might also be involved in perpetuating disease manifestations [105]. In this study, sex-stratified PCS prevalence was estimated at 47.23% (95% CI: 44.03%-50.42%) in male and 52.77% (95% CI: 49.58%-55.97%) in female, which are in line with the findings from most publications with similar subject.
Populations with comorbidities such as respiratory problems, hypertension, and diabetes also had higher PCS prevalence, which indicated the role of these diseases in influencing the persistence of COVID-19 symptoms. Multiple studies also reported that high body mass index (BMI) was associated with higher hospitalisation rates and increased COVID-19 illness severity, resulting in a higher risk of developing persistent COVID-19 symptoms. Patients with known obese BMI were more likely to experience moderate or severe dyspnoea (37.5%) than those with BMI < 30 kg/m2 (27.0%), leading to a higher risk for post-acute COVID-19 [101]. Studies conducted prior to the COVID-19 pandemic era also identified inadequate humoral and cellular immune responses to vaccination against various different viruses in individuals with higher BMI [106, 107]. Another study reported a weak association between obesity and persisting fatigue post-COVID infection [108], even though this might have been due to the higher risk of chronic fatigue among overweight people, particularly obese individuals [109]. Apart from that, hospitalisation during the acute phase might also contribute towards higher PCS prevalence, whereby individuals hospitalised during the acute phase of the infection had higher prolonged symptom prevalence (54%) compared with non-hospitalised patients (34%). In addition to all of the reported cases, there are also a substantial number of undetected infections due to several circumstances, which include silent infections, diagnostic challenges, and underreporting [110,111,112].
Geographical region-specific PCS-prevalence
In this review, the estimated pooled prevalence based on continental regions was found highest in Asia (49.79%), followed by America (46.29%), Europe (46.28%), and Australia (42.41%). In a meta-analysis published in April 2022, which had focused on post-COVID-19 condition prevalence at > 28 days after infection, Chen et al. reported that the regional pooled prevalence estimates were highest in Asia 51% (95% CI: 37%-65%), followed by Europe 44% (95% CI: 32%-56%), and USA 31% (95% CI: 21%–43%). The regional differences described in another meta-analysis showed that the pooled prevalence among hospitalised population across continents was significantly higher in Europe 62.7% (95% CI: 56.5%–68.5%) compared to both North America 38.9% (95% CI:24.0%–56.3%) and Asia 40.9% (95% CI: 34.5%–47.7%) [94]. There were less studies on PCS prevalence in Australian and African continental regions published compared to Asian, European, and American regions. The fact that Australia is the only country in the Australian continent might be the cause of the smaller number of related publications from the region. For African region, a study included in this review reported that the prevalence of persistent symptoms 3 months following acute SARS-CoV-2 infection was 50.2% in Liberia [59]. Based on a meta-analysis conducted using long-COVID studies with 4-weeks minimum duration after the COVID-19 acute onset, Müller S. A. et. al. reported that the prevalence of long COVID in African countries varied widely, from 2% in Ghana to 86% in Egypt [113]. The scarcity of published studies on this health condition in African region might be due to varied factors influencing the reporting, including inadequate clinical data and diagnostics, accessibility to healthcare services and lack of awareness [113].
Strengths and limitations
Numerous post-COVID studies did not use similar term to refer to PCS. In this review, the inclusion criteria used in the study selection process allowed more PCS-specific prevalence data to be captured, contributing as a strength to this study. In addition, further few subgroup analyses conducted in this study allows more additional information on PCS prevalence based on the certain factors studied. Among the limitations in this study is that some of the studies potentially relevant for inclusion might not have been identified during the database search or might have been eliminated during the screening process, due to the different keywords and titles used. This review might have been subjected to language bias too, as only articles in English were included. Other limitation might include the issue of the high between-study heterogeneity in the meta-analysis, which might be a true heterogeneity due to various reasons such as differences in the assessment timepoints, the differences of sociodemographic factors worldwide, plus the smaller number of studies in certain geographical regions, such as studies in Australia as it is the only country in the continental region, and studies in resource-poor countries in Africa and certain parts of Asia.
Conclusions
This meta-analysis determined that the estimated pooled prevalence for PCS worldwide was 41.79% (95% CI: 39.70%-43.88%). The included studies had a significant moderate heterogeneity (I2 = 51%, p = 0.03). The estimated prevalence could be used in further related comprehensive studies, including more comprehensive analyses stratifying the prevalence based on symptom-specific risk factors too, which might enable the development of a better healthcare management plan for individuals with PCS. The provision of proper health, social, and economic protections for the higher-risk population is essential, as PCS affects population health and concurrently contributes to the higher economic burden on such patients and countries.
Availability of data and materials
Data relevant to the study were included in the article.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence Interval
- COVID-19:
-
Coronavirus disease 2019
- I2 :
-
I-squared
- JBI:
-
Joanna Briggs Institute
- MERS:
-
Middle East Respiratory Syndrome
- MeSH:
-
Medical Subject Headings
- NICE:
-
National Institute for Health and Care Excellence
- PACS:
-
Post-acute COVID-19 syndrome
- PCS:
-
Post-COVID Syndrome
- PHEIC:
-
Public Health Emergency of International Concern
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews & Meta-Analyses
- RCGP:
-
Royal College of General Practitioners
- RdRp:
-
RNA-dependent RNA polymerase
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- SIGN:
-
Scottish Intercollegiate Guidelines Network
- T2DM:
-
Type 2 Diabetes Mellitus
- WHO:
-
World Health Organization
- WOS:
-
Web of Science
- τ2 :
-
Tau-squared
References
Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44.
Lee A. Wuhan novel coronavirus (COVID-19): why global control is challenging? Elsevier Public Heal Emerg Collect. 2020;179:A1.
Summers J, Cheng HY, Lin HH, Barnard LT, Kvalsvig A, Wilson N, et al. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. Lancet Reg Heal – West Pacific. 2020;4:44.
World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020. 2020.
Ministry of Health Malaysia. Post Covid-19 Management Protocol. 2021.
Kayaaslan B, Eser F, Kalem AK, Kaya G, Kaplan B, Kacar D, et al. Post-COVID syndrome: a single-center questionnaire study on 1007 participants recovered from COVID-19. J Med Virol. 2021;93(12):6566–74.
Lancet T. Facing up to long COVID. Lancet. 2020;396(10266):1861.
Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15.
Felicity C, Elisa P. How and why patients made Long Covid. Soc Sci Med. 2021;268.:113426.2021;27(4):601–15.
Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737–54. https://doi.org/101080/2374423520211924397.
Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: a living systematic review. BMJ Glob Heal. 2021;6(9):e005427.
World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
NICE Guidelines, editor. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE Guidelines. 2020.
Alinaghi SAS, Bagheri AB, Razi A, Mojdeganlou P, Mojdeganlou H, Afsah AM, et al. Late complications of covid-19; an umbrella review on current systematic reviews. Arch Acad Emerg Med. 2023;11(1):e28.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46.
Mehraeen E, SeyedAlinaghi SA, Karimi A. The post-Omicron situation: The end of the pandemic or a bigger challenge? J Med Virol. 2022;94(8):3501–2.
Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med20&NEWS=N&AN=34312178.
Bellan M, Baricich A, Patrucco F, Zeppegno P, Gramaglia C, Balbo PE, et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11(1):22666. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med20&NEWS=N&AN=34811387.
Bliddal S, Banasik K, Pedersen OB, Nissen J, Cantwell L, Schwinn M, et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep [Internet]. 2021;11(1):13153. Available from:. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med20&NEWS=N&AN=34162913
Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10):1507–13. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med20&NEWS=N&AN=34111579.
Zayet S, Zahra H, Royer P-YY, Tipirdamaz C, Mercier J, Gendrin V, et al. Post-COVID-19 syndrome: Nine months after SARS-CoV-2 infection in a cohort of 354 patients: data from the first wave of COVID-19 in nord franche-comté hospital, France. Microorganisms. 2021;9(8):1719.
Fjelltveit EB, Blomberg B, Kuwelker K, Zhou F, Onyango TB, Brokstad KA, et al. Symptom burden and immune dynamics 6 to 18 months following mild severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2): a case-control study. Clin Infect Dis. 2022;76:60–70.
Fumagalli C, Zocchi C, Tassetti L, Silverii MV, Amato C, Livi L, et al. Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med. 2022;97:36–41.
Helmsdal G, Hanusson KD, Kristiansen MF, Foldbo BM, Danielsen ME, Steig BÁ, et al. Long COVID in the long run - 23-month follow-up study of persistent symptoms. Open Forum Infect Dis. 2022;9(7). Available from:. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85136307702&doi=10.1093%2Fofid%2Fofac270&partnerID=40&md5=cbcb5cc2ee6b5652237920f2edfe20ea
Kingery JR, Safford MM, Martin P, Lau JD, Rajan M, Wehmeyer GT, et al. Health status, persistent symptoms, and effort intolerance one year after acute COVID-19 infection. J Gen Intern Med. 2022;37(5):1218–25. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med21&NEWS=N&AN=35075531.
Knight DRT, Munipalli B, Logvinov II, Halkar MG, Mitri G, Dabrh AMA, et al. Perception, prevalence, and prediction of severe infection and post-acute sequelae of COVID-19. Am J Med Sci. 2022;363(4):295–304. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85123740987&doi=10.1016%2Fj.amjms.2022.01.002&partnerID=40&md5=4f42b267a8346594bf423562cea9f026.
Nehme M, Braillard O, Chappuis F, Courvoisier DS, Kaiser L, Soccal PM, et al. One-year persistent symptoms and functional impairment in SARS-CoV-2 positive and negative individuals. J Intern Med. 2022;292(1):103–15.
Petersen MS, Kristiansen MF, Hanusson KD, Foldbo BM, Danielsen ME, Steig BA, et al. Prevalence of long COVID in a national cohort: longitudinal measures from disease onset until 8 months’ follow-up. Int J Infect Dis. 2022;122:437–41.
Rivera-Izquierdo M, Lainez-Ramos-Bossini AJ, de Alba IGF, Ortiz-Gonzalez-Serna R, Serrano-Ortiz A, Fernandez-Martinez NF, et al. Long COVID 12 months after discharge: persistent symptoms in patients hospitalised due to COVID-19 and patients hospitalised due to other causes-a multicentre cohort study. BMC Med. 2022;20(1):92.
Tisler A, Stirrup O, Pisarev H, Kalda R, Meister T, Suija K, et al. Post-acute sequelae of COVID-19 among hospitalized patients in Estonia: Nationwide matched cohort study. PLoS One. 2022;17(11):e0278057. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med22&NEWS=N&AN=36417409.
Titze-de-Almeida R, da Cunha TR, Dos Santos Silva LD, Ferreira CS, Silva CP, Ribeiro AP, et al. Persistent, new-onset symptoms and mental health complaints in Long COVID in a Brazilian cohort of non-hospitalized patients. BMC Infect Dis. 2022;22(1):133. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med21&NEWS=N&AN=35135496.
Wu Q, Ailshire JA, Crimmins EM. Long COVID and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci Rep. 2022;12(1):11647. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med22&NEWS=N&AN=35804058.
Babicki M, Kołat D, Kapusta J, Kałuzińska-Kołat Ż, Jankowski P, Mastalerz-Migas A, et al. Prevalence and assessment of risk factors among Polish adults with post-COVID-19 syndrome: a 12-month follow-up study. Polish Arch Intern Med. 2023;133(12):16512.
Boglione L, Poletti F, Rostagno R, Moglia R, Cantone M, Esposito M, et al. Long-COVID syndrome in hospitalized patients after 2 years of follow-up. J Public Heal Emerg. 2023;7:4.
Daniel CL, Fillingim S, James J, Bassler J, Lee A. Long COVID prevalence and associated characteristics among a South Alabama population. Public Health. 2023;221:135–41.
Fatima S, Ismail M, Ejaz T, Shah Z, Fatima S, Shahzaib M, et al. Association between long COVID and vaccination: A 12-month follow-up study in a low- to middle-income country. PLoS One. 2023;18(11):e0294780.
Feter N, Caputo EL, Leite JS, Delpino FM, Silva LS da, Vieira YP, et al. Prevalence and factors associated with long COVID in adults from Southern Brazil: Findings from the PAMPA cohort. Cad Saude Publica. 2023;39(12):e00098023.
Gaspar P, Dias M, Parreira I, Gonçalves HD, Parlato F, Maione V, et al. Predictors of long-COVID-19 and its impact on quality of life: longitudinal analysis at 3, 6 and 9 months after discharge from a Portuguese centre. In: Acta Medica Portuguesa. 2023;36(10):647–60.
Hastie CE, Lowe DJ, McAuley A, Mills NL, Winter AJ, Black C, et al. True prevalence of long-COVID in a nationwide, population cohort study. Nat Commun. 2023;14(1):7892.
Hua MJ, Gonakoti S, Shariff R, Corpuz C, Acosta RAH, Chang H, et al. Prevalence and Characteristics of Long COVID 7–12 Months After Hospitalization Among Patients From an Urban Safety-Net Hospital: A Pilot Study. AJPM Focus. 2023;2(3):100091.
Jayasekera MMPT, De Silva NL, Edirisinghe EMDT, Samarawickrama T, Sirimanna SWDRC, Govindapala BGDS, et al. A prospective cohort study on post COVID syndrome from a tertiary care centre in Sri Lanka. Sci Rep. 2023;13(1):15569.
Jogdand MS, Bhondwe MR, Jogdand KS, Yerpude PN, Tathe GR, Wadiyar SS. Prevalence and Determinants of Long COVID among the COVID-19 Survivors: A Cross-sectional Study from A Rural Area of Maharashtra. Indian J Community Heal. 2023;35(2):193–8.
Khanafer N, Henaff L, Bennia S, Termoz A, Chapurlat R, Escuret V, et al. Factors Associated with Long COVID-19 in a French Multicentric Prospective Cohort Study. Int J Environ Res Public Health. 2023;20(17):6678.
Kim Y, Bae S, Chang HH, Kim SW. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. 2023;13(1):11207.
Krishnadath I, Harkisoen S, Gopie F, van der Hilst K, Hollum M, Woittiez L, et al. Prevalence of persistent symptoms after having COVID-19 in a cohort in Suriname. Rev Panam Salud Publica/Pan Am J Public Heal. 2023;47:e79.
Lapa J, Rosa D, Mendes JPL, Deusdará R, Romero GAS. Prevalence and Associated Factors of Post-COVID-19 Syndrome in a Brazilian Cohort after 3 and 6 Months of Hospital Discharge. Int J Environ Res Public Health. 2023;20(1):848.
Martínez-Ayala MC, Proaños NJ, Cala-Duran J, Lora-Mantilla AJ, Cáceres-Ramírez C, Villabona-Flórez SJ, et al. Factors associated with long COVID syndrome in a Colombian cohort. Front Med. 2023;10:1325616.
Montoy JCC, Ford J, Yu H, Gottlieb M, Morse D, Santangelo M, et al. Prevalence of symptoms ≤12 months after acute illness, by COVID-19 testing status among adults — United States, December 2020–March 2023. MMWR Morb Mortal Wkly Rep. 2023;72(32):859–65. https://www.cdc.gov/mmwr/volumes/72/wr/mm7232a2.htm.
Peghin M, De Martino M, Palese A, Chiappinotto S, Fonda F, Gerussi V, et al. Post-COVID-19 syndrome 2 years after the first wave: the role of humoral response, vaccination and reinfection. Open Forum Infect Dis. 2023;10(7):ofad364.
Rodríguez Onieva A, Vallejo Basurte C, Fernández Bersabé A, Camacho Cerro L, Valverde Bascón B, Muriel Sanjuan N, et al. Clinical characterization of the persistent COVID-19 symptoms: a descriptive observational study in primary care. J Prim Care Community Heal. 2023;14:21501319231208283.
Silva KM, Freitas DCA, Medeiros SS, Miranda LVA, Carmo JBM, Silva RG, et al. Prevalence and predictors of COVID-19 long-term symptoms: a cohort study from the Amazon Basin. Am J Trop Med Hyg. 2023;109(2):466–70.
Talhari C, Criado PR, Castro CCS, Ianhez M, Ramos PM, Miot HA. Prevalence of and risk factors for post-COVID: Results from a survey of 6,958 patients from Brazil. An Acad Bras Cienc. 2023;95(1):e20220143.
Tran TK, Truong SN, Thanh LT, Gia NLH, Trung HP, Dinh BT. Post-COVID condition: a survey of patients recovered from COVID-19 in Central Vietnam. J Infect Dev Ctries. 2023;17(9):1213–20.
van der Maaden T, Mutubuki EN, de Bruijn S, Leung KY, Knoop H, Slootweg J, et al. Prevalence and Severity of Symptoms 3 Months After Infection With SARS-CoV-2 Compared to Test-Negative and Population Controls in the Netherlands. J Infect Dis. 2023;227(9):1059–67.
Wahlgren C, Forsberg G, Divanoglou A, Östholm Balkhed Å, Niward K, Berg S, et al. Two-year follow-up of patients with post-COVID-19 condition in Sweden: a prospective cohort study. Lancet Reg Heal - Eur. 2023;28:100595.
Wong MCS, Huang J, Wong YY, Wong GLH, Yip TCF, Chan RNY, et al. Epidemiology, Symptomatology, and Risk Factors for Long COVID Symptoms: Population-Based, Multicenter Study. JMIR Public Heal Surveill. 2023;9:e42315.
Bello-Chavolla OY, Fermín-Martínez CA, Ramírez-García D, Vargas-Vázquez A, Fernández-Chirino L, Basile-Alvarez MR, et al. Prevalence and determinants of post-acute sequelae after SARS-CoV-2 infection (Long COVID) among adults in Mexico during 2022: a retrospective analysis of nationally representative data. Lancet Reg Heal - Am. 2024;30:100688.
Gwaikolo C, Sackie-Wapoe Y, Badio M, Glidden D V., Lindan C, Martin J. Prevalence and determinants of post-acute sequelae of COVID-19 in Liberia. Int J Epidemiol. 2024;53(1):dyad167.
Jangnin R, Ritruangroj W, Kittisupkajorn S, Sukeiam P, Inchai J, Maneeton B, et al. Long-COVID prevalence and its association with health outcomes in the post-vaccine and antiviral-availability era. J Clin Med. 2024;13(5):1208.
Keng Tok PS, Kang KY, Ng SW, Rahman NA, Syahmi MA, Pathmanathan MD, et al. Post COVID-19 condition among adults in Malaysia following the omicron wave: a prospective cohort study. PLoS One. 2024;19(1):e0296488.
Nguyen KH, Bao Y, Mortazavi J, Allen JD, Chocano-Bedoya PO, Corlin L. Prevalence and Factors Associated with Long COVID Symptoms among U.S. Adults, 2022. Vaccines. 2024;12(1):99.
Patro M, Gothi D, Anand S, Priyadarshini DPDK, Ojha UC, Pal RS, et al. Follow-up study of COVID-19 sequelae (FOSCO study). Lung India. 2024;41(2):103–9.
Salmon D, Slama D, Linard F, Dumesges N, Le Baut V, Hakim F, et al. Patients with Long COVID continue to experience significant symptoms at 12 months and factors associated with improvement: A prospective cohort study in France (PERSICOR). Int J Infect Dis. 2024;140:9–16.
Tan S, Pryor AJG, Melville GW, Fischer O, Hewitt L, Davis KJ. The lingering symptoms of post-COVID-19 condition (long-COVID): a prospective cohort study. Intern Med J. 2024;54(2):224–33.
Woldegiorgis M, Cadby G, Ngeh S, Korda RJ, Armstrong PK, Maticevic J, et al. Long COVID in a highly vaccinated but largely unexposed Australian population following the 2022 SARS-CoV-2 Omicron wave: a cross-sectional survey. Med J Aust. 2024;220(6):323–30.
Higgins JPT. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–60.
Melsen WG, Bootsma MCJ, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–9. Available from: http://dx.doi.org/10.1111/1469-0691.12494.
Tang JL, Liu JL. Misleading funnel plot for detection of bias in meta-analysis. J Clin Epidemiol. 2000;53(5):477–84.
Moy FM, Hairi NN, Lim ERJ, Bulgiba A. Long COVID and its associated factors among COVID survivors in the community from a middle-income country-An online cross-sectional study. PLoS One. 2022;17(8):e0273364. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med22&NEWS=N&AN=36040960.
Arjun MC, Singh AK, Pal D, Das K, Venkateshan M. Characteristics and predictors of Long COVID among diagnosed cases of COVID-19. PLoS One. 2022;17(12):e0278825. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medl&NEWS=N&AN=36538532.
Jabali MA, Alsabban AS, Bahakeem LM, Zwawy MA, Bagasi AT, Bagasi HT, et al. Persistent Symptoms Post-COVID-19: An Observational Study at King Abdulaziz Medical City, Jeddah, Saudi Arabia. CUREUS J Med Sci. 2022;14(4):e24343.
Alkwai HM, Khalifa AM, Ahmed AM, Alnajib AM, Alshammari KA, Alrashidi MM, et al. Persistence of COVID-19 symptoms beyond 3 months and the delayed return to the usual state of health in Saudi Arabia: A cross-sectional study. Sage Open Med. 2022;10:20503121221129918.
Baris SA, Toprak OB, Cetinkaya PD, Fakili F, Kokturk N, Kul S, et al. The predictors of long-COVID in the cohort of Turkish Thoracic Society-TURCOVID multicenter registry: One year follow-up results. Asian Pac J Trop Med. 2022;15(9):400–9. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85139758970&doi=10.4103%2F1995-7645.354422&partnerID=40&md5=2e324372c1b34f9b8d857275f3aebbe1.
Kim Y, Bitna Ha, Kim SW, Chang HH, Kwon KT, Bae S, et al. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. 2022;22(1):1–12. https://doi.org/10.1186/s12879-022-07062-6.
Imoto W, Yamada K, Kawai R, Imai T, Kawamoto K, Uji M, et al. A cross-sectional, multicenter survey of the prevalence and risk factors for Long COVID. Sci Rep. 2022;12(1):22413. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medl&NEWS=N&AN=36575200.
Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, Lomelin-Gascon J, Morales-Juárez L, de la Cerda MLR, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
Estrada-Codecido J, Chan AK, Andany N, Lam PW, Nguyen M, Pinto R, et al. Prevalence and predictors of persistent post-COVID-19 symptoms. JAMMI. 2022;7(3):208–19. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85139237175&doi=10.3138%2Fjammi-2022-0013&partnerID=40&md5=476625042f96602c577e2016e65b5de9.
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–31. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med19&NEWS=N&AN=33692530.
Peter RS, Nieters A, Krausslich HG, Brockmann SO, Gopel S, Kindle G, et al. Post-acute sequelae of covid-19 six to 12 months after infection: population based study. August D Blankenhorn B, Bopp-Haas U, Bunk S, Deibert P, Dietz A, Friedmann-Bette B, Giesen R, Gotz V, Grote S, Gruner B, Junginger A, Kappert O, Kirsten J, Kuhn A, Malek NP, Muller B, Niess A, Pfau S, Piechotowski I, Rieg S, Rottele S, Schellenberg J, Sc BC, Group EP 1 S, editors BMJ. 2022;379:e071050. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med22&NEWS=N&AN=36229057.
Kostev K, Smith L, Koyanagi A, Konrad M, Jacob L. Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19. Pediatr Res. 2022;95:182–7.
Forster C, Colombo MG, Wetzel AJ, Martus PJ, Joos S. Persisting symptoms after COVID-19. Dtsch Arztebl Int. 2022;119(10):167-+.
Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Capriotti V, D’Alessandro A, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021;11(12):1685–8. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85107434855&doi=10.1002%2Falr.22832&partnerID=40&md5=906d5f4f7fc87c42348fe39371f1395b.
Fischer A, Zhang L, Elbéji A, Wilmes P, Oustric P, Staub T, et al. Long COVID symptomatology after 12 months and its impact on quality of life according to initial coronavirus disease 2019 disease severity. Open Forum Infect Dis [Internet]. 2022;9(8). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85141512873&doi=10.1093%2Fofid%2Fofac397&partnerID=40&md5=9e13b2fa20e3f1ebd3deac40523cbd4d.
Montenegro P, Moral I, Puy A, Cordero E, Chantada N, Cuixart L, et al. Prevalence of Post COVID-19 Condition in Primary Care: A Cross Sectional Study. Int J Environ Res Public Health. 2022;19(3):1836.
Moreno-Perez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jimenez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. group C-A research, editor. J Infect. 2021;82(3):378–83. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med19&NEWS=N&AN=33450302.
Ballering AV, van Zon SKR, Hartman TC, Rosmalen JGM. Initiative LCR. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–61 WE-Science Citation Index Expanded (SCI.
Menges D, Ballouz T, Anagnostopoulos A, Aschmann HE, Domenghino A, Fehr JS, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS One. 2021;16(7):e0254523.
Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Abdeeva E Antsiferova E, Artigas E, Bairashevskaia A, Belkina A, Bezrukov V, Bordyugov S, Bratukhina M, Chen J, Deunezhewa S, Elifkhanova K, Ezhova A, Filippova Y, Frolova A, Ganieva J, Gorina A, Kalan Y, Kirillov B, Korgunova M, Krupina A, Kuznetsova A, AN, Team SSR, editors. Clin Exp Allergy. 2021;51(9):1107–20. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med20&NEWS=N&AN=34351016.
Ghosn J, Piroth L, Epaulard O, Le Turnier P, Mentré F, Bachelet D, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27(7):1041.e1–1041.e4.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Reports 111. 2021;11(1):1–12. https://www.nature.com/articles/s41598-021-95565-8. [cited 2023 Jan 23].
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226(9):1593–607. https://academic.oup.com/jid/article/226/9/1593/6569364.
Rahmati M, Udeh R, Yon DK, Lee SW, Dolja-Gore X, McEVoy M, et al. A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: a call to action for neurological, physical, and psychological sciences. J Med Virol. 2023;95(6):e28852.
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. 2023;55:101762.
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz J V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. The Lancet Infectious Diseases. 2022;22(4):e102–7.
O’Sullivan O. Long-term sequelae following previous coronavirus epidemics. Clin Med J R Coll Physicians London. 2021;21(1):e68–e70.
Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(5):657–66. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med21&NEWS=N&AN=35124265.
Halpin S, O’Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242–3.
Bell ML, Catalfamo CJ, Farland L V., Ernst KC, Jacobs ET, Klimentidis YC, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS One. 2021;16(8):e0254347.
Pazukhina E, Andreeva M, Spiridonova E, Bobkova P, Shikhaleva A, El-Taravi Y, et al. Prevalence and risk factors of Post-COVID-19 Condition in adults and children at 6 and 12 months after hospital discharge: a prospective, cohort study in moscow (Stop COVID). SSRN Electron J. 2022;20:244.
Parkin A, Davison J, Tarrant R, Ross D, Halpin S, Simms A, et al. A Multidisciplinary NHS COVID-19 Service to Manage Post-COVID-19 Syndrome in the Community. 2021. https://doi.org/10.1177/21501327211010994.
Martin-Loeches I, Motos A, Menéndez R, Gabarrús A, González J, Fernández-Barat L, et al. ICU-Acquired Pneumonia Is Associated with Poor Health Post-COVID-19 Syndrome. J Clin Med. 2022;11(1):224.
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. -month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32.
Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28(4):611.e9-611.e16.
Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020;92(10):2050–4.
Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36(8):1072–7.
Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33(36):4422–9.
Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784.
Flores S, Brown A, Adeoye S, Jason LA, Evans M. Examining the impact of obesity on individuals with chronic fatigue syndrome. Work Heal Saf. 2013;61(7):299–307.
McElfish PA, Purvis R, James LP, Willis DE, Andersen JA. Perceived barriers to covid-19 testing. Int J Environ Res Public Health. 2021;18(5):2278.
Zimmermann L, Bhattacharya S, Purkayastha S, Kundu R, Bhaduri R, Ghosh P, et al. SARS-CoV-2 infection fatality rates in india: systematic review, meta-analysis and model-based estimation. Stud Microeconomics. 2021;9(2):137–79.
Rahmandad H, Lim TY, Sterman J. Behavioral dynamics of COVID-19: estimating underreporting, multiple waves, and adherence fatigue across 92 nations. Syst Dyn Rev. 2021;37(1):5–31.
Müller SA, Isaaka L, Mumm R, Scheidt-Nave C, Heldt K, Schuster A, et al. Prevalence and risk factors for long COVID and post-COVID-19 condition in Africa: a systematic review. Lancet Glob Heal. 2023;11(11):e1713–24.
Acknowledgements
The authors express their sincere gratitude to Ministry of Higher Education (MoHE) Malaysia for the funding of this research via the Fundamental Research Grant Scheme under grant number (FRGS/1/2022/SKK04/UKM/02/2), and to those who had contributed to the production of the article.
Funding
This research was funded by the Ministry of Higher Education (MoHE) Malaysia through Fundamental Research Grant Scheme under the grant number (FRGS/1/2022/SKK04/UKM/02/2).
Author information
Authors and Affiliations
Contributions
Conception of the work: RR and AI. Initial search, data extraction, screening process, quality assessment, and data analysis: RR, NIS, and AI. Results interpretation: AI, AFAA, LSS, AA, and RR. Drafting the article: RR and NIS. Critical revision of the manuscript: AI, AFAA, LSS, AA, and RR. Final approval of the manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. This study is a systematic review based on published studies. Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this review.
Consent for publication
Not applicable (patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this review). However, all authors had approved and consented for the publication of this review.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sk Abd Razak, R., Ismail, A., Abdul Aziz, A.F. et al. Post-COVID syndrome prevalence: a systematic review and meta-analysis. BMC Public Health 24, 1785 (2024). https://doi.org/10.1186/s12889-024-19264-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19264-5