Abstract
Background
This study explored the association of cardiovascular disease (CVD) with cancer mortality risk in individuals with or without a history of cancer, to better understand the interplay between CVD and cancer outcomes.
Methods
Utilizing data from the National Health and Nutrition Examination Survey (NHANES) spanning 1999 to 2018, a retrospective cohort analysis was conducted. This analysis accounted for the survey’s complex design to ensure national representativeness. The association of CVD with cancer mortality was assessed through multivariable Cox proportional hazards models.
Results
The present study included 59,653 participants, of whom 54,095 did not have cancer and 5558 had a history of cancer. In individuals without cancer, heart failure (HF) was associated with an increased risk of mortality from cancer (HR, 1.36; 95% CI, 1.09–1.69; P = 0.005). In participants with cancer, HF correlated with a higher risk of mortality from cancer (HR, 1.76; 95% CI, 1.32–2.34; P < 0.001). Diabetes (DM), hypertension (HBP) and coronary heart disease (CHD) were not significantly associated with an increased risk of mortality from cancer. Significant differences were observed in the interaction between cancer and CHD (HR, 0.68; 95% CI, 0.53–0.87; P = 0.002). For cancer and HBP, a similar trend was noted (HR, 0.75; 95% CI, 0.62–0.91; P = 0.003). No significant differences were found in interactions between HF, DM and cancer.
Conclusions
HF was associated with an increased risk of mortality from cancer, regardless of cancer history, while HBP, CHD and DM showed no significant association. These findings underscore the importance of understanding the mechanisms behind the increased risk of cancer mortality following HF.
Similar content being viewed by others
Background
Cancer and cardiovascular disease (CVD) are the leading non-communicable causes of global morbidity and mortality. In 2015, CVD resulted in 17.7 million deaths globally, while cancer was responsible for 8.8 million deaths [1,2,3]. Since the 1990s, there has been a notable decline in cancer-related mortality, with projections indicating that the number of cancer survivors in the United States will exceed 26 million by 2040 [4,5,6,7]. This growing population of cancer survivors faces an increased risk of developing CVD, with cardiac risk factors significantly influencing treatment-related cardiotoxicity. Both CVD and cancer share common risk factors, such as obesity and diabetes (DM), suggesting a potential shared pathobiology—a concept supported by emerging evidence [8, 9]. This intersection of cancer and CVD has led to the development of the specialized field of cardio-oncology [10,11,12].
Despite the recognized link between cancer and CVD, the evidence guiding clinical decisions in cardio-oncology remains sparse. Extensive research has been conducted on cancer treatment-induced cardiotoxicity, the impact of pre-existing CVD on cancer mortality, especially among cancer patients, is less understood [13,14,15]. Recognizing this gap, our study seeks to provide empirical evidence on the role of CVD in cancer mortality, utilizing data from the National Health and Nutrition Examination Survey (NHANES). We hypothesize that CVD significantly increases the risk of cancer mortality and aimed to exam the association between CVD and cancer mortality in individuals with or without a history of cancer.
Methods
Study population
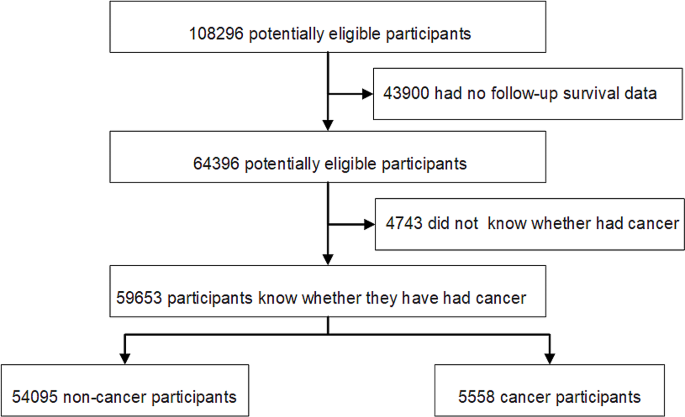
This study utilized data from the NHANES, a representative, multistage, and stratified health survey conducted in the United States [16,17,18,19,20]. This study received ethical approval from the National Center for Health Statistics (NCHS) Institutional Review Board and informed consent was obtained from all participants. The research adhered to the Tenets of the Declaration of Helsinki. Ethical considerations have been rigorously followed to ensure that participants confidentiality was not impacted. We included participants from the NHANES database spanning 1999 to 2018, exclusion criteria were set to omit individuals without clear cancer status or those missing follow-up survival data. Participants were categorized into cancer and non-cancer groups based on physician-reported cancer diagnoses (Fig. 1). NCHS linked the survey data with death certificate records from the National Death Index (NDI) for mortality follow-up. Follow-up time was calculated in person-months from the interview date to either the date of death, the end of the mortality follow-up period, or December 31, 2019, whichever occurred first. The linked mortality files classified causes of death into nine categories using (ICD)-10 codes. Our primary focus was on deaths due to malignant neoplasms (ICD-10 codes: C00-C97) and all-cause mortality.
Sociodemographic characteristics and covariates
Participants provided information on age, gender, race and ethnic group (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), education level (< High school, High school, some college or Associates degree, College graduate) and marital status (never married, married or living with a partner, separated or divorced or widowed). The ratio of family income to the poverty level was categorized as < 1, 1 to 3, or > 3. Smoking status was categorized as current, past or never. Body mass index (BMI), calculated as the weight in kilograms divided by the square of the height in meters, was classified into three weight-status groups: normal (BMI < 25), overweight (BMI 25 ∼ 30), or obese (BMI ≥ 30). Creatinine data were obtained from the original database. The presence of various comorbidities, such as DM, hypertension (HBP), coronary heart disease (CHD), heart failure (HF), stroke, chronic bronchitis and chronic liver disease, was determined based on reported diagnoses from a physician.
Statistical analysis
We employed complex survey design adjustments from NHANES data to ensure representative estimates for the US population, accounting for sample weights, clustering, and stratification [21, 22]. Data analysis was conducted using R software version 4.3.1. Categorical variables were analyzed using Rao-Scott adjusted Chi-square test and continuous variables were analyzed using weighted mean comparisons. Kaplan-Meier survival curves provided weighted comparisons of the cumulative incidence of cancer-related deaths and all-cause deaths. We rigorously tested the proportional hazards assumption through the examination of martingale residuals and the application of time-dependent covariate tests. Cox proportional hazards models, incorporating survey sample weights, were utilized to estimate hazard ratio (HR) for cancer mortality, adjusting for potential confounders including gender, age, BMI, race, education, marital status, income level and CVD conditions including CHD, HF, HBP, DM. Missing data were addressed using the fully efficient fractional imputation technique, with less than 3% missing values for most variables, except for BMI (6.3% missing), family income-to-poverty ratio (9.9% missing) and Creatinine (11.8% missing) [23]. Sensitivity analyses excluded subjects with missing values in BMI, marital status, creatinine, and DM, HBP, CHD, HF statuses. Statistical significance was set at p ≤ 0.05.
Results
Participants characteristics
The study cohort comprised 59,653 individuals who were categorized into the non-cancer group (N = 54,095) and the cancer group (N = 5558). Table 1 presents the clinical characteristics of non-cancer and cancer participants. Compared to non-cancer participants, those with cancer were older, had a higher proportion of females, and exhibited elevated systolic blood pressure levels. Diastolic blood pressure levels were lower in the cancer group. A lower percentage of smokers was noted in the cancer group, and this group had a shorter follow-up time. In terms of education level, individuals with or above a college education were more prevalent in the cancer group, while those with an education level below high school were less frequent in the cancer group. The proportion of participants living alone was notably higher in the cancer group. Creatinine levels were higher in cancer participants than in non-cancer participants. Cancer participants, in comparison with non-cancer participants, were significantly more likely to have DM (15.5% vs. 8.2%, respectively; P < 0.001), HBP (50.4% vs. 28.3%, respectively; P < 0.001), HF (6.2% vs. 2.0%, respectively; P < 0.001), CHD (8.1% vs. 3.0%, respectively; P < 0.001), Stroke (6.5% vs. 2.4%, respectively; P < 0.001), Chronic bronchitis (11.2% vs. 5.6%, respectively; P < 0.001) and Liver condition (5.3% vs. 3.3%, respectively; P < 0.001). Among all cancer participants, skin cancer had the highest proportion, accounting for 28.3%, followed by breast cancer (15.8%), prostate cancer (9.4%), cervix cancer (8.1%), melanoma (7.4%), colon cancer (4.7%), uterus cancer (3.6%), lung cancer (2.2%), other types of cancer accounting for 20.5%.
Association of CVD with cancer mortality
Examination of martingale residuals showed the proportional hazards assumption is reasonable for data of the present study (Supplemental figure). Among all participants, presence of cancer was associated with higher risk of cancer mortality among cancer participants, when compared with participants without cancer (HR 2.35, 95%CI 2.14 ∼ 2.58, P < 0.001). Presence of HF was associated with higher risk of cancer mortality among all participants, when compared with participants without HF (HR 1.52, 95%CI 1.34 ∼ 1.71, P < 0.001). DM, HBP, CHD were not associated with significant increased cancer mortality risk. There was statistically significant difference in the associations of cancer× CHD interaction (HR 0.68, 95%CI 0.53 ∼ 0.87, P = 0.002) and cancer× HBP interaction (HR 0.75, 95%CI 0.62 ∼ 0.91, P = 0.003). There was no statistically significant difference in the associations of cancer× HF interaction (P = 0.891) and cancer× DM interaction (P = 0.56) (Table 2).
Among non-cancer participants, presence of HF was associated with higher risk of cancer mortality among cancer participants, when compared with participants without HF (HR 1.36, 95%CI 1.09 ∼ 1.69, P = 0.005). DM, HBP, CHD were not associated with significant increased cancer mortality risk. Among cancer participants, presence of HF was associated with higher risk of cancer mortality among cancer participants, when compared with participants without HF (HR 1.76, 95%CI 1.32 ∼ 2.34, P < 0.001). DM, HBP, CHD were not associated with significant increased cancer mortality risk (Table 3).
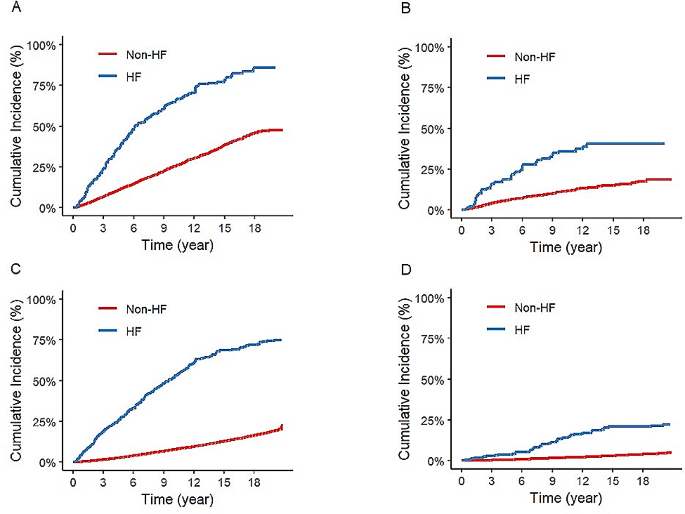
Kaplan-Meier curves showed cumulative all-cause mortality and cancer mortality. Cancer participants with HF had a higher all-cause mortality compared with cancer participants without HF. Cancer participants with HF also had a higher cancer mortality compared with cancer participants without HF. Non-cancer participants with HF had a higher all-cause mortality compared with non-cancer participants without HF. Non-cancer participants with HF also had a higher cancer mortality compared with non-cancer participants without HF (Fig. 2).
Kaplan-Meier Survival Curves for All-Cause and cancer Mortality. Cumulative mortality rates were estimated with use of imputation-adjusted survey weights. (A) All-cause mortality among cancer participants with heart failure (HF) versus those without. (B) Cancer mortality among cancer participants with HF versus those without. (C) All-cause mortality among non-cancer participants with HF versus those without. (D) Cancer mortality among non-cancer participants with HF versus those without. Mortality rates are adjusted for imputation and survey weights to reflect the NHANES cohort accurately
In sensitivity analyses, presence of HF was associated with higher risk of cancer mortality among all participants, when compared with participants without HF (HR 1.56, 95%CI 1.18 ∼ 2.06, P < 0.001). DM, HBP, CHD were not associated with significant increased cancer mortality risk. Among non-cancer participants, presence of HF was associated with higher risk of cancer mortality among cancer participants, when compared with participants without HF (HR 1.47, 95%CI 1.02 ∼ 2.13, P = 0.03). Among cancer participants, presence of HF was associated with higher risk of cancer mortality among cancer participants, when compared with participants without HF (HR 1.69, 95%CI 1.03 ∼ 2.79, P = 0.037).
Discussion
The present study embarked on an exploration of the associations between CVD and cancer mortality, leveraging a comprehensive retrospective cohort analysis of data from NHANES spanning 1999 to 2018. Confirming our hypotheses, we found that HF was associated with a 37% increased risk of cancer mortality in participants without cancer and a 73% increase in those with cancer, compared to those without HF. The findings of this investigation revealed that HF is a notable predictor of increased cancer mortality risk irrespective of cancer history, a discovery that underscores the intricate and potentially bidirectional relationship between HF and cancer. Conversely, DM, HBP, and CHD did not exhibit a statistically significant association with cancer mortality, highlighting the unique position of HF within the spectrum of CVD affecting cancer outcomes.
Our findings concur with prior research that has indicated a heightened cancer risk associated with HF [24,25,26,27] and may be linked to shared risk factors, such as the association of chronic kidney disease with increased cancer risk in the elderly [28]. We observed that common risk factors like HBP, obesity, DM, and tobacco use are shared between cancer and heart failure. Similarly, Symptoms such as fatigue, dyspnea, and weight loss also present in both HF and cancer, adding complexity to their management [29,30,31,32]. Koene et al. elucidated the shared risk factors and biological mechanisms between CVD and cancer, suggesting a unified pathobiological framework that may contribute to the co-occurrence of these diseases [9]. Chronic inflammation and immune modulation in HF could promote tumor progression. Experimental models have shown a causal relationship between ischemic HF and tumor growth, possibly mediated by factors released from failing myocardium [8, 33,34,35,36,37]. Sympathetic nervous system activation in HF, as observed in breast cancer mouse models, is associated with increased metastasis, which can be mitigated by beta-blocker therapy [38]. This suggests a potential therapeutic role for beta-blockers in cancer patients with elevated heart rates [39].
Our study uniquely identified that CHD and HBP demonstrated an interactive effect with cancer, which may provide a protective influence. This interaction may be linked to the protective effects of medications used in the treatment of HBP and CHD. Angiotensin Receptor Blocker (ARB) hold anti-tumor potential by inhibiting the action of angiotensin II, as do Angiotensin-Converting Enzyme Inhibitors (ACEI), which block the generation of angiotensin II and are considered to have anti-tumor effects. Long-term ARB and ACEI use was significantly associated with a reduced risk of incident cancer [40]. Statins, such as atorvastatin, simvastatin, rosuvastatin and pravastatin, have demonstrated anticancer activity across various cancer types in laboratory studies. These drugs exert direct effects on cancer cells, influencing tumor initiation, progression, metastasis, and response to therapy. While the role of statins in cancer prevention is debated, robust research confirms their potential as repurposed drugs in the fight against cancer. Recent systematic reviews and meta-analyses indicate that statin treatment is linked to a decreased risk of overall mortality and cancer-specific mortality in advanced-stage cancer patients. The multifaceted effects of statins, including antiproliferative and apoptotic-inducing properties, position them as promising agents in cancer therapy, introducing innovative perspectives and novel treatment targets [41,42,43,44,45].
Our study, while providing valuable insights into the association between CVD and cancer mortality, is subject to several limitations. Firstly, relying on data from NHANES introduces potential biases such as recall bias and inaccuracies in self-reported health and lifestyle factors. The retrospective cohort design, though robust, cannot establish causality between CVD conditions and cancer mortality, highlighting the need for prospective or randomized controlled designs in future research. Despite adjusting for multiple confounders, residual or unmeasured confounding factors could still influence the observed associations. The study primarily focuses on HF, DM, HBP and CHD, with limited data on other CVD conditions and specific types of cancer, which constrains our understanding of these associations. Additionally, the span of data from 1999 to 2018 encompasses significant changes in healthcare and lifestyle, the implications of which may not be fully captured in our analysis. Addressing these limitations in future studies is crucial for refining our understanding of the complex interplay between cardiovascular health and cancer outcomes.
Conclusions
In conclusion, we found that HF exhibited an elevated risk of cancer mortality, irrespective of a patient’s cancer history. This association underscores the importance of integrating cardiovascular health management into cancer care strategies. Conversely, DM, HBP and CHD did not demonstrate a significant correlation with increased cancer mortality risk, highlighting the specificity of HF ‘s impact on cancer outcomes. Our findings contribute to the burgeoning field of cardio-oncology, emphasizing the need for a multidisciplinary approach to patient care that addresses both cardiovascular health and cancer risk. The nuanced understanding of the relationship between specific cardiovascular conditions and cancer mortality could lead to more effective prevention, management, and treatment strategies that holistically address patient health. As the interplay between CVD and cancer continues to reveal its complexity, ongoing research in this intersection is imperative for advancing patient care and improving outcomes.
Data availability
The data and the simulation results that support the findings of this study are available in Figshare with the identifie. All National Health and Nutrition Examination Survey data were accessed from https://www.cdc.gov/nchs/nhanes.htm.
Abbreviations
- CVD:
-
Cardiovascular disease
- NHANES:
-
National Health and Nutrition Examination Survey
- HF:
-
Heart failure
- DM:
-
Diabetes
- HBP:
-
Hypertension
- CHD:
-
Coronary heart disease
- NCHS:
-
National Center for Health Statistics
- NDI:
-
National Death Index
- ICD:
-
International Classification of Diseases
- BMI:
-
Body mass index
- ARB:
-
Angiotensin Receptor Blocker
- ACEI:
-
Angiotensin-Converting Enzyme Inhibitors
References
WHO. Cardiovascular diseases. http://www.hoint/mediacentre/factsheets/fs317/en/(18 October 2019).
Cancer W. October: http://www.who.int/mediacentre/factsheets/fs297/en/ (18 2019).
Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Syst. 2017;66(6):1–75.
Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clinic proceedings 2014;89(9):1287–1306.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Coleman MP, Gatta G, Verdecchia A, Estève J, Sant M, Storm H, Allemani C, Ciccolallo L, Santaquilani M, Berrino F. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Annals Oncology: Official J Eur Soc Med Oncol. 2003;14(Suppl 5):v128–149.
Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the silver tsunami: prevalence trajectories and Comorbidity Burden among Older Cancer survivors in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Cosponsored Am Soc Prev Oncol. 2016;25(7):1029–36.
de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019;21(12):1515–25.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk factors in Cardiovascular Disease and Cancer. Circulation. 2016;133(11):1104–14.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801.
Lancellotti P, Suter TM, López-Fernández T, Galderisi M, Lyon AR, Van der Meer P, Cohen Solal A, Zamorano JL, Jerusalem G, Moonen M, et al. Cardio-oncology services: rationale, organization, and implementation. Eur Heart J. 2019;40(22):1756–63.
del Pozo Cruz B, Ahmadi MN, Lee I-M, Stamatakis E. Prospective associations of Daily Step counts and Intensity with Cancer and Cardiovascular Disease incidence and mortality and all-cause mortality. JAMA Intern Med. 2022;182(11):1139–48.
Townsend N, Kazakiewicz D, Lucy Wright F, Timmis A, Huculeci R, Torbica A, Gale CP, Achenbach S, Weidinger F, Vardas P. Epidemiology of cardiovascular disease in Europe. Nat Reviews Cardiol. 2022;19(2):133–43.
Wang Y, Liu B, Han H, Hu Y, Zhu L, Rimm EB, Hu FB, Sun Q. Associations between plant-based dietary patterns and risks of type 2 diabetes, cardiovascular disease, cancer, and mortality– a systematic review and meta-analysis. Nutr J. 2023;22(1):46.
Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital and health statistics Series 2, Data evaluation and methods research 2012(155):1–39.
Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat Ser 2 Data Evaluation Methods Res 2013(160):1–23.
Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat Ser 2 Data Evaluation Methods Res 2014(162):1–33.
Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation procedures. Vital Health Stat Ser 2 Data Evaluation Methods Res 2020(184):1–35.
Patel CJ, Pho N, McDuffie M, Easton-Marks J, Kothari C, Kohane IS, Avillach P. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci data. 2016;3:160096.
Centers for Disease Control and Prevention (CDC). About the National Health and Nutrition Examination Survey. 2017 https://www.cdcgov/nchs/nhanes/about_nhaneshtm.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat Ser 2 Data Evaluation Methods Res 2013(161):1–24.
Pan S, Chen S. Empirical Comparison of Imputation Methods for Multivariate Missing Data in Public Health. Int J Environ Res Public Health 2023, 20(2).
Riley JP, Beattie JM. Palliative care in heart failure: facts and numbers. ESC Heart Fail. 2017;4(2):81–7.
Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail. 2017;4(4):492–8.
Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, Bekfani T, Sandek A, Lainscak M, Doehner W, et al. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). ESC Heart Fail. 2017;4(4):448–57.
von Haehling S. Co-morbidities in heart failure beginning to sprout-and no end in sight? Eur J Heart Fail. 2017;19(12):1566–8.
Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, Craig JC. Association of CKD and cancer risk in older people. J Am Soc Nephrology: JASN. 2009;20(6):1341–50.
Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62(10):881–6.
Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is Associated with increased risk of Cancer. J Am Coll Cardiol. 2016;68(3):265–71.
Banke A, Schou M, Videbaek L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail. 2016;18(3):260–6.
Malhotra J, Boffetta P. Association of increased Cancer risk with heart failure. J Am Coll Cardiol. 2016;68(3):272–3.
Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, Jong Sd, Haubner BJ, Nagengast WB, Lyon AR, Vegt Bvd. Heart failure stimulates Tumor Growth by circulating factors. Circulation. 2018;138(7):678–91.
Cuomo A, Pirozzi F, Attanasio U, Franco R, Elia F, De Rosa E, Russo M, Ghigo A, Ameri P, Tocchetti CG, et al. Cancer Risk in the Heart failure Population: Epidemiology, mechanisms, and clinical implications. Curr Oncol Rep. 2020;23(1):7.
Soman A, Asha Nair S. Unfolding the cascade of SERPINA3: inflammation to cancer. Biochim et Biophys Acta (BBA) - Reviews Cancer. 2022;1877(5):188760.
Ausoni S, Azzarello G. Development of Cancer in Patients With Heart Failure: How Systemic Inflammation Can Lay the Groundwork. Frontiers in cardiovascular medicine 2020, 7.
Israr MZ, Bernieh D, Salzano A, Cassambai S, Yazaki Y, Heaney LM, Jones DJL, Ng LL, Suzuki T. Association of gut-related metabolites with outcome in acute heart failure. Am Heart J. 2021;234:71–80.
Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–52.
van Bilsen M, Patel HC, Bauersachs J, Böhm M, Borggrefe M, Brutsaert D, Coats AJS, de Boer RA, de Keulenaer GW, Filippatos GS, et al. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2017;19(11):1361–78.
Cho IJ, Shin JH, Jung MH, Kang CY, Hwang J, Kwon CH, Kim W, Kim DH, Lee CJ, Kang SH et al. Antihypertensive drugs and the risk of Cancer: a Nationwide Cohort Study. J Clin Med 2021, 10(4).
Barbalata CI, Tefas LR, Achim M, Tomuta I, Porfire AS. Statins in risk-reduction and treatment of cancer. World J Clin Oncol. 2020;11(8):573–88.
Ricco N, Kron SJ. Statins in Cancer Prevention and Therapy. Cancers 2023, 15(15).
Jiang W, Hu J-W, He X-R, Jin W-L, He X-Y. Statins: a repurposed drug to fight cancer. J Experimental Clin Cancer Res. 2021;40(1):241.
Zhou Q, Jiao Z, Liu Y, Devreotes PN, Zhang Z. The effects of statins in patients with advanced-stage cancers - a systematic review and meta-analysis. Front Oncol 2023, 13.
Matusewicz L, Czogalla A, Sikorski AF. Attempts to use statins in cancer therapy: an update. Tumor Biology. 2020;42(7):1010428320941760.
Acknowledgements
We would like to acknowledge the participants and investigators of National Health and Nutrition Examination Survey. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
There was no funding to state.
Author information
Authors and Affiliations
Contributions
C G chose the topic. Z J, B L, Q L provided methodological support. C G completed the subsequent data analysis and article writing. Y H provided guidance and assistance throughout the process. All authors revised the manuscript for important intellectual content, participated in the decision to submit the manuscript for publication, and approved the final submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research has followed the Tenets of the Declaration of Helsinki. Ethical considerations have been rigorously followed, with all data sourced from the publicly available NHANES, which obtained informed consent from all participants and received ethical approval from the NCHS Research Ethics Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ge, C., Jiang, Z., Long, B. et al. Associations between cardiovascular diseases and cancer mortality: insights from a retrospective cohort analysis of NHANES data. BMC Public Health 24, 1049 (2024). https://doi.org/10.1186/s12889-024-18498-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-18498-7