Abstract
Introduction
Despite several strategies exist for anemia prevention and control, it has been the major public health important problem in the world. Numerous immediate and long-term health issues were reported in children who have history of anemia including decreased work productivity in adult hood period. Although analyzing data on burden and risk factors of anemia are the recommended action areas of World Health Organization framework for accelerating anemia reduction, the aggregated national burden and contributors of anemia in Ethiopia has not been determined so far. There for, this systematic and meta-analysis study is aimed to assess the pooled prevalence and associated factors of anemia among children aged 6–23 months in Ethiopia.
Methods
The electronic databases including PubMed, Scopus, EMBASE, Web of Science, Science Direct, Google scholar and institutional repositories were searched using search terms. The studies that reported the prevalence and/or risk factors of anemia in children 6–23 months of age were included. The JBI quality assessment tool was used to evaluate the quality of each study. The data was extracted with Microsoft Excel, 2019 and analyzed with STATA 17.0 statistical software. A random effect model was used to estimate the pooled prevalence of anemia and its associated factors. The Cochrane Q-test statistics and I2 test were used to measure heterogeneity between the included studies. Furthermore, publication bias was examined using the funnel plot graph and statistical tests (Egger’s and begg tests). Outliers also visualized using Galbraith plot. When necessary, sensitivity analysis was also employed to detect small study effect.
Result
Ten studies with a total population of 14, 733 were included for analysis. The pooled prevalence of anemia among children aged 6–23 months of age in Ethiopia was found to be 57.76% (95%CI; 51.61–63.91; I2 = 97.192%; p < 0.001). Having history of diarrhea AOR = 2.44 (95%CI: 1.03–3.85), being stunted AOR = 2.00 (95%CI: 1.38–2.61), living in food insecure house hold AOR = 2.08 (95%CI: 1.10–3.07), consuming less diversified food AOR = 2.73 (95%CI: 2.06–3.39) and being 6–11 months of age AOR = 1.59 (95%CI: 1.23–1.95) were associated with anemia.
Conclusion and recommendation
The prevalence of anemia is in the range of severe public health problem among children aged 6–23 months in Ethiopia. Diarrhea, stunting, house hold food insecurity, dietary diversity, and age were the predictors of anemia. Further, prospective cohort and random controlled trial studies are recommended. Further, random controlled trial especially effectiveness of nutritional education interventions trial is important. To reduce prevalence of anemia, strengthening diarrhea reduction program, securing household food insecurity, preventing stunting, giving special attention for infants age 6–11 months and encouraging food diversification are important.
Similar content being viewed by others
Introduction
Anemia is a strong indicator of overall health and defined as a condition in which the number of red blood cells or the hemoglobin concentration within them is lower than normal and insufficient to meet an individual’s physiological needs [1, 2]. It is one of the public health important problems in both developed and developing nations [3]. World Health Organization (WHO) considered anemia as mild, moderate and severe public health problem when its prevalence is 5-19.9%, 20-39.9% and 40% of the population, respectively [2].
There is geographical difference in anemia distribution. Africa, South-East Asia region, and Eastern Mediterranean region were the major contributor regions of anemia in the globe. Regarding to its burden, 27% of the world’s population were anemic in 2013 [4]. Women in reproductive age and children 6–59 months were the major contributors of anemia in the general population [2, 5]. Another global study on anemia predicted that 40% of all children aged 6–59 months were anemic in 2019 which was 48% in 2000 and 42.6% in 2011 [5, 6]. Anemia accounts 89% of all disability in developing countries [4]. Based on anemia severity 21%, 18% and 1% of children aged 6–59 months had mild, moderate, and severe anemia, respectively in 2019 [7]. A previous study report also showed that the burden is highest in Central and Western Sub-Saharan Africa(SSA) [4]. In East Africa, the prevalence of anemia was reported as 53% in 2019 [7]. According to the global burden disease report 8.2 million, 10 million, 1.02 million in 1990 and 12.35 million, 14.39 million, 1.28 million in 2013 children were with mild, moderate, and severe anemia, respectively in Ethiopia [4]. The Ethiopian Demographic and Health Survey (EDHS) report also described that 57% of the children under the age of 5 years were anemic in 2016 [8]. Anemia levels were highest in those aged 6–23 months, with 72% of these children having anemia [8]. Even though iron‐deficiency remains the dominant cause of anemia in all populations33%, 40% and 42% of non-pregnant women, pregnant women, and children were affected by anemia worldwide, respectively [4]. Hemoglobinopathies, infections, chronic kidney diseases, gastrointestinal disorders, and gynecological issues are also the other more significant causes of anemia [4, 9]. World Health Organization considered, the cause of anemia in area where prevalence of anemia greater than 40% were multifactorial, no single cause responsible for anemia [10]. During early childhood period, iron needs due to rapid growth, inadequate iron intake due to exclusively breastfed without iron supplementation, inadequate dietary iron intake, prematurity (low iron store), low availability of dietary sources of iron secondary to low socioeconomic status and dietary restrictions were the possible causes of anemia [11, 12].Despite inconsistency among existing studies, several factors were associated with high prevalence of anemia among children aged 6–23 months in Ethiopia. Age, food insecurity, poor dietary diversity, time of complementary feeding initiation (late or early), diarrhea, cow milk initiation before one-year, poor breast-feeding practice, having poor wealth index, maternal age and educational status were the predictors of anemia in this age group [13,14,15,16,17,18,19,20].
Numerous immediate and long-term health issues affect children who have or have history of anemia. In anemic children, the oxygen-carrying capacity of blood is low which leads to lack of oxygen supply to vital organs including the fast-growing children’s brain and heart [3, 11]. Anemia negatively affects mental, motor, and cognitive development, which in turn lead to social withdrawal, attention deficit, poor growth, and impaired school performance (learning difficulties) of children which may be irreversible, especially in children less than 2 years, despite adequate therapy [11, 21]. In severe cases, anemia in children is ends with heart failure and death [11, 22]. the harmful impact of anemia is not only to young children but also it hits women who are pregnant or planning a pregnancy [4]. Anemia has also a tendency to hinder behavioral development and raising the likelihood of illness including high risk of infection [3, 21, 22]. Anemia can also reduce work productivity later in life [3, 23]. In the long run, children who were anemic during infancy had a discrepancy of 5–10 points on intelligence tests and other cognitive performance examinations in school than children who were not anemic.
Several strategies exist for anemia prevention and control. These include improvement of dietary intake, food diversification, food fortification, supplementation with iron and other micronutrients, appropriate infectious disease prevention and control, delayed cord clamping and health education [23]. Optimization of maternal nutritional status can also decrease inter-generational iron depletion anemia during infancy [3]. Despite implementation of several strategies, a significant number of children have been living with anemia.
Although high proportion of anemia reported in infants and preschool children in the previous global study [24], the burden of anemia among children less than 2 years has not been determined in Ethiopia. In addition, analyzing data on cause and risk factors of anemia is one of the action areas of WHO framework for accelerating anemia reduction. Therefore, having knowledge about the pooled prevalence and risk factors of anemia in the population (children 6–23 months) helps to decide on appropriate interventions, resource mobilization and monitor and evaluate the impact and safety of public health programs. It may also contribute to the national strategy for child health and wellbeing. Furthermore, it informs policy by providing information to health practitioners, policy makers and other stake holders that will provide appropriate measures to improve the health services particularly to childhood anemia in the study area.
Research question
What is the national burden of anemia among children aged 6–23 months in Ethiopia?
What are the risk factors of anemia among children aged 6–23 months in Ethiopia?
Objective
To perform a systematic review of available research literature regarding the prevalence and associated factors of anemia among children aged 6–23 months in Ethiopia, 2023.
Specific objective
To estimate the pooled prevalence of anemia among children aged 6–23 months in Ethiopia.
To determine the risk factors that exposed children aged 6–23 months for anemia in Ethiopia.
Methods
Study design and setting
All the studies which have been conducted on anemia among children aged 6–23 months in Ethiopia were cross-sectional by study design. There is inconsistency in prevalence report and controversies in reporting the risk factors that exposed children for anemia. In addition, cross-sectional studies are lower in evidence generation than systematic review. Systematic review and meta-analysis are driven by evidence-based medicine movement and Cochrane collaboration. They are advantageous in reducing bias, replicability, in resolving controversies, and in providing reliable basis for decision makings than cross-sectional studies. Therefore, in this systematic review and meta-analysis, the authors reviewed studies conducted on anemia among children aged 6–23 months in Ethiopia to estimate the pooled prevalence of anemia and to resolve the existing controversies between studies. Cochrane Handbook for Systematic Reviews of Interventions recommends at least 10 studies for publication bias assessment. Only two studies may be enough to do a meta-analysis study. Cochrane Handbook for Systematic Reviews of Interventions also recommends at least two studies to run forest plot [25]. The Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guideline was used to review the existing article about anemia in the population described [26]. Ethiopia is one of the east African countries situated in the horn of Africa having a total population of 117.88 million, 58.98 million males and 58.90 million females in 2021 [27]. By age distribution, nearly half of Ethiopians are under age 15 (47%), while 4% are age 65 and older [8]. In 2021, Ethiopia will have 3.65 million births. That is 10,014 per day, ranking eighth. accounting for 22.17% of the total population. In terms of urbanization, it is also placed 219th with the urban population of 25.59 million [27]. Administratively, it has 10 regions and two administrative cities [8].
Searching strategy
The presence of systematic review protocol on the topic of prevalence and associated factors of anemia among children aged 6–23 months in Ethiopia was checked via searching different databases including PubMed, Scopus, EMBASE, Web of Science, ScienceDirect, Google scholar, the national health center review and institutional dissemination databases and Prospero for systematic review. As far as our search strategy is concerned, there was no systematic review conducted on our topic of interest. Thus, the actual search was conducted from March 2, 2023 to May 14, 2023. In order to minimize time-lag bias, the searching process was updated on June 15, 2023. Our search strategy focused on all published and unpublished studies with epidemiological data on the prevalence and associated factors of anemia among children aged 6–23 months in Ethiopia without restriction for publication year. key terms: children, anemia and Ethiopia with combinations of MeSH terms were used as searching strategies. These key terms were combined using Boolean operators “AND/OR’’ to narrow the search in the databases. The searching using Boolean operators in PubMed was(“Prevalence OR Frequency OR proportion”) AND (“Associated Factors” OR “determinants” OR “risk factors” OR “causes”) AND (“anemia” OR “anaemia”) AND (“child**”OR “pediatric**”OR “paediatric**”) AND “6–23 month**”) AND “Ethiopia” and Science direct and google scholar “Prevalence ”AND (“Associated Factors” OR “determinants”) AND “anemia” AND “children”AND “6–23 month”) AND “Ethiopia”were used. In addition, the electronic searching strategy was supplemented by manual searches to identify relevant unpublished studies. Search terms used included “Anemia”, “young children” or “infant” or “children aged 6-23month”,” and “Ethiopia.” The reference lists of included studies were also screened for the presence of additional studies. Three authors (MA, DT and AK) conducted the searching process. References were managed using End Note software version X8. This systematic review and meta-analysis study was not registered with PROSPERO.
Study selection and eligibility
The three authors independently and meticulously appraised the contents of each of the identified article (B.B.A., A.T.A. and A.T.). The retrievals were appraised for inclusion in the final review by using their titles and abstracts. Finally, the appraisal process completed by reviewing full text of the papers. Those studies which met the following inclusion criteria were considered to be included in the study.
Inclusion criteria
Population
Studies carried out in children aged 6–23 months.
Study design
All observational studies (all article or publication reporting prevalence of anemia and/or associated factors were considered for inclusion).
Study area
Those studies conducted only in Ethiopia.
Study design and outcome definition
Original studies and government surveys, EDHS which reported at least the prevalence and/or one associated factors of anemia among children aged 6–23 months in Ethiopia were included. Studies defined anemia as hemoglobin levels of < 11 g/dl, and for duplicate publications, only the most recent, comprehensive publication with the largest sample size were included.
Language
only studies reported in English language were included.
Publication year- all articles published before June 23,2023 were included.
Exclusion criteria
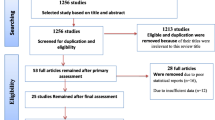
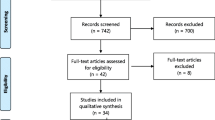
The four reviewers (S.D.H, B.B., K.M. and A.B) performed the selection of the studies independently and blindly after thorough screening of the abstracts and the full texts of the studies. Methodologically flawed articles were excluded. Any differences arose throughout the review process were settled by consensus, and if they persisted despite debate, a third party was consulted to resolve the issue. Review studies, commentaries were also excluded. In addition, studies conducted in a population with hemoglobinopathies like sickle cell anemia and with no information on the tools used to diagnose anemia were also excluded. The processes of identifying, screening and including or excluding records were done using the PRISMA flow diagram guideline (Fig. 1).
Publication condition
Studies which meet the eligibility criteria were included regardless of their publication year and publication status (published, unpublished and grey literature, etc.)
Quality assessment
The quality of the included studies were independently assessed by three authors (A.K., M.A., and G.K.) using the Joanna Briggs Institute (JBI) critical appraisal tool for cross-sectional studies. We categorized the overall quality scores as low quality (high risk of bias), moderate quality (moderate risk of bias), and high quality (low risk of bias) for scores of < 50, 50–75, and > 75%, respectively [28]. The appraisal tool has the following 8 criteria: (1) inclusion criteria, (2) description of study subject and setting, (3) valid and reliable measurement of exposure, (4) objective and standard criteria used, (5) identification of confounder, (6) strategies to handle confounder, (7) outcome measurement, and (8) appropriate statistical analysis. Studies were considered low risk whenever fitted to 50% and/or above in the quality assessment checklist criteria [28, 29].
Outcome
The primary outcome variable of this review was prevalence of anemia. The outcome was measured based on the proportion of children aged 6–23 months with their hemoglobin level less than 11 g/dl. The prevalence of anemia reported in different studies were presented by pooling the prevalence reported in each included article. To take the study-specific true effects across the included studies into consideration, the random effect meta-analysis model was employed. A random effects model for the reported proportion was used to present the pooled prevalence of anemia. Furthermore, different factors that are reported which affect the prevalence of anemia were also identified. These factors were presented using a narrative synthesis through generation of different themes with their pooled effect size (adjusted Odds Ratio, AOR).
Data analysis
The pooled prevalence of anemia with 95% confidence intervals (CIs) was calculated using a random-effects model. In addition, the Cochran’s Q test was utilized to assess the significance of the heterogeneity (p≤0.05 was considered as statistically significant). I -Squared (I2) values of < 25%, 25–50%, 50.01-75% and > 75% were interpreted as a low, medium, and high heterogeneity, respectively. To identify publication bias, funnel plots were created and then applied Egger’s test. Publication bias was declared when the Egger’s and begg test became significant or p-value<0.05. Next, a Galbraith plot was formed to locate any studies that had statistical outliers.
Result
Study selection
A total of 187 studies were identified using electronic searches (through Databases searching (n = 187)) that were conducted up to June, 2023. Of the articles accessed, the duplicated studies were handled using both End Note software version X8 reference manager software and manual comparison of titles/abstracts. After combining all reference from all data bases, using ‘find duplicates’ from End Note software version X8 “reference” menu bar, the duplicated references were removed. After duplication removed, a total of 94 articles remained (93 duplicated). Then, 94 studies were screened using their title and abstract for full-text review. Of the articles screened, 18 were excluded because they are not our population interest (their study extended to children age > 23 month), not conducted in Ethiopia (n = 41), not about human(n = 7) and about specific anemia (iron, megaloblastic…) (n = 18). The rest 10 articles were checked for their quality. Fortunately, all 10 articles with (n = 14, 733 study participants) fulfil the quality assessment (no article left due to quality assessment). The authors considered them for the prevalence and/ or associated factors analysis (Fig. 1).
Characteristics of the studies
Out of 10 eligible studies two of them were conducted in similar population and season [14, 15]. Among the two studies, the one which has small sample size, Gebrehaweria Gebremeskel M et al [14] was not considered in the analysis. The characteristics of included studies were described in Table 1 [13,14,15,16,17,18,19,20]. Four studies were Ethiopia Demographic Health Survey [14, 15], 2 in Amhara [17, 20], 3 in Oromia [18, 19, 30] and 3 in SNNPE [13, 16, 31]. Of the total population 14, 733, the major contributor of the population was EDHS (9878) followed by 1992 from SNNPE, 1920 from Oromia and 943 from Amhara. All studies were cross-sectional in study design and 1 institutional and 9 community-based studies by setting. The studies were published from 2015 to 2022. The number of participants included in the studies, ranging from 216 [30] to 2970 [15] (Table 1). The population age distribution of the included studies was similar, ranged from 6 to 23 months. Although, Woldie, H. et al [20], Sorsa, Aet al [18] and Heinrichs, H. et al [15] did not report the mean age, it ranged from 11.58 ± 2.75 [19] to 14.7 ± 5.1 months [17]. The reported prevalence of anemia ranged from 44.4% [18] up to 72.3% [14] across the included studies (Table 1). The discrepancy between number of eligible study and number of studies listed in the table (Table 1) is due to one study report. From which three rounds of EDHS report which are in one article were considered for analysis [15].
Prevalence of anemia
As described in the study characteristic section of this paper, study conducted by Heinrichs et al. splited as 2005, 2011 and 2016. Study conducted by Gebrehaweria Gebremeskel M. et al. (EDHS 2016) had small sample size as compared to study done by Heinrichs, H. et al. (EDHS 2016) so that study conducted by Gebrehaweria Gebremeskel M et al. was not considered in the pooled prevalence analysis. Most of the studies (n = 10) had reported the prevalence of anemia [13,14,15,16,17,18,19,20, 30, 31]. The random-effects model analysis from those studies revealed that, the pooled prevalence of anemia in Ethiopia was found to be 57.76% (95%CI; 51.61–63.91; I2 = 97.192%; p < 0.001) (Fig. 2). I2 (97.81%) indicated high heterogeneity. Galbraith plot did not show any significant outlier study (Supplementary Fig. 1).
Subgroup analysis
The subgroup analysis was done through stratification by region. Based on this, the prevalence of anemia among the study participant was found to be 57% in Amhara, 69.07% in EDHS, 56.88% in SNNPE, 47.88% in Oromia regions (Supplementary Fig. 2).
Sensitivity analysis
We employed a leave-one-out sensitivity analysis to identify the potential source of heterogeneity in the analysis of the prevalence of anemia with in the study population in Ethiopia. The results of this sensitivity analysis showed that our findings were not dependent on a single study. The estimated pooled prevalence of anemia varied between 56.31(95% CI, 50.33–62.29) [15] and 59.14 (95% CI, 52.00-66.28) [18] after deletion of a single study (Supplementary Fig. 3).
Publication bias
We had also checked publication bias using a funnel plot. It did not show any obviously observed publication bias (Fig. 3). Egger’s regression and begg test p-value were 0.11 and 0.44, respectively, which also indicated the absence of publication bias.
Factors associated with anemia
All of the included [13,14,15,16,17,18,19,20] except two studies [30, 31] reported at least one factor associated with anemia among children aged 6–23 months in Ethiopia. Even though studies reported that being male, being underweight, poor wealth quantile and early complementary feeding as a significant predictor of anemia in children aged 6–23 months of age; the pooled effect size of these factors did not show any association with anemia. The pooled estimate of having history of diarrhea, being stunted, poor dietary diversification, living in food insecure house hold and being aged between 6 and 11 months were significant predictors of anemia in children 6–23 months of age. For further information, the significant variables were discussed in detail here in the coming sub-topics.
History of diarrhea
Three studies found significant association between diarrhea and anemia. Of these, the highest and lowest risk factors for children aged 6–23 month with diarrhea were AOR = 4.9 (1.63, 4.9) Woldie, H. et al. [20] and AOR = 1.7(1.18, 2.44) Tegegne, M. et al. [19], respectively, compared to those who had no diarrhea. The forest plot combining result of three studies showed that the overall estimate of AOR of history of diarrhea was 2.44 (95%CI: 1.03–3.85); I2 = 56.12%; P = 0.001). I2 and P-value showed moderate heterogeneity (Fig. 4).
Regarding to publication bias, the funnel plot analysis showed symmetrical distribution (Supplementary Fig. 4). The Galbraith plot also did not show any outlier (Supplementary Fig. 5). During the Egger’s regression and begg test, the p-value was 0.17 and 1.00, respectively, which indicated the absence of publication bias. Additionally, we employed a leave-one-out sensitivity analysis to identify the potential source of heterogeneity in the analysis of the pooled estimate of history of diarrhea as a risk factor for anemia. The results of this sensitivity analysis showed that pooled effect size was not dependent on a single study. The pooled estimate of history of diarrhea varied between 1.73(95%CI, 1.10–2.36) and 3.35(95%CI, 1.93–4.77) after deletion of a single study (Supplementary Fig. 6).
Children with stunting
A total of three studies were reported a significant association between stunting and anemia [17, 19, 20]. Among the significant studies, the highest and lowest risk factors for anemia, in children with stunting were AOR = 2.7 (1.2, 6.05) Woldie, H. et al. [20] and AOR = 1.88(1.31, 2. 7), Tegegne, M. et al [19], respectively, compared to those who had no stunting. The forest plot result of three studies showed that the overall estimate of AOR of stunting was 2.00 (95%CI: 1.38–2.61); I2 = 0.0%; P = 0.001). I2 and P-value showed homogeneity of the studies (Fig. 5). Regarding to publication of bias, the funnel plot analysis showed symmetrical distribution (Supplementary Fig. 7). The Galbraith plot also did not show any outlier (Supplementary Fig. 8). During the Egger’s regression test, the p-value was 0.45, which indicated the absence of publication bias. The begg test also support absence of publication bias, p-value = 0.30.
House hold food insecurity
Malako, B.G. et al., Molla et al., and Tegegne, M et al. were reported a significant association of house hold food insecurity and anemia in children aged 6–23 months of age [16, 17, 19]. Among the significant studies, the highest and lowest risk factors for anemia in children living in food insecure house hold were AOR = 2.74 (1.62, 4.65), Malako, B.G. et al. [16] and AOR = 1.44(1.01, 2. 04), Tegegne, M. et al [19], respectively, compared to their counter parts. The heterogeneity test for house hold food insecurity, the combining result of three studies forest plot revealed that the overall estimate of AOR was 2.08 (95%CI: 1.10–3.07); I2 = 0.0%; P = 0.001) (Fig. 6). I2 and P-value showed homogeneity of the studies. Regarding to publication bias, the funnel plot analysis showed symmetrical distribution (Supplementary Fig. 9). The Galbraith plot also did not show any outlier (Supplementary Fig. 10). During the Egger’s regression and begg test p-value were 0.03 and 1.00, respectively; which indicated the presence of publication bias. Thus, we employed a leave-one-out sensitivity analysis to identify the possible source of bias in the analysis of the pooled estimate of house hold food insecurity as a risk factor for anemia. The results of this sensitivity analysis disclosed that our findings were depend on a single study, Tegegne, M. et al [19] (Supplementary Fig. 11). Our pooled estimate of house hold food insecurity varied between 1.88 (95%CI, 0.7–3.06) and 2.72(95%CI, 1.67–3.77) after deletion of a single study.
Dietary diversity
Malako, B.G. et al., Molla et al., Woldie, H. et al. and Tegegne, M. et al. were stated a significant association of dietary diversity and anemia in children aged 6–23 months of age [16, 17, 19, 20]. Among the significant studies, the highest and lowest risk factors were AOR = 3.2 (1.35, 7.38), Woldie, H. et al. [20] and AOR = 1.4(1.03, 1.92), Menaseb Gebrehaweria, et al. [14], respectively, compared to those who took diversified food (Fig. 7). The heterogeneity test for dietary diversity, the combining result of three studies forest plot presented the overall estimate of AOR was 2.73 (95%CI: 2.06–3.39); I2 = 0.0%; P = 0.001) (Fig. 7). I2 and P-value showed homogeneity. Regarding to publication of bias, the funnel plot analysis showed symmetrical distribution (Supplementary Fig. 12). The Galbraith plot also did not show any outlier (Supplementary Fig. 13). During the Egger’s regression test, the p-value was 0.81, which indicated the absence of publication bias.
Age
Gebrehaweria Gebremeskel M et al., et al., Woldie, H. et al., Sorsa, A. et al., Heinrichs, H. et al. and Tegegne, M et al. were described a significant association of age 6–23 months and anemia in children aged 6–23 months of age [14, 15, 18,19,20]. The highest and lowest risk factors for children aged 6–11 months were AOR = 9.6 (3.61, 25.47), Woldie, H. et al. [20]and AOR = 1.31(1.07, 1.63), Gebrehaweria Gebremeskel M et al [14], respectively, compared to those whose age ranged from 18 to 23 months among the significant studies (Fig. 8). The heterogeneity test for the combining result of three studies forest plot showed that the overall estimate of AOR for children aged 6–11 months was 1.59 (95%CI: 1.23–1.95); I2 = 50.15%; P = 0.001). I-squared and P-value showed heterogeneity of the studies (Fig. 8). Regarding to publication of bias, the funnel plot analysis showed asymmetrical distribution (Supplementary Fig. 14). The Galbraith plot also showed an outlier (Supplementary Fig. 15). During the Egger’s regression test, the p-value was 0.01, which indicated the presence of publication bias. Thus, we employed a leave-one-out sensitivity analysis to identify the potential source of heterogeneity in the analysis of the pooled estimate of 6-11months of age as a risk factor for anemia. The results of this sensitivity analysis showed that our findings were depend on single study (Supplementary Fig. 16). Our pooled estimate of age 6–11 months varied between 1.45(95%CI, 1.17–1.74) and 1.75(95%CI, 1.3–2.2) after deletion of a single study. Then, subgroup analysis was done using study setting. The pooled estimate of AOR for community-based study was 1.75 (95%CI, 1.3–2.2), I2 = 37.11% and p = 0.001 and AOR for health institution was 1.31 (95%CI, 1.03–1.59) (Fig. 8).
Discussion
This systematic and meta-analysis study included 10 studies to assess the pooled prevalence of anemia and associated factors among children 6–23 months of age in Ethiopia which were published from 2015 to 2022. As far as our knowledge is concerned, this systematic and meta-analysis study is the first of its kind in Ethiopia among children aged 6–23 months.
In this study, the pooled prevalence of anemia among children aged 6–23 month was 57.76% which is in the range of WHO severe public health problem [32]. The pooled prevalence is much higher than a previous study done in China [33] and Romania [34]. The discrepancy might be due to socio-economic (absence of iron supplementation in Ethiopia but in China,Ying Yang Bao program which is a free government micronutrient supplementation program that has been in operation since 2009 to improve the health of children in China’s disadvantaged rural communities [35]. High parasitic infestation and inappropriate complementary feeding initiation (early cereal based feeding prolonged milk feeding) were also the possible contributors of discrepancy [10]. A previous study in Latin America and the Caribbean countries also reported that the prevalence of anemia is higher in preschool children than school age children [22]. Similarly, a higher proportion of anemia was also reported in children 1–2 years of age in United States of America [36]. Additionally, the prevalence of anemia is also higher than the previous systematic and meta-analysis study among children aged 6–59 months done in Ethiopia [37]. This discrepancy might be due to growing tissues need an absolute increase in iron content to maintain an iron repletion state, young children’s blood volume must expand, their muscle and tissue mass must rise, and their neurodevelopment and brain development are at a critical stage compared to old child. In addition, iron requirement decreased at the age of third year secondary to decreased growth velocity [10]. This implies that children in early childhood period requires anemia screening as early as possible and the key population, children under 2 years old need to be considered to combat anemia. Furthermore, low amounts of important minerals such as iron, zinc, and folate, as well as vitamin A and B12 in the anemic mother’s breast milk, may affect the hemoglobin level of the breastfeeding child [38]. On the other side, the prevalence of anemia in the population of interest is lower than the previous study, standardized demographics health survey of multiple countries report of anemia (70%) [39]. The discrepancy might be due to methodology including sampling and quality of sample collection in demographic health surveys. Another study report in Myanmar reported that 77.2% of the children aged 6–23 month had anemia which is higher than this study finding [40]. Genetic, ethnic, and geographical lifestyle factors may all interact and can contribute for the discrepancy.
Having history of diarrhea, being stunted, living in food insecure house hold, using less dietary diversity and children aged 6–11 monthswere significantly associated with anemia prevalence.
The odds of anemia among children having history of diarrhea were 2.44 times more likely to develop anemia than children not having diarrhea. A previous studies also reported a similar finding [41]. In normal physiology, iron is absorbed in the proximal doudneum. The increased peristalisis of gastro intestinal system during diarrhea may reduce nutrient absorbtion including iron. Iron distribution regulation functions as an innate defense response against invading microorganisms may also contribute for iron deficiency related anemia. Even in the absence of infection, certain aspects of human iron metabolism guarantee that pathogenic microbes have little access to iron. First, in humans, the majority of iron is sequestered intracellularly, complexed within hemoglobin within erythrocytes. As a result, several diseases have evolved ways to free hemoglobin by lysing erythrocytes and extracting iron from heme. Hemolytic pathogens, on the other hand, must contend with haptoglobin and hemopexin, two host glycoproteins that scavenge freed hemoglobin and heme, respectively [42, 43]. The other hypothesis for anemia secondary to infection is linked to effects of various cytokine mediators of inflammation, such as tumor necrosis factor (TNF), interleukin (IL-1 and IL-6), and the interferons (IFNs)-IFNγ. Neocytolysis (selective hemolysis of the youngest red cells) appears to be a consequence of relative or absolute erythropoietin deficiency. Although mediators of inflammation including cytokines causes anemia secondary to infection, hepcidin is the most significant mediator in the pathogenesis of anemia. It promotes macrophage iron retention by causing internalization of the iron export protein, ferroprotein.
Furthermore, those stunted children age 6–23 months of age were also 2.00 times more likely to be anemic than their counter part. The finding was supported by previous studies [44, 45]. Since, all of the studies included in this study were cross-sectional, they did not show the real causal relationship between anemia and stunting. However, the interrelationships between diet and anemia in young infants may be explained by iron metabolism and the innate immunological response to infection. Iron is an important but harmful component in the human body due to its ability to exist in one of two oxidation forms, ferrous (Fe2+) or ferric (Fe3+). In the ferrous state, iron serves as an electron donor, while in the ferric state, it works as an electron acceptor. Iron’s redox potential can produce reactive oxygen species, which can then produce free radicals that damage lipids, DNA, and protein, especially under conditions of iron excess [46]. Another hypothesis, in low-resource settings, where newborn iron stores may be suboptimal and there is reduced access to food including iron-rich foods which may leads to stunting [47]. In addition, intrauterine growth restriction and preterm delivery, both of which contribute to LBW and small size at birth [47]. Those neonates born prematurely have a tendency to low iron stores and short stature [10, 47, 48].
Similarly, food-insecure children were 2.08 times more likely to develop anemia than secured once. The Food and Agriculture Organization [49] definition of household food insecurity has two broad components: insufficient access to a nutritionally adequate and safe food supply and underutilization of these foods by household members. The access part comprises three main domains: “anxiety and uncertainty about household food supply, inadequate quality of food, and insufficient food to eat by household members” [50, 51]. The utilization component is affected mostly by nutritional knowledge and beliefs; however, access to healthcare, water, sanitation services, hygiene and childhood illness management are also factors [49]. The negative effects of household food insecurity are: decreased food consumption, which comprises of reduced dietary variety and nutrient intake, and under nutrition of household members including micronutrient deficiencies. Children in food-insecure households typically consume diets high in refined sugars and deficient in minerals including iron which result in anemia [52, 53]. In addition, lack of adequate iron consumption; a diet lacking in micronutrients that may facilitate iron absorption and utilization such as vitamin C, vitamin A, folate, vitamin B-12, and carotenoids and consuming foods high in phytic acid, which may reduce iron absorption [52]. Furthermore, previous research on house hold food insecurity and micronutrient deficits in the United States suggests that diets in food insecure households are lower in iron and other micronutrients and higher in carbohydrate and fat [54]. In addition, food insecurity may lead to anemia due to a lack of nutritious diets with high protein quality, enough micronutrient content and bioavailability, macro minerals, iron, and critical fatty acids in children from food insecure households, which increases the likelihood of childhood anemia [55]. Generally, household members live in food insecure house hold obligated to consume less diversified foods [16].
Children who received less diversified food were more likely to be anemic than those who received diversified one. The finding was supported by studies done in Ghana [56] and China [57]. WHO defined minimum dietary diversity as the proportion of children aged 6–23 months who received foods from at least four out of seven food groups in a 24 h time period [58].
Children whose age 6–11 month were 1.59 times more likely to develop anemia as compared with children whose age 18–23 months. Breast milk has inadequate iron to meet the nutritional iron need of growing infant [59]. Provision breast milk alone coupled with rapid iron depletion beyond six months also increases risk of anemia for younger infant. The main source of iron for young infant is their iron store during intra uterine life. After birth, they are prone for iron deficiency due to the increased postnatal iron required to facilitate rapid growth and an earlier onset of erythropoiesis, which occurs 1-3months earlier in preterm than term infants [12]. Unless appropriate complementary feeding is initiated and iron supplemented for preterm and risk term, the existing iron store become gradually depleted and fail to meet the demand. Iron supplementation is recommended after 1 months of age in preterm and 4 month of age for term infants, especially those infants live in anemia prevalent area [10, 60]. This study finding was supported by previous studies [33, 61, 62]. This might be due to increased nutrition demand for growth at late infant(6–11 months) age [63], maternal anemia, early cord clamping prenatal iron store depletion and poor knowledge of care givers in complementary feeding composition (iron poor food) and complementary feeding initiation (being late or early) [64,65,66]. American academic of pediatrics also reason out that the amount of iron lost was added to the amounts of iron necessary for increased blood volume, increased tissue mass, and storage iron throughout the age group of 6–12 months, mostly from sloughed epithelial cells from the skin and the intestinal and urinary tracts [67]. Being born from mother with maternal anemia may exposed young infants early for anemia due to low iron store [10]. This implies that anemia screening and iron reach food or supplementation requirement with in the first year of life.
Limitation of study
Since, all of the included studies were cross-sectional, the result cannot show the real cause-effect relationship between anemia and identified factors. In Ethiopia, there is variation in demography (low land and high land) and living standard (pastoralists and agrarian). These may contribute for variety in food composition and accessibility. Since, most of the included studies except EDHS were from only three productive administrative regions, the pooled prevalence of anemia may be under estimated in Ethiopia. As a result, it is difficult to represent the true effect within the country because research from more than half of the areas were scarce. The use of varied data collection tools in the included research may have an impact on generalizing the findings. Furthermore, the study’s pooling of prevalence and odds ratio despite significant variability is one of its limitations. Even though no language exist other than English is available for health and health related article publications in Ethiopia, restriction of searching to English language may be the limitation of this study.
Conclusion
According to this study report, the prevalence of anemia is high as compared to the world health organization anemia grading. Diarrhea, stunting, house hold food insecurity, dietary diversity, and age were the predictors of anemia. Anemia has a tendency to hinder the children capacity for learning, psychomotor development, cognitive development, behavioral development, physical growth, and raising the likelihood of illness including high risk of infection and work productivity later in life. To halt this effect of anemia, strengthening diarrhea reduction program, securing household food insecurity, preventing stunting, giving special attention for infants and encouraging food diversification are important.
Recommendation
An interventional random control trial is needed to determine cause-effect relationship of anemia and predictors. Since Most iron-rich foods are available in Ethiopia and iron deficiency is most cause of anemia in children, effectiveness of nutritional education interventions trial is recommended.
Strengthening nutritional intervention including appropriate complementary feeding composition and initiation, anemia screening during infancy period, nutritional education programs including dietary diversification, encouraging hygiene and sanitation practices, prevention of anemia in pregnant women and launching iron supplementation program based on World Health Organization guideline could help to prevent childhood anemia in Ethiopia and possibly reduce the prevalence. In addition, infants (preterm and risk term at one month and four months of birth, respectively) require a special consideration for iron supplementation program to prevent anemia. The health care providers need to screen children age 6–23 months for anemia who presents with diarrhea.
Furthermore, care givers need to be encouraged to include iron reach foods in the complementary food preparation like lentil, soybean, spinach, meat etc. In the same way, they need to be aware about hinders of iron absorption like tea which contain tannin.
Lastly, since anemia has no single cause, it requires the involvement individual to community at large to reduce the burden and its sequels. This can be achieved through strengthening frequent communication using the most accessible media to create awareness on importance of food diversity, infant and young children feeding, producing nutrient rich agricultural products, health seeking behaviors (early detection of chronic infection) of the community and working on food insecurity in collaboration with health care and agricultural stakeholders targeted to anemia reduction.
Implication to policy
Evidences suggest that the prevalence of anemia is very high among children aged 6–23 months. The finding of this study, anemia prevalence 57.76%, strengthen the previous studies report on prevalence of anemia in children aged 6–23 months. If children aged 6–23 months are harmed by nutrition-related illness; technological innovation, work productivity, discovery, and societal advancement cannot be expected. Even though, 2 mg per day after 1 month of age for preterm and 1 mg per day after 4 months for term neonate iron supplementation is recommended by American Academy of Pediatrics which requires commitment of programmers working on the areas and the government to launch iron supplementation program and mobilize resource for preterm and risk term neonates. all alternative intervention need to be implemented as soon as feasible to prevent anemia in children. This could be accomplished through strengthening training on infant and young children feeding practice for front line health care workers and health education for care givers, launching anemia screening at late infancy age and education on food diversification to the community, education on complimentary feeding composition and timing, conducting a serious of effectiveness of nutritional education interventions and strengthening maternal anemia prevention programs (malaria prevention and iron supplementation). Generally, the following points can be recommended as priority action areas to reduce the prevalence of anemia and its consequence: Conducting a study on effectiveness of nutritional education interventions trials at national level; launching iron supplementation program for all preterm neonate and risk term neonate (like born from mother with anemic, in food insecure household, with diabetic) and due to multifactorial nature of anemia causes, launching anemia screening program at late infancy period may reduce the long-term impact of anemia. This could be integrated with measle vaccination. The implementation of these anemia reduction program may require commitment of the government, donors, high resource mobilization and inter sectoral collaboration like health sector and agricultural sectors. However, anemia prevention and control programs may face several challenges from the side of policy maker(political priority, poor coordination of resources to support multisectoral response and less financial commitment), resource-human and financial (challenge of integration of anemia control program into existing programs, poor awareness of need for anemia prevention and control) and directly the beneficiary-care givers (lack of education and information about anemia prevention, restricted financial access to participate in anemia control and prevention strategies (iron-rich food sources, iron supplements, health services, etc.), poor awareness of benefits of interventions and poor compliance with iron supplementation).
Implication to further research
The meta-analysis found that the prevalence of anemia was quite high among children aged 6–23 months and the primary reasons for anemia burden were identified. However, the included studies were too diverse, and cross-sectional research also failed to demonstrate a temporal association between anemia and its factors. In addition, the measurements of exposure and their effect on the outcome of this study were made at the same time. It is not easy to assess the reasons for existing directions of associations. The study finding may also be prone for mediator and confounding variables effect. As a result, there is a potential for reverse causality of the identified factors. So, prospective studies assessing the predictors and the outcomes of anemia in children 6–23 months such as effectiveness of nutritional education interventions trials are required by stratifying the possible confounding and mediator variables.
Data Availability
All relevant data are available within the paper. In addition, the corresponding author will provide anonymized data upon reasonable request from researchers who require more information.
References
Irwin J, Kirchner JT. Anemia in children. Am Family Phys. 2001;64(8):1379.
Organization WH. Accelerating anaemia reduction: a comprehensive framework for action 2023.
Balarajan Y, et al. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–35.
Kassebaum NJ, Collaborators GA. The global burden of anemia. Hematol Oncol Clin N Am. 2016;30(2):247–308.
Stevens GA, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000-19: a pooled analysis of population-representative data. Lancet Glob Health. 2022;10(5):e627–39.
Organization WH. How together we can make the world’s most healthy and sustainable public food procurement, in How together we can make the world’s most healthy and sustainable public food procurement. 2022.
Stevens GA, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–19: a pooled analysis of population-representative data. The Lancet Global Health. 2022;10(5):e627–39.
EDHS, Ethiopian Demographic Health Survey 2016.
Safiri S, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the global burden of Disease Study 2019. J Hematol Oncol. 2021;14(1):1–16.
Organization WH. Guideline daily iron supplementation in infants and children. World Health Organization; 2016.
Akalu Y, et al. Iron-rich food consumption and associated factors among children aged 6–23 months in sub-saharan Africa: a multilevel analysis of demographic and health surveys. PLoS ONE. 2021;16(6):e0253221.
McCarthy EK, Dempsey EM, Kiely ME. Iron supplementation in preterm and low-birth-weight infants: a systematic review of intervention studies. Nutr Rev. 2019;77(12):865–77.
Alemayehu M, et al. Prevalence and correlates of anemia among children aged 6–23 months in Wolaita Zone, Southern Ethiopia. PLoS ONE. 2019;14(3):e0206268.
Gebrehaweria Gebremeskel M, Lemma Tirore L. Factors Associated with Anemia among Children 6–23 months of age in Ethiopia: a multilevel analysis of data from the 2016 Ethiopia demographic and Health Survey. Pediatr Health Med Ther. 2020;11:347–57.
Heinrichs H, et al. Anaemia and its determinants among young children aged 6–23 months in Ethiopia (2005–2016). Matern Child Nutr. 2021;17(2):e13082.
Malako BG, Teshome MS, Belachew T. Anemia and associated factors among children aged 6–23 months in Damot Sore District, Wolaita Zone, South Ethiopia. BMC Hematol. 2018;18:14.
Molla A et al. Prevalence of Anemia and Associated Factors among Infants and Young Children Aged 6–23 Months in Debre Berhan Town, North Shewa, Ethiopia J Nutr Metab, 2020. 2020: p. 2956129.
Sorsa A, Habtamu A, Kaso M. Prevalence and predictors of Anemia among children aged 6–23 months in Dodota District, Southeast Ethiopia: A Community-based cross-sectional study. Pediatr Health Med Ther. 2021;12:177–87.
Tegegne M, Abate KH, Belachew T. Anaemia and associated factors among children aged 6–23 months in agrarian community of Bale zone: a cross-sectional study. J Nutr Sci. 2022;11:e96.
Woldie H, Kebede Y, Tariku A. Factors Associated with Anemia among Children Aged 6–23 Months Attending Growth Monitoring at Tsitsika Health Center, Wag-Himra Zone, Northeast Ethiopia J Nutr Metab, 2015. 2015: p. 928632.
WHO, T.W. 2025: anaemia policy brief. Geneva: World Health Organization; 2014.
Iglesias Vázquez L, et al. Prevalence of anemia in children from latin America and the caribbean and effectiveness of nutritional interventions: systematic review and meta–analysis. Nutrients. 2019;11(1):183.
Balarajan Y, et al. Anaemia in low-income and middle-income countries. The Lancet. 2011;378(9809):2123–35.
McLean E, et al. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–54.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 2008.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Nation U. World Population Prospects 2019 2021.
Institute JB. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews checklist for analytical cross sectional studies. North Adelaide, Australia The Joanna Briggs Institute; 2017.
Kasa AS, et al. Precancerous cervical lesion in Ethiopia: systematic review and meta-analysis. Syst Reviews. 2021;10(1):287.
Roba KT et al. Anemia and undernutrition among children aged 6–23 months in two agroecological zones of rural Ethiopia Pediatric health, medicine and therapeutics, 2016: p. 131–140.
Malako BG, et al. Stunting and anemia among children 6–23 months old in Damot Sore district, Southern Ethiopia. BMC Nutr. 2019;5(1):1–11.
WHO, W. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015.
Liu J et al. Prevalence and Temporal Trend (2016–2018) of Anaemia among 6–23-Month-Old Infants and Young Children in China International journal of environmental research and public health, 2021. 18(4): p. 2041.
Stativa E, et al. Prevalence and predictors of anaemia in Romanian infants 6–23 months old. J Public Health. 2016;38(3):e272–81.
Wu Q et al. Monitoring and evaluating the adherence to a complementary food supplement (Ying Yang Bao) among young children in rural Qinghai, China: a mixed methods evaluation study. J Global Health, 2017. 7(1).
Gupta PM et al. Iron, Anemia, and Iron Deficiency Anemia among Young Children in the United States. Nutrients, 2016. 8(6).
Belachew A, Tewabe T. Under-five anemia and its associated factors with dietary diversity, food security, stunted, and deworming in Ethiopia: systematic review and meta-analysis. Syst Reviews. 2020;9(1):1–9.
Wang J, et al. The influence of Malnutrition and micronutrient status on anemic risk in children under 3 years old in poor areas in China. PLoS ONE. 2015;10(10):e0140840.
Prieto-Patron A, et al. Association between anaemia in children 6 to 23 months old and child, mother, household and feeding indicators. Nutrients. 2018;10(9):1269.
Kang Y, Kim J. Age-specific risk factors for child anaemia in Myanmar: analysis from the demographic and Health Survey 2015–2016. Matern Child Nutr. 2019;15(4):e12870.
Huang Z, et al. Prevalence and risk factors of anemia among children aged 6–23 months in Huaihua, Hunan Province. BMC Public Health. 2018;18:1–11.
Nairz M, Weiss G. Iron in Infection and immunity. Mol Aspects Med. 2020;75:100864.
Parrow NL, Fleming RE, Minnick MF. Sequestration and scavenging of iron in Infection. Infect Immun. 2013;81(10):3503–14.
Engle-Stone R et al. Predictors of anemia in preschool children: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project The American journal of clinical nutrition, 2017. 106(suppl_1): p. 402S–15.
Sanou D, Ngnie-Teta I. Risk factors for anemia in preschool children in sub-Saharan Africa. 2012.
Cassat JE, Skaar EP. Iron in Infection and immunity. Cell Host Microbe. 2013;13(5):509–19.
Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biology. 2017;29(2):e22952.
Pasricha SR, et al. Iron Defic Lancet. 2021;397(10270):233–48.
FAO., Declaration on world food security, World Food Summit, Rome 1996.
Wolfe WS. Building household food-security measurement tools from the ground up. Food Nutr Bull. 2001;22:5–12.
Chambers D et al. Proceedings of the 8th Annual Conference on the Science of Dissemination and Implementation: Washington, DC, USA. 14–15 December 2015 Implement Sci, 2016. 11 Suppl 2(Suppl 2): p. 100.
Fischer NC, et al. Household food insecurity is associated with anemia in adult Mexican women of reproductive age. J Nutr. 2014;144(12):2066–72.
Benz EJ Jr, Berliner N, Schiffman FJ. Anemia. Cambridge University Press; 2018.
Kaiser LL, et al. Food security and nutritional outcomes of preschool-age Mexican-American children. J Am Diet Assoc. 2002;102(7):924–9.
Parlesak A, Geelhoed D, Robertson A. Toward the prevention of childhood undernutrition: diet diversity strategies using locally produced food can overcome gaps in nutrient supply. FoodNutr Bull. 2014;35(2):191–9.
Saaka M, Galaa SZ. How is dietary diversity related to haematological status of preschool children in Ghana? Food & Nutrition Research. 2017;61(1):1333389.
Zou S-h, et al. Associations between dietary patterns and anaemia in 6-to 23-month-old infants in central South China. BMC Public Health. 2021;21(1):1–9.
Organization WH. Indicators for assessing infant and young child feeding practices: part 2: measurement 2010.
Wiseman G. Nutrition and health. 2002.
Baker RD, Greer FR, Nutrition Co. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics, 2010. 126(5): p. 1040–1050.
Pasricha S-R, et al. Determinants of anemia among young children in rural India. Pediatrics. 2010;126(1):e140–9.
Huang Y, et al. Prevalence and causes of anaemia in children aged 6–23 months in rural Qinghai, China: findings from a cross-sectional study. BMJ open. 2019;9(9):e031021.
Lozoff B. Iron Deficiency and child development. FoodNutr Bull. 2007;28(4suppl4):S560–71.
Zhang Y, et al. Effectiveness of complementary food supplements and dietary counselling on anaemia and stunting in children aged 6–23 months in poor areas of Qinghai Province, China: a controlled interventional study. BMJ open. 2016;6(10):e011234.
Wang J, et al. Effectiveness of community-based complementary food supplement (Yingyangbao) distribution in children aged 6–23 months in poor areas in China. PLoS ONE. 2017;12(3):e0174302.
Locks LM, et al. Using formative research to design a context-specific behaviour change strategy to improve infant and young child feeding practices and nutrition in N epal. Matern Child Nutr. 2015;11(4):882–96.
Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics, 2010. 126(5): p. 1040-50.
Acknowledgements
Initially, we would like to forward our thanks for all authors of the included studies. This paper was nothing without their effort.
In addition, all authors contributed to finalizing the manuscript were also acknowledged. Lastly, we also extend our thanks to editors and reviewers for their unpaid attention to improve this manuscript.
Funding
The authors did not receive any fund for publication.
Author information
Authors and Affiliations
Contributions
All authors agreed to be held accountable for all elements of the work and took part at any points of the study’s idea conception, article searching, article quality assessment, data extraction, analysis, and interpretation. They also contributed to the writing and revision of the paper. Based on this, the research idea was conceptualized by M.A. The three authors also independently and meticulously appraised the contents of each of the identified articles (B.B.A., A.T.A. and A.T.). The four reviewers (S.D.H, B.B., K.M. and A.B) performed the selection of the studies independently and blindly after thorough screening of the abstracts and the full texts of the studies. The data was extracted by M.A, D.T.A and D.A. The quality of the included studies was independently assessed by three authors (A.K., M.A., and G.K.). The data analysis and interpretation was conducted by S.D.H, M.A., B.B.A and A.K. Finally, all authors gave their final review and approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since the study did not use primary data, ethical approval is not applicable.
Consent for publication
Not applicable.
Competing interests
In this paper, the authors have no any conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azmeraw, M., Kassaw, A., Habtegiorgis, S.D. et al. Prevalence of anemia and its associated factors among children aged 6–23 months, in Ethiopia: a systematic review and meta analysis. BMC Public Health 23, 2398 (2023). https://doi.org/10.1186/s12889-023-17330-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-17330-y