Abstract
Background
The economic crisis that began in 2008 has severely affected Southern (Greece, Italy, Portugal, Spain) Western European (SWE) countries of Western Europe (WE) and may have affected ongoing efforts to eliminate viral hepatitis. This study was conducted to investigate the impact of the economic crisis on the burden of HBV and HCV disease.
Methods
Global Burden of Diseases 2019 data were used to analyse the rates of epidemiological metrics of HBV and HCV acute and chronic infections in SWE and WE. Time series modelling was performed to quantify the impact of healthcare expenditure on the time trend of HBV and HCV disease burden in 2000–2019.
Results
Declining trends in incidence and prevalence rates of acute HBV (aHBV) and chronic HBV were observed in SWE and WE, with the pace of decline being slower in the post-austerity period (2010–2019) and mortality due to HBV stabilised in SWE. Acute HCV (aHCV) metrics and chronic HCV incidence and mortality showed a stable trend in SWE and WE, whereas the prevalence of chronic HCV showed an oscillating trend, decreasing in WE in 2010–2019 (p < 0.001). Liver cancer due to both hepatitis infections showed a stagnant burden over time. An inverse association was observed between health expenditure and metrics of both acute and chronic HBV and HCV.
Conclusions
Epidemiological metrics for HBV and HCV showed a slower pace of decline in the post-austerity period with better improvement for HBV, a stabilisation of mortality and a stagnant burden for liver cancer due to both hepatitis infections. The economic crisis of 2008 had a negative impact on the burden of hepatitis B and C. Elimination of HBV and HCV by 2030 will be a major challenge in the SWE countries.
Similar content being viewed by others
Background
Viral hepatitis has been recognised as a health and development priority with the adoption of two United Nations resolutions in 2010 and 2014 [1]. In response to these resolutions, WHO launched the first global health sector strategy for viral hepatitis in 2016 [2]. The strategy aims to articulate the synergistic actions needed to coordinate national responses to eliminate hepatitis and support the 2030 Agenda for Sustainable Development [3, 4]. Specifically, WHO has set two targets for 2030: to reduce the incidence of chronic viral infection by 90% and the number of deaths from viral hepatitis by 65%, and to ensure equitable access to prevention, testing, care and treatment services for all [2]. Viral hepatitis is caused by five hepatitis viruses (A to E) and is responsible for an estimated 1.3 million deaths per year, mainly from chronic liver disease and liver cancer due to hepatitis B virus (HBV) and hepatitis C virus (HCV) [5].
Despite the availability of a preventive HBV vaccine since 1982, recent data on global HBV prevalence estimate that there are 316 million chronic hepatitis B surface antigen (HBsAg) carriers (4.1% of the world’s population), including 6 million children under the age of five, with peak prevalence observed in Africa (6.5%) and the Western Pacific region (7.1%) [6]. It is estimated that 15–40% of people living with HBV will develop serious sequelae of infection, mainly affecting the liver [7]. HCV has a lower prevalence compared to HBV, with 58 million chronic infections in 2019 (0.8% of the world population), with peak prevalence observed in the Eastern Mediterranean (1.6%) and European regions (1.3%) [5, 8]. Up to 85% of people with acute HCV will develop chronic infection [9]. Estimated incidence in 2019 was similar for both viruses (1.5 million new infections each) [5]. Recent data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) from 2010 to 2019 showed declining trends in the incidence of acute HBV and HCV, and HBV cirrhosis worldwide until 2015, as well as overall declines in disability-adjusted life years (DALYs) and mortality for HBV and HCV cirrhosis, although analysis of macro areas showed disparities in disease epidemiology [10, 11].
The Great Recession that hit Europe in 2008–2009 particularly affected Greece, Italy, Portugal and Spain, weakening their economic systems and worsening their deficits [12]. As a result, a sovereign debt crisis was triggered, forcing their governments to implement austerity measures and increase tax revenues from 2010 onwards [13, 14]. In these countries, health systems have been severely affected by the crisis, with significant reductions in public health expenditure between 2009 and 2017 (between -7% and -4%) [14]. A number of examples of the impact of the crisis can be highlighted. In Italy, the number of hospital beds fell from 4.6 to 3.6 per 1,000 inhabitants between 2000 and 2010, lower than the European average from the same period (5.5), and continued to fall, reaching 3.2 in 2015, after which it stabilised at 3.1 [15]. In Portugal, self-reported access to healthcare deteriorated [16] and excess mortality associated with influenza and cold weather was reported in early 2012, probably related to low home heating capacity [17]. The health status of the population worsened significantly, especially among vulnerable groups (e.g. increased rates of stillbirth [18], anxiety and alcohol-related disorders [17, 19], heart attacks [20], suicide [21,22,23] and some communicable diseases, among others). For example, an HIV outbreak among people who inject drugs (PWID) occurred in Greece in 2011–2012, mainly due to a reduction in preventive measures [24, 25], while delays in the approval of innovative drugs for HCV have been denounced in Portugal [26]. In this context, we have performed a time series analysis of the burden of HBV- and HCV-associated diseases and investigated whether austerity measures have affected the burden of these diseases in Greece, Italy, Portugal and Spain.
Methods
Overview and metrics
GBD 2019 provides a standardised approach to estimating annual updates of epidemiological trends in the global burden of 369 diseases and injuries and 87 risk factors from 1990 to 2019 [27, 28]. Raw input data from 2000 to 2019 were obtained from a comprehensive set of sources, including population censuses, disease notifications and registries, household surveys, civil registration and vital statistics, health service utilisation, and others. The data are made available through the Global Health Data Exchange, a catalogue of global health and demographic data. Six GBD codes were used to extract estimates: acute HBV (A.5.8.2) and acute HCV (A.5.8.3) causes; cirrhosis and other chronic liver diseases due to hepatitis B (B.4.1.1) and due to hepatitis C (B.4.1.2), collectively referred to as cirrhosis in this paper; liver cancer due to hepatitis B (B.1.7.1) and due to hepatitis C (B.1.7.2). Cases of cirrhosis and other chronic liver diseases due to alcohol consumption (code B.4.1.3) were excluded. The burden of these diseases was assessed using the following epidemiological metrics which were the primary outcomes of analysis: prevalence, incidence, mortality, years lived with disability (YLDs), years of life lost to premature mortality (YLLs), and disability-adjusted life years (DALYs) for HBV and HCV diseases.
The sites studied were four Southern (Greece, Italy, Portugal, Spain) Western European (SWE) countries. For comparison, we also extracted metrics for the GBD Western Europe (WE) region: Andorra, Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, the Netherlands, Norway, Portugal and Spain (Appendix). The four SWE countries analysed were also included in the trends for Western Europe because GBD estimates are available for the whole of the Western Europe region. All metrics were disaggregated by sex: female, male or both, and by 5 age groups. The upper and lower limits of the 95% confidence interval (95% CI) were also extracted for each parameter. These were derived from 1000 draws of the distribution of each estimation step by age, sex and location for each year included in the analysis and represent the ordinal 25th and 975th draws of each quantity [27]. In order to assess the impact of austerity measures, we used current health expenditure as defined by the World Bank as a percentage of gross domestic product (%GDP) for each country studied [29]. This variable was not included in the analysis for WE due to the lack of %GDP for the WE region as a whole. HIV burden was also included in the analysis. HIV incidence and prevalence data were extracted from the Global Health Data Exchange, using GBD code B.1.7.2, to control for potential confounding by the relationship between HIV and viral hepatitis, due to the prevalence of co-infection and the impact of HIV on the clinical course of hepatitis [30].
Statistical analysis
To quantify the role of austerity, the impact of current health expenditure on the time trend of HBV and HCV disease burden, interrupted time series models were performed. The primary outcomes were considered as the dependent variables (prevalence, incidence, mortality, YLDs, YLLs, and DALYs) and the independent variables or covariates were %GDP, sex, age group, period trend, post-austerity period trend and HIV burden. To account for the delayed effect of austerity, %GDP was introduced with a five-year lag compared to the epidemiological metrics. For HIV burden, HIV incidence was used in the models for acute HBV and HCV, whereas HIV prevalence was used in the models for cirrhosis and cancer due to HBV and HCV. Once the model was defined, multiple linear regressions were fitted to estimate the effect, 95% CI and p values for each independent variable for outcome, causes and countries [27, 28]. The period from 2000 to 2010 represents the pre-austerity period, while the years from 2010 to 2019 include both the austerity and post-austerity periods [12]. Detailed methods for the entire study are available in the GBD 2019 summary publications [27, 28], each of which follows the recommendations of the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) to ensure that data and methods are adequately documented [31]. Table 1 provides brief descriptions of the methods used to derive each of the GBD metrics included in this analysis. All data were retrieved from the Global Burden of Disease Study 2019 between September and December 2020 [32]. Analyses and plots were performed using R version R-4.0.3 [33].
Ethics approval and consent to participate
This study does not require ethical approval or informed consent. It does not contain data on individuals, but only epidemiological estimates.
Consent for publication
Not applicable.
Role of funding source
Funding was provided by the Bill & Melinda Gates Foundation. The funder of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Hepatitis B Virus
Acute hepatitis B virus infection
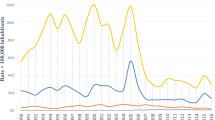
Overall, a decreasing trend in incidence and prevalence rates of acute hepatitis B virus (aHBV) in WE was observed during the study period (Fig. 1A, Table 2). In 2019, aHBV incidence and prevalence rates in Greece were 448.9 (95% CI: 354.3 – 555.2) and 51.7 (40.8; 64.0), respectively, three times higher than in Portugal and Spain and five times higher than in Italy (Appendix). Regarding aHBV incidence, a significant downward trend was observed in Greece from 2000 to 2019 [-23.17 cases per 100,000 for each consecutive year (-40.41 – -5.94); p = 0.01], despite the upward trend during the post-austerity period [24.00 (4.03 – 43.97); p = 0.02]. The downward trend was also observed in the other countries analysed, although it was not statistically significant. Incidence rates were higher in males in WE, Greece, Italy, Portugal, reaching up to 40.11 (9.77 – 70.46) cases per 100,000 for each case in females (p < 0.001) (Table 4). Each age group showed significantly lower incidence rates compared with the previous age group in WE [-6.01 (-10.05 – -1.96); p < 0.001], while an opposite trend was observed in SWE [from 14.70 (7.88 – 21.53) cases per 100,000 in Portugal to 86.92 cases (76.32 – 97.52) in Greece (p < 0.001)]. The incidence rate increased significantly from 8.10 (7.22 – 8.99) to 19.16 (17.93 – 20.40) cases per 100,000 for each HIV case reported in Portugal, Italy, Spain and WE, and up to 159.30 (149.06 – 169.53) in Greece. aHBV prevalence showed a similar trend, although at lower rates. Regarding mortality, a stable trend was observed in WE and SWE during 2000 – 2019, despite the significant upward trend observed in Greece [0.19 (0.08 – 0.29) cases per 100,000 for each consecutive year; p < 0.001], which was compensated during the post-austerity period [-0.26 (-0.38 – -0.13); p < 0.001] (Table 4). DALYs and YLLs showed a similar trend in Greece (p < 0.001). Notably, the rates have stabilised since 2015 (Fig. 1A, Supplementary Appendix). No changes were observed in the other countries. Males and older age groups were more affected in WE and SWE (p < 0.001) (Table 4, Appendix).
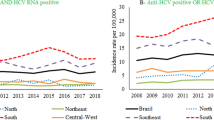
Trends of acute (A, B) and chronic (C-F) hepatitis B and hepatitis C age-standardized rates of prevalence, incidence, mortality, years lived with disability (YLDs), years of life lost (YLLs) and disability-adjusted life years (DALYs) per 100 000 population in Greece, Italy, Portugal, Spain and Western Europe from 2000 to 2019 (Global Burden of Disease Study 2019). The shadows around the lines represent the 95% confidence interval of the estimates
Chronic hepatitis B virus infection
Both WE and SWE showed a decreasing trend between 2000 and 2019 in the six investigated metrics of cirrhosis and other chronic liver diseases (CCLD), which was significant for WE and Greece (Fig. 1C, Table 2, Appendix). Greece showed a decrease in incidence [-0.29 (-0.43 – -0.15); p < 0.001] and prevalence [-55.81 (-95.15 – -16.47); p = 0.006] cases per 100,000 inhabitants each consecutive year, followed by a stabilisation during the post-austerity period (p < 0.001) (Table 4). Italy had the highest incidence rate of CCLD throughout the study period [3.20 cases per 100,000 persons (2.42 – 4.15) in 2019] (Appendix). Incidence rates were lower in males in Greece, Portugal and Spain, reaching up to -0.44 (-0.78 – -0.10) cases per 100,000 for each case in females (p < 0.01), whereas prevalence rates were much higher in males in WE and SWE countries, reaching up to 223.23 (150.31 – 296.16) cases per 100,000 for each case in females (p < 0.001) in Greece, which was two times higher than in Portugal and four times higher than in Italy and Spain. In addition, age group was negatively associated with CCLD incidence in Greece [-0.12 (-0.21 – -0.03); p = 0.01] and Portugal [-0.20 (-0.28 – -0.11); p < 0.001]. The incidence of CCLD due to HBV was weakly positively associated with HIV prevalence. For CCLD prevalence, age group showed a positive effect, highlighting Greece with 596.48 cases per 100,000 (571.06 – 621.89; p < 0.001), twice more than the other SWE countries and 3.5 times more than WE. CCLD prevalence was positively associated with HIV prevalence in WE and SWE, ranging from 1.24 (1.14 – 1.34) in Portugal to 17.86 (16.23 – 19.50) in Greece (p < 0.001).
Regarding CCLD mortality, a decreasing trend was observed from 2000 to 2019 in WE and SWE, reaching statistical significance in WE [-0.11 (-0.18 – -0.05) cases per 100,000 for each consecutive year; p < 0.001] and Greece [-0.20 (-0.35 – -0.05; p = 0.01], despite a slight increase during the post-austerity period, which was significant only in Greece [0.20 (0.03 – 0.38); p = 0.02]. YLLs showed a similar trend. Males had significantly higher mortality rates than females in all countries, reaching the highest rates in Italy [2.04 (1.59 – 2.49) cases per 100,000 for each female case (p < 0.001)] and Portugal [2.08 (0.23 – 9.23); p < 0.001], similar to YLLs. Older age groups showed higher mortality and YLLs rates in WE and SWE, up to 1.86 [(1.73 – 2.00; p < 0.001] and 26.66 [(23.07; 30.25); p < 0.001], respectively, in WE (Table 4).
The prevalence rates of liver cancer (LC) due to HBV were at least 290–690-fold lower than CCLD across all sites in 2019 (Appendix). This is comparable to WE, where LC prevalence was 360-fold lower in 2019. LC burden remained stable for the six metrics evaluated over the period 2000 – 2019 (Fig. 1E), while males had higher rates compared to females in WE and SWE, particularly in Greece, where mortality was more than twofold higher compared to other SWE countries [4.02 (3.33 – 4.72); p < 0.001]. When stratified by age, a significant increase in rates was associated with older age groups for the six metrics, which were always about 2.5 times higher in Greece [mortality: 2.78 (2.50 – 3.07); p < 0.001] (Appendix, Table 4). A negligible effect of HIV on the LC metrics was observed (Table 4).
Hepatitis C virus
Acute hepatitis C virus infection
The incidence and prevalence rates of acute HCV (aHCV) showed a generally stable trend in WE and SWE. Italy had the highest prevalence and incidence rates in both sexes in 2000, and estimates were still 1.7 times higher than in WE in 2019 [7.81 (6.68 – 9.27) vs 4.72 (4.13 – 5.45)] (Fig. 1B; Appendix).
Males had significantly higher incidence and prevalence rates compared to females in WE [8.17 (6.85 – 9.49) and 0.94 (0.79 – 1.10) respectively; p < 0. 001], Portugal [10.58 (5.25 – 15.91) and 1.22 (0.61 – 1.84) respectively; p < 0.001] and Spain [11.61 (5.21 – 18.02) and 1.34 (0.60; 2.08) respectively; p < 0.001]. Conversely, in Greece, the incidence and prevalence rates were comparable between sexes although slightly higher in females than in males [-1.65 (-5.94; 2.65) and -0.19 (-0.69; 0.31) respectively; p = 0.45]. These metrics reached statistical significance for age, with estimates increasing in older classes (p < 0.001). Stable trends in mortality, DALYs and YLLs due to aHCV in WE and SWE were observed from 2000 to 2019 (Table 3, Table 4, Appendix). Although statistically significant, there were few differences between sex and age groups (p < 0.001). A negligible effect of HIV on aHCV metrics was observed, with the exception of a negative association between HIV and aHCV incidence in WE and SWE (Table 4).
Chronic hepatitis C virus infection
The incidence rates of CCLD showed a generally stable trend in WE and SWE, whereas the prevalence showed an oscillating trend, resulting in a decrease in WE, which differed from the stabilisation observed in Portugal and Spain at the end of the study period (Fig. 1D, Table 4). Greece showed an increasing prevalence rate, while Italy showed a decreasing trend that did not reach statistical significance. Nevertheless, Italy still had the highest prevalence of CCLD in 2019 with 1214.1 (981.9 – 1497.7) cases per 100,000 population, 3 times higher than Greece and almost 2 times higher than Portugal, Spain and WE (Appendix). Males had significantly higher prevalence rates than females in WE, Portugal and Spain [124.34 (90.51 – 158.16), 120.14 (69.56 – 170.71) and 123.60 (56.86 – 190.34), respectively; p < 0.001]. Older individuals showed greater prevalence rate, reaching values of 972.41 cases per 100,000 (937.19; 1007.64; p < 0.001) in Italy, 2 to 3 times higher than in the other countries. Overall, a stable trend was observed for mortality and a slight reduction was observed for WE and SWE for DALYs, YLLs, YLDs, and males were more affected than females, reaching the highest values in Italy [(mortality: 6.36 (4.83 – 7.89) and DALYs: 113.83 (89.70 – 137.97); p < 0.001]. Older individuals showed greater mortality rate, with Italy standing out with 7.12 cases per 100,000 (6.57; 7.67; p < 0.001), between 1.4 and 3.6 times more than WE and the other SWE countries.
The incidence and prevalence rates of LC showed a stable trend in WE and SWE (Table 3, Table 4, Appendix). Age groups and males showed a positive association, although to a lesser extent than for CCLD. A general slight increase was observed for mortality, DALYs, YLL and YLD in WE and SWE. Males showed significantly higher mortality and DALY rates compared to females in Italy, Portugal and Spain, with mortality being 8- and tenfold higher in Italy [7.84 (6.16 – 9.52)] and Spain [10.03 (8.03 – 12.04)] and twofold higher in Portugal [4.41 (3.44 – 5.38)] (p < 0.001). Similar trends in mortality and DALYs were observed for age groups, with Italy and Spain being 2 – 5 times higher than Portugal and Greece (p < 0.001) (Table 4; Appendix). A negligible effect of HIV on CCLD and LC metrics was observed (Table 4).
Association between the burden of viral hepatitis and health expenditure
Overall, an inverse association was observed between health expenditure and both HBV and HCV acute infections, with results close to significance for aHBV DALYs [-2.54 (-5.09 – 0.01); p = 0.05)] and aHBV YLLs [-2.53 (-5.09 – 0.03); p = 0.05)] in Greece. For aHCV, the results showed that one unit (percentage) increase in health expenditure was associated with a more beneficial effect on reducing incidence cases in Italy [-8.93 (-34.31 – 16.46); p = 0.492], up to 80 times higher than in the other SWE countries. A similar inverse association between health expenditure and CCLD metrics was found for both HBV and HCV, except for CCLD-HBV prevalence in Portugal and Spain. In addition, a positive but not significant association was found between health expenditure and LC metrics for both HBV and HCV (Table 4).
Discussion
This is the first study to analyse the impact of austerity measures related to the 2008 economic crisis on the burden of hepatitis B and hepatitis C infections and related diseases in WE from 2000 to 2019, focusing on the EU countries most affected by austerity measures, namely Greece, Italy, Portugal and Spain. Overall, an inverse association was observed between health expenditure and both HBV and HCV acute infection and CCLD metrics, with a stronger impact on reducing aHBV DALYs and YLLs in Greece and aHCV incidence in Italy. Epidemiological metrics for HBV and HCV showed mixed trends, with better improvement for HBV than HCV, albeit with some country-specific differences, a slower pace of decline in the post-austerity period (2010–2019) and a stabilisation of mortality. An exception was observed for liver cancer due to both hepatitis, with a stagnant burden over time.
In WE, despite a much higher prevalence rate of acute HBV compared to acute HCV, the prevalence of cirrhosis was comparable between HBV and HCV over the study period (2000 – 2019). The higher rate of spontaneous HBV seroclearance (up to 95%) compared to HCV (up to 20% – 30%) [34, 35] may explain the observed convergence in cirrhosis burden between the two infections. The introduction of the HBV vaccine has led to significant improvement in all metrics related to CCLD-HBV [35]. The continuous development and availability of effective antiviral treatment for chronic HBV since the mid-1990s has also contributed to the decline in the burden of sequelae of HBV infection. It has been one of the main factors explaining the disparity between HBV and HCV burdens for decades, until the availability of direct-acting antiviral agents (DAAs), which cure over 95% of HCV cases. The incidence and prevalence rates of acute HBV infection decreased over time in WE and SWE, but most slowly in Greece. Similarly, DALYs, YLLs, YLDs and mortality due to aHBV remained stable over time in WE and SWE, except in Greece, where a sharp increase was observed until 2010, followed by an abrupt decrease. This is likely to be related to the observed inverse association between health expenditure and DALYs, YLLs, YLDs and mortality metrics in SWE, notably in Greece, where austerity measures implemented during the economic crisis had subsequent negative health consequences [36]. Moreover, the highest aHBV mortality in Greece may be at least partly explained by the late introduction of HBV vaccination in 1998 [37], which remained below the recommended 95% coverage for 24-month-old children until 2015 [38, 39]. In contrast, Italy showed the lowest incidence trend, which may be due to the early introduction of universal HBV vaccination in infants in 1991 and in children aged 12 years until 2003 [40]. In Spain, mandatory vaccination was introduced in 1992 for children aged ≥ 12 years and from birth in 1998 [41], and in Portugal in 1995 [42].
However, during the last decade, universal HBV vaccination coverage in children has improved remarkably in all four SWE countries, reaching the 2020 prevention target (95% or more) [38]. In addition, the general improvement in hygiene and healthcare standards, safety standards for blood and its components used for transfusion [43], the use of disposable syringes, the implementation of universal HBsAg screening during pregnancy and prophylaxis of vertical transmission, and information campaigns on HIV/AIDS may also have contributed to the decline in aHBV incidence in the study countries, and more markedly in Italy [44]. The introduction of universal HBV vaccination probably affected the chronic stage of cirrhosis, where a general downward trend, less pronounced in recent years, was observed in WE and SWE, although mortality estimates were extremely low and stable.
Acute HCV incidence and prevalence remained stable over time in WE and SWE, whereas a similar trend to aHBV was found for DALYs, YLLs, YLDs and mortality, with a point of inflection in the post-austerity period for Greece, although to a lesser extent compared to aHBV. The lower burden of aHCV could be related to the marginalisation of high-risk groups, leading to under-diagnosis of HCV disease [34]. Secondly, the later implementation of mandatory notification may explain the different rate between countries, highlighting the earlier mandatory surveillance in Italy in 1990 as opposed to Spain in 2015 [45, 46]. The later mandatory reporting of HCV in some countries may have biased the estimation of the association with HIV incidence, resulting in a negligible effect of HIV on HCV metrics. On the contrary, HIV incidence was positively associated with both aHBV and CCLD-HBV incidence and prevalence. This finding could be explained by the fact that HIV screening contributes to the detection of hepatitis B cases. Similar to aHBV, an inverse association was observed between health expenditure and epidemiological metrics.
The prevalence of HCV cirrhosis stage showed a smaller decrease than HBV cirrhosis stage in WE and SWE over the period 2000 – 2019. Three main factors could explain this observation. First, the constant incidence of acute HCV and the lack of a preventive vaccine. Second, the low rate of spontaneous seroclearance of chronic HCV infection (about 1%) [47, 48]. Third, the low efficacy of conventional treatment based on ribavirin and interferon (about 40% for HCV genotype 1) [49]. However, during the post-austerity period 2010–2019, a more pronounced decrease was observed for CCLD-HCV, which could be explained by the implementation of national HCV treatment plans based on DAAs since 2012 in Greece [50], 2015 in Italy, Portugal and Spain [51,52,53]. The viral eradication rate reached levels above 90% in the four countries, meeting the 2020 target for the proportion of treated patients achieving a sustained viral response [38, 50, 54].
Recent global GBD data showed a decline in the prevalence of chronic HBV infection over time [6]. On the contrary, our data showed a stable trend of HBV- and HCV-induced liver cancer in WE and SWE. Under reporting of chronic viral hepatitis may partly explain the differences in burden. For example, in Greece 80% of chronic HCV patients are unaware of their status [55], while in Spain the undiagnosed proportion of active HCV infection is 29% [56]. Italy has an estimated 280,000 undiagnosed patients [54]. The new global cancer data from the International Agency for Research on Cancer have estimated that the number of new cases and deaths from liver cancer could increase by > 55% over the next twenty years. However, these estimates are not directly comparable with our results because they include all causes of liver cancer [57].
Sex and age have a major impact on the epidemiological burden of HBV and HCV disease. Males and older people have the highest rates of HBV and HCV disease, except for chronic HBV, which is higher in females in Greece, Portugal and Spain, in line with previously reported data from US veterans [58]. Highest peak of prevalence in older adults as observed in Italy is most probably associated to high transmission in the past through unsafe injections, blood transfusions or other nosocomial transmission routes which has been reported to be the case in Italy. Nowadays, that specific screening test was available for identifying infected donors, people who inject drugs are presently the main target population for infection along with other risk groups, such as migrants.
Notably, health expenditure as a percentage of GDP, used as a proxy for austerity, had a negative impact on acute HBV and HCV infection, mainly on aHBV DALYs and YLLs in Greece and on aHCV incident in Italy. Similar results were found between health expenditure and CCLD metrics for both HBV and HCV.
With the advent of DAAs, new “test and treat” interventions have been introduced recently for key populations at high risk of infection [59]. There are several key populations, such as people living with HIV, sex workers, migrant populations, MSM, and also people who belong to more than one group [60]. For example, HCV prevalence in Portugal is 84% among PWID [61]. In addition, there is growing concern about men who have sex with men (MSM) as a risk group in the HBV/HCV epidemic [62,63,64,65,66,67]. In 2017, Greece launched the first plan to respond to hepatitis C among PWID, prisoners, sex workers, MSM, refugees and immigrants [68]. In the same year, a key policy document in Portugal recognised the principle of equivalence (United Nations Resolution 45/111 of 14 December 1990), i.e. that prisoners have a right to infectious disease healthcare equivalent to that provided to the general population [69]. This should lead to a significant reduction in the burden of HCV and HBV. For example, interventions piloted in the Italian prison system have shown that micro-elimination [70] of hepatitis C is feasible in both PWID and non-PWID prisoners [71, 72]. The promotion of micro-elimination has also been implemented in Spain over the last decade. For example, a universal test-and-treat intervention supported by telemedicine showed high acceptance among people living in prison and achieved a high HCV cure rate [73,74,75]. In Portugal, since 2018, a new model of care has been implemented in Portuguese prisons to provide on-site healthcare to eliminate hepatitis C among the vulnerable population of people living in prison [76]. However, several barriers still limit the provision of healthcare in prisons [73, 74].
The impact of DAAs on HCV infection worldwide should be evaluated with longer follow-up, as it may have a major beneficial effect at the public health level. However, despite the high efficacy of DAAs, the risk of reinfection is not eliminated. In Spain, for example, 1.1% of those cured were reinfected [77]. The COVID-19 pandemic could further jeopardise the elimination of HCV and HBV hepatitis by 2030 [78, 79] in several ways, affecting national health systems and their resilience. Approximately 43% – 48% of countries responding to a WHO global survey reported between 5% and > 50% disruption in HBV and HCV diagnosis and treatment in 2021 [80, 81]. In addition, in reallocating resources in response to COVID-19, many countries deprioritised their national responses for critical harm reduction services [80, 81]. In this context, renewed efforts and structured and harmonised policies are needed to provide the framework for eliminating viral hepatitis as a public health threat. Importantly, scaling up testing should be prioritised. This will benefit both the infected person and the community by preventing further transmission. A multi-dimensional approach based on the development of novel point-of-care (POC) diagnostic virological tests and improved screening and linkage-to-care strategies for those most at risk should be promoted. New studies should assess the feasibility and impact of rapid, simple and cost-effective POC technologies on the enrolment and retention of infected individuals in the treatment cascade. POC can change the paradigm of conventional blood testing based on centralised laboratory facilities, widen access to testing and self-testing in community and harm reduction settings or low-resource settings, and engage hard-to-reach populations. At the same time, opioid substitution therapy and needle and syringe programmes should be scaled up in both community and prison settings to counter the negative impact of the current global economic slowdown on people with substance use disorders and vulnerable groups.
The main limitation of this study is the availability of primary data on which the GBD estimates depend. The data sources used in the GBD study are large and comprehensive, including censuses, population registers, vital registration, sample registration (i.e., vital registration covering a sample), demographic data, surveillance systems, verbal autopsies, hospital data, health insurance claims data, surveys, disease registries, morbidity notification data, police records, published literature. In particular, the completeness of the vital registration system in recent years was > 99% in the four countries studied. However, there are still limitations. In fact, the much lower rates of both acute and chronic HCV in Greece, especially at the beginning of the study period, may reflect the scarcity of available data. The accuracy of cause of death and verbal autopsy data depends on death certificates being coded correctly according to international standards and the practices of physicians completing them. Co-morbidities at the time of death can complicate this process, potentially impacting the accuracy of these data sources. The ability of a country to report acute hepatitis may also depend on its testing practices. This may have a direct impact on the confidence intervals, which are wide for Greece for aHBV and aHCV compared to the other countries. Geographical subnational patterns or subgroup-specific analyses were not included in this work, partly because of limited data availability. In terms of methods, the trends for Western Europe included the four countries studied because GBD estimates are available for the whole of Western Europe, which may have introduced a bias in the interpretation of the results. The study was not designed to assess the specific impact of each national policy implemented on the observed epidemiological trends, while a longer study period might have improved the assessment of the impact of DAAs introduction. Surveillance data, such as timely access to therapies for both HBV and HCV, should be examined in future studies as they are released by the relevant national institutions.
Conclusions
In conclusion, an inverse association was observed between health expenditure and both HBV and HCV acute infection and CCLD metrics, with a stronger impact on reducing aHBV DALYs and YLLs in Greece and aHCV incidence in Italy. Epidemiological metrics for HBV and HCV showed mixed trends, a slower pace of decline in the post-austerity period (2010–2019), a stabilisation of mortality and a stagnant burden for liver cancer due to both hepatitis over time. The 90% reduction in the incidence of chronic HBV and HCV infection and the 65% reduction in attributable mortality from the 2015 baseline recommended by WHO for the 2030 Hepatitis elimination plan have not been achieved [2]. Thus, the elimination of HBV and HCV infection, as endorsed by the Global Health Sector Strategy, remains a challenge, highlighting the critical importance of strong health systems and sustainable funding to address these persistent public health issues.
Availability of data and materials
The data presented in this manuscript have been made publicly available through the Global Health Data Exchange (http://ghdx.healthdata.org/).
Abbreviations
- aHBV:
-
Acute HBV
- aHCV:
-
Acute HCV
- CCLD:
-
Cirrhosis and other chronic liver diseases
- 95% CI:
-
95% Confidence interval
- DAAs:
-
Direct-acting antiviral agents
- DALYs:
-
Disability-adjusted life years
- EU:
-
European Union
- GATHER:
-
Guidelines for Accurate and Transparent Health Estimates Reporting
- GBD:
-
Global Burden of Diseases, Injuries, and Risk Factors Study
- HBV:
-
Hepatitis B virus
- HBsAg:
-
Hepatitis B surface antigen
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- LC:
-
Liver cancer
- MSM:
-
Men who have sex with men
- PWID:
-
People who inject drugs
- SWE:
-
Southern (Greece, Italy, Portugal and Spain) Western European countries
- YLDs:
-
Years lived with disability
- YLLs:
-
Years of life lost to premature mortality
- WE:
-
Western Europe
- WHO:
-
World Health Organization
References
Sixty-Seventh World Health Assembly. 67th World Health Assembly Resolution on Hepatitis (WHA67.6). 2014;67.6(May):1–6. Available from: https://apps.who.int/gb/ebwha/pdf_files/WHA67-REC1/A67_2014_REC1-en.pdf.
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Glob Hepat Program Dep HIV/AIDS. 2016;(June):56. Available from: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
Sixty-Ninth World Health Assembly. World Health Assembly resolution WHA69.11, Agenda item 13.2 on Health in the 2030 Agenda for Sustainable Development. Wha6911. 2016;(May). Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_R11-en.pdf.
World Health Organization. Ensure healthy lives and promote well-being for all at all ages. 2016; Available from: https://www.who.int/sdg/targets/en/.
World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/item/9789240027077.
GBD 2019 Hepatitis B Collaborators. Global regional and national burden of hepatitis B, 1990 – 2019 : a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;1253(22):1–34.
Burns GS, Thompson AJ. Viral Hepatitis B: Clinical and Epidemiological Characteristics. Cold Spring Harb Lab Press. 2014;1–14. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4292086/pdf/cshperspectmed-HEP-a024935.pdf.
Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, Muljono DH, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2468125316301819.
Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1):S58–68. Available from: https://doi.org/10.1016/j.jhep.2014.07.012.
Veracruz N, Gish RG, Cheung R, Chitnis AS, Wong RJ. Global trends and the impact of chronic hepatitis B and C on adjusted life years. Liver Int. 2022;42(June):2145–53.
Veracruz N, Gish RG, Cheung R, Chitnis AS, Wong RJ. Global incidence and mortality of hepatitis B and hepatitis C acute infections, cirrhosis and hepatocellular carcinoma from 2010 to 2019. J Viral Hepat. 2022;29(December 2021):352–65.
Szczepanski M. A decade on from the crisis: Main responses and remaining challenges. European Parliament. 2019. Available from: https://www.europarl.europa.eu/RegData/etudes/BRIE/2019/642253/EPRS_BRI(2019)642253_EN.pdf.
Council on foreign Relations. The Eurozone in Crisis. 2015. Available from: https://www.cfr.org/backgrounder/eurozone-crisis.
Serapioni M, Hespanha P. Crisis and Austerity in Southern Europe: Impact on Economies and Societies. e-cadernos CES [Online]. 2019;31.
Italian Ministry of Health. Statistics yearbook 2019. 2019. Available from: https://www.istat.it/it/files//2019/12/Asi-2019.pdf.
Legido-Quigley H, Karanikolos M, Hernandez-Plaza S, de Freitas C, Bernardo L, Padilla B, et al. Effects of the financial crisis and Troika austerity measures on health and health care access in Portugal. Health Policy (New York). 2016;120(7):833–9. Available from: https://www.sciencedirect.com/science/article/pii/S0168851016300860.
Sakellarides C, Castelo-Branco L, Barbosa P, Azevedo H. European Observatory on Health Systems and Policies. The impact of the financial crisis on the health system and health in Portugal. 2014. Available from: https://www.euro.who.int/__data/assets/pdf_file/0006/266388/The-impact-of-the-financial-crisis-on-the-health-system-and-health-in-Portugal.pdf.
Vlachadis N, Kornarou E. Increase in stillbirths in Greece is linked to the economic crisis. BMJ. 2013;346. Available from: https://www.bmj.com/content/346/bmj.f1061.
Gili M, Roca M, Basu S, McKee M, Stuckler D. The mental health risks of economic crisis in Spain: evidence from primary care centres, 2006 and 2010. Eur J Public Health. 2013Feb;23(1):103–8.
Costa G, Marra M, Salmaso S, Gruppo AIE su crisi e salute. Health indicators in the time of crisis in Italy. Epidemiol Prev. 2012;36(6):337—366. Available from: http://europepmc.org/abstract/MED/23293258.
Santana P, Costa C, Cardoso G, Loureiro A, Ferrão J. Suicide in Portugal: Spatial determinants in a context of economic crisis. Health Place. 2015;35:85–94. Available from: https://www.sciencedirect.com/science/article/pii/S1353829215001033.
Fountoulakis KN, Savopoulos C, Siamouli M, Zaggelidou E, Mageiria S, Iacovides A, et al. Trends in suicidality amid the economic crisis in Greece. Eur Arch Psychiatry Clin Neurosci. 2013Aug;263(5):441–4.
De Vogli R, Marmot M, Stuckler D. Excess suicides and attempted suicides in Italy attributable to the great recession. J Epidemiol Community Health. 2013 Apr 1;67(4):378 LP – 379. Available from: http://jech.bmj.com/content/67/4/378.1.abstract.
European Centre for Disease Prevention and Control. Risk assessment on HIV in Greece. Stockholm: ECDC; 2012. Available from: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/20121130-Risk-Assessment-HIV-in-Greece.pdf.
Kondilis E, Giannakopoulos S, Gavana M, Ierodiakonou I, Waitzkin H, Benos A. Economic Crisis, Restrictive Policies, and the Population’s Health and Health Care: The Greek Case. Am J Public Health. 2013;103(6):973–9. Available from: https://doi.org/10.2105/AJPH.2012.301126.
European AIDS treatment Group. The impact of economic austerity on the HIV response in Portugal: a community perspective. 2014. Available from: https://www.gatportugal.org/public/uploads/tomadasposicao/Country%20Brief_Portugal%20EN.doc.pdf.
Abbafati C, Machado DB, Cislaghi B, Salman OM, Karanikolos M, McKee M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Abbafati C, Machado DB, Cislaghi B, Salman OM, Karanikolos M, McKee M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49.
World Bank. Current health expenditure (% of GDP) - Italy, Greece, Spain, Portugal. Available from: https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS?locations=IT-GR-ES-PT.
Ganesan M, Poluektova LY, Kharbanda KK, Osna NA. Human immunodeficiency virus and hepatotropic viruses comorbidities as the inducers of liver injury progression. World J Gastroenterol. 2019;25(4):398–410.
Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;6736(16):30388–9. Available from: http://gather-statement.org.
The Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2019 (GBD 2019) Data Resources. 2019. Available from: source: http://ghdx.healthdata.org/gbd-2019.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.r-project.org/.
World Health Organization. Hepatitis C - Key facts. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
World Health Organization. Hepatitis B - Key facts. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
Global Burden of Disease 2016 Greece Collaborators. The burden of disease in Greece, health loss, risk factors, and health financing, 2000–16: an analysis of the Global Burden of Disease Study 2016. Lancet Public Heal. 2018;3:e395-406.
Papatheodoridis G. Epidemiology of hepatitis B and D in Greece. 2008. Available from: http://www.vhpb.org/files/html/Meetings_and_publications/Presentations/ATHS41.pdf.
European Centre for Disease Prevention and Control. The sustainable development goals and hepatitis B and C in the EU/EEA. ECDC: Stockholm. 2021;(March). Available from: https://www.ecdc.europa.eu/en/publications-data/hepatitis-b-and-c-sustainable-development-goals-eu-eea.
Organisation for Economic Co-operation and Development. Health Statistics 2021. Health Care Utilisation 2019 Survey. 2021. Available from: https://oe.cd/ds/health-statistics.
Gazzetta Ufficiale della Repubblica Italiana. Legge 27 maggio 1991, n. 165. Available from: https://www.gazzettaufficiale.it/atto/stampa/serie_generale/originario.
Instituto de Salud Carlos III. Protocolos de la Red Nacional de Vigilancia Epidemiológica. Protocolo de vigilancia de la Hepatitis B. Versión 1 de junio de 2013. Revisado el de 3 de septiembre de 2016. Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/PROTOCOLOS/PROTOCOLOS EN BLOQUE/PROTOCOLOS_RENAVE-ciber.pdf.
Direção-Geral da Saúde. Programa nacional para as hepatites virais. 2017. Available from: https://www.dgs.pt/documentos-e-publicacoes/relatorio-do-programa-nacional-para-as-hepatites-virais-2017.aspx.
European Blood Alliance. Italy. Available from: https://europeanbloodalliance.eu/country/italy/.
Zuccaro O, Tosti ME, et al. Epidemiology of acute viral hepatitis in Italy: results of the surveillance through SEIEVA (Sistema Epidemiologico Integrato dell’Epatite Virale Acuta). Rapporti ISTISAN 12/4. 2012. Available from: https://www.iss.it/documents/20126/45616/12_4_web.pdf/36d495ed-ee41-8ef7-24d3-67d33aee6789?t=1581095228731.
Ministero della Sanità. Decreto Ministeriale 15 dicembre 1990 Sistema informativo delle malattie infettive e diffusive. Gazzetta Ufficiale del 8 gennaio 1991, n. 6. 1991. Available from: https://www.epicentro.iss.it/infettive/pdf/DM_151290.pdf.
Ministerio de Sanidad. Orden SSI/445/2015, de 9 de marzo, por la que se modifican los anexos I, II y III del Real Decreto 2210/1995, de 28 de diciembre, por el que se crea la Red Nacional de Vigilancia Epidemiológica, relativos a la lista de enfermedades de declaración obligato. 2015. Available from: https://www.boe.es/buscar/doc.php?id=BOE-A-2015-2837.
Bulteel N, Partha Sarathy P, Forrest E, Stanley AJ, Innes H, Mills PR, et al. Factors associated with spontaneous clearance of chronic hepatitis C virus infection. J Hepatol. 2016Aug;65(2):266–72.
Watanabe H, Saito T, Shinzawa H, Okumoto K, Hattori E, Adachi T, et al. Spontaneous elimination of serum hepatitis C virus (HCV) RNA in chronic HCV carriers: a population-based cohort study. J Med Virol. 2003Sep;71(1):56–61.
Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: Efficacy, side effects, and complications. Gut. 2006;55(9):1350–9.
Papatheodoridis G. Aiming towards hepatitis C virus elimination in Greece. Ann Gastroenterol. 2019;32(4):321–9.
Gruppo multidisciplinare sui farmaci per l’epatite C cronica della Regione Emilia-Romagna. Nuovi antivirali diretti nella terapia dell’epatite C cronica. 2016. Available from: https://salute.regione.emilia-romagna.it/ssr/strumenti-e-informazioni/ptr/archivio/atti-regionali-e-documenti-ptr/229-epatite-c-cronica-giugno-2016.
Direção-Geral da Saúde. Tratamento da Hepatite C Crónica no Adulto. 2017;028:1–44. Available from: https://www.dgs.pt/.../norma-n-0282017-de-28122017-pdf.aspx%0A.
Ministerio de sanidad igualdad y asuntos sociales. Plan estratégico para el abordaje de la hepatitis c en el sistema nacional de salud. Ministerio de Sanidad, Servicios Sociales e Igualdad. 2015. Available from: https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/plan_estrategico_hepatitis_C.pdf.
Kondili L, Quaranta MG, Ferrigno L, Galli M, Andreoni M, Puoti M, et al. Eliminazione dell’epatite C cronica in italia: strategie di screening gratuito. Vol. 34, Istituto Superiore di Sanità. 2021.
Papatheodoridis G, Sypsa V, Kantzanou M, Nikolakopoulos I, Hatzakis A. Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey. J Viral Hepat. 2015;22(4):409–15.
Estirado Gómez A, Justo Gil S, Limia A, Avellón A, Arce Arnáez A, González-Rubio R, et al. Prevalence and undiagnosed fraction of hepatitis C infection in 2018 in Spain: results from a national population-based survey. Eur J Public Health. 2021Dec;31(6):1117–22.
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–606.
Kramer JR, El-serag H, Taylor TJ, White D, Frayne S, Cao Y, et al. HCV-related complications are increasing in women veterans: A national cohort study. 2017;24(11):955–65.
Forns X, Colom J, García-Retortillo M, Quer JC, Lens S, Martró E, et al. Point-of-care hepatitis C testing and treatment strategy for people attending harm reduction and addiction centres for hepatitis C elimination. J Viral Hepat. 2022Mar;29(3):227–30.
World Health Organization. Consolidated guidelines on HIV, viral hepatitis and STI prevention, diagnosis, treatment and care for key populations. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/item/9789240052390.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Statistical Bulletin 2017. 2017. Available from: http://www.emcdda.europa.eu/data/stats2017.
Urbanus AT, Van De Laar TJW, Geskus R, Vanhommerig JW, Van Rooijen MS, Schinkel J, et al. Trends in hepatitis C virus infections among MSM attending a sexually transmitted infection clinic; 1995–2010. AIDS. 2014Mar;28(5):781–90.
European Centre for Disease Prevention and Control. Hepatitis C. In: ECDC. Annual epidemiological report for 2019. Stockholm. ECDC; 2021. 2021. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/AER-Hepatitis-C-2019.pdf.
Garriga C, Manzanares-Laya S, de Olalla P García, Gorrindo P, Lens S, Solà R, et al. Evolution of acute hepatitis C virus infection in a large European city: Trends and new patterns. PLoS One. 2017;12(11).
Midgard H, Weir A, Palmateer N, Lo Re V, Pineda JA, Macías J, et al. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65(1):S33–45. Available from: https://doi.org/10.1016/j.jhep.2016.07.012.
van de Laar TJW, Matthews GV, Prins M, Danta M. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS. 2010Jul;24(12):1799–812.
Jin F, Dore GJ, Matthews G, Luhmann N, Macdonald V, Bajis S, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021 Jan 1;6(1):39–56. Available from: https://doi.org/10.1016/S2468-1253(20)30303-4.
Hellenic Ministry of Health. Hellenic National Plan for the Management of Hepatitis C. 2017. Available from: http://www.moh.gov.gr/articles/ministry/grafeio-typoy/press-releases/4865-ethniko-sxedio-drashs-gia-thn-antimetwpish-ths-hpatitidas-c.
Saúde J e S-G da S de EA e da J e do S de EA e da. Despacho n.o 6542/2017. Diário da República n.o 145/2017, Série II de 2017–07–28. 2017. Available from: https://dre.pt/application/conteudo/107774607.
Lazarus JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM, et al. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin Liver Dis. 2018;38(3):181–92.
Giuliani R, Casigliani V, Fornili M, Sebastiani T, Freo E, Arzilli G, et al. HCV micro-elimination in two prisons in Milan. Italy : A model of care. 2020;27(12):1444–54.
Fiore V, Matteis G De, Ranieri R, Saderi L, Pontali E, Muredda A, et al. HCV testing and treatment initiation in an Italian prison setting : A step-by-step model to micro-eliminate hepatitis C. Int J Drug Policy. 2021;90:103055. Available from: https://doi.org/10.1016/j.drugpo.2020.103055.
Cuadrado A, Llerena S, Cobo C, Pallás JR, Mateo M, Cabezas J, et al. Microenvironment Eradication of Hepatitis C : A Novel Treatment Paradigm. 2018;113(11):1639–48.
Jiménez-galán G, Alia-alia C, Vegue-gonzález M, García-berriguete RM, Fernández-rodríguez C, González-fernández M, et al. The contribution of telemedicine to hepatitis C elimination in a correctional facility. 2019;111(7):550–5.
Cuadrado A, Cobo C, Mateo M, Blasco AJ, Cabezas J, Llerena S, et al. Telemedicine efficiently improves access to hepatitis C management to achieve HCV elimination in the penitentiary setting. Int J Drug Policy. 2021;88:103031. Available from: https://www.sciencedirect.com/science/article/pii/S0955395920303698.
World Health Organization. Improved access to health services to eliminate hepatitis C in Portuguese prisons. 2018. Available from: https://www.euro.who.int/en/health-topics/Health-systems/health-workforce/news/news/2018/8/improved-access-to-health-services-to-eliminate-hepatitis-c-in-portuguese-prisons#.
Secretaría General de Sanidad y Consumo Ministerio de Sanidad, Consumo y Bienestar Social. Plan Estratégico para el Abordaje de la Hepatitis C en el Sistema Nacional de Salud (PEAHC). 2018. Available from: https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/Plan_Estrategico_Abordaje_Hepatitis_C_(PEAHC).pdf.
World Health Organization. New Report Highlights Global Progress on Reducing HIV, Viral Hepatitis and Sexually Transmitted Infections and Signals Need for Renewed Efforts to Reach 2030 Targets. 2021. Available from: https://www.who.int/news/item/20-05-2021-new-report-highlights-global-progress-on-reducing-hiv-viral-hepatitis-and-sexually-transmitted-infections-and-signals-need-for-renewed-efforts-to-reach-2030-targets.
Wingrove C, James C, Wang S. The impact of COVID-19 on hepatitis services and civil society organisations. Lancet Gastroenterol Hepatol. 2021 Sep 1;6(9):682–4. Available from: https://doi.org/10.1016/S2468-1253(21)00263-6.
World Health Organization. Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic: January-March 2021. Interim report. 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS-continuity-survey-2021.1.
World Health Organization. Third round of the global pulse survey on continuity of essential health services during the COVID-19 pandemic: November–December 2021. Interim report 7. 2022. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2022.1.
Acknowledgements
The authors are solely responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. This manuscript was prepared as part of the GBD Collaborator Network and in accordance with the GBD protocol. The Global Burden of Disease Study is primarily funded by the Bill & Melinda Gates Foundation (OPP1152504). The funder of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the publication. The authors would like to thank the Senior Data Scientist MEng. Óscar Rodríguez Villaamil for the fruitful discussions and for his valuable IT support.
Funding
Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Consortia
Contributions
Providing data or critical feedback on data sources. C. Palladino, I.J. Ezeonwumelu, N. Taveira, S. Hassan, Z. Kabir, A.H. Mokdad, L. Monasta, F. Mulita, M.J. Postma, and R. Tabarés-Seisdedos. Developing methods or computational machinery. C. Palladino, A.H. Mokdad, and F. Mulita. Providing critical feedback on methods or results. C. Palladino, R. Ramis, I.J. Ezeonwumelu, N. Taveira, V. Briz, G. Carreras, F. Fischer, D. Golinelli, S. Hassan, A. Koyanagi, J.V. Lazarus, Z. Kabir, AF.A. Mentis, A.H. Mokdad, F. Mulita, M.J. Postma, R. Tabarés-Seisdedos, and A. Thiyagarajan. Drafting the work or revising it critically for important intellectual content. C. Palladino, R. Ramis, I.J. Ezeonwumelu, N. Taveira, M.J. Postma, V. Briz, A. Biondi, F. Fischer, S. Gallus, D. Golinelli, G. Gorini, A. Koyanagi, AF.A. Mentis, T.J. Meretoja, A.H. Mokdad, L. Monasta, F. Mulita, and A. Thiyagarajan. Managing the estimation or publications process. C. Palladino, N. Taveira, V. Briz, and A.H. Mokdad.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
C Palladino reports contract funding support from Fundação para a Ciência e a Tecnologia (FCT), I.P. (national funding), under a contract-programme as defined by DL No. 57/2016 and Law No. 57/2017 (DL57/2016/CP1376/CT0004), https://doi.org/10.54499/DL57/2016/CP1376/CT0004 (https://doi.org/10.54499/DL57/2016/CP1376/CT0004). I Ezenowumelu reports financial support for presenting at the 2023 Conference on Retroviruses and Opportunistic Infections (CROI 2023) by the CROI New Investigator Scholarship. V Lazarus reports grants or contracts from AbbVie, Gilead Sciences, MSD, Roche Diagnostics; royalties or licenses from Novavax; payment or honoraria for lectures from AbbVie, Gilead Sciences, Intercept, Janssen, Novo Nordisk; participation on a Data Safety Monitoring Board or Advisory Board with Same-visit hepatitis C testing and treatment to accelerate cure among people who inject drugs (The QuickStart Study): a cluster randomised control trial – Australia; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with EASL Public Health and Policy Committee as a member, HIV Outcomes as a co-chair, and SHARE Global Health Foundation; all outside the submitted work. A-F A Mentis reports grants or contracts from ‘MilkSafe: A novel pipeline to enrich formula milk using omics technologies’, a research co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T2EDK-02222), as well as from ELIDEK (Hellenic Foundation for Research and Innovation, MIMS-860); payment for expert testimony from saving as an external peer-reviewer for Fondazione Cariplo, Italy; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid as an editorial board member for Systematic Reviews and Annals of Epidemiology, and as an associate editor for Translational Psychiatry; and other financial or non-financial interests having served as a scientific officer for “BGI Group” over the last year, all outside the submitted work. L Monasta reports support for the present manuscript from the Italian Ministry of Health (Ricerca Corrente 34/2017), payments made to the Institute for Maternal and Child Health IRCCS Burlo Garofolo. M J Postma reports stock or stock options in PAG BV (Groningen, Netherlands) and HealthEcore (Zeist, Netherlands). R Tabares-Sesdedos reports grants or contracts from the Spanish Ministry of Innovation, Institute of Health Carlos III (PID2021-129099OB-I00) and Generalitat Valenciana (CIPROM/2022/58), payments made to the University of Valencia, all outside the submitted work. V Briz reports financial support by Instituto de Salud Carlos III (PI18CIII/00020). All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Palladino, C., Ramis, R., Ezeonwumelu, I.J. et al. Impact of the 2008 economic crisis on the burden of hepatitis B and C diseases in Southern European countries. BMC Public Health 24, 1642 (2024). https://doi.org/10.1186/s12889-024-18912-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-18912-0