Abstract
Background
Despite the close relationship between pre-pregnancy body mass index (BMI), gestational weight gain (GWG) and postpartum weight (PPW), these factors are often studied separately. There are no data characterising longitudinal weight trajectories among pregnant and postpartum women in urban African populations. We examined maternal weight trajectories from pregnancy through to 12 months postpartum, factors associated with higher weight trajectory class membership and associations of weight trajectories with infant growth at 12 months.
Methods
Data from 989 women were examined for weight trajectories from first antenatal care visit in pregnancy to 12 months postpartum using latent-class growth models. Baseline factors associated with class membership were assessed using multinomial logistic regression. Of the enrolled women, 613 of their infants were assessed for growth at 12 months. Anthropometry measurements for mothers and infants were conducted by a trained study nurse. Associations between maternal weight trajectory class and infant weight-for-age (WAZ), length-for-age (LAZ), weight-for-length (WLZ) at 12 months of age were analysed using linear regression.
Results
Four distinct classes of maternal weight trajectories were identified. The classes included consistent low (29%), consistent medium (37%), medium–high (24%) and consistent high (10%) trajectories. Similar to trends observed with medium–high trajectory, baseline factors positively associated with consistent high class membership included age (OR 1.05, 95% CI 1.01–1.09), pre-pregnancy BMI (OR 2.24, 95% CI 1.97–2.56), stage 1 hypertension (OR 3.28, 95% CI 1.68–6.41), haemoglobin levels (OR 1.39, 95% CI 1.11–1.74) and parity (OR 1.39, 95% CI 1.15–1.67); living with HIV (OR 0.47, 95% CI 0.30–0.74) was inversely associated. In adjusted analyses, compared to consistent medium weight trajectory, consistent low weight trajectory (mean difference -0.41, 95% CI -0.71;-0.12) was associated with decreased, and consistent high weight trajectory (mean difference 1.21, 95% CI 0.59–1.83) with increased infant WAZ at 12 months of age.
Conclusion
Identification of unique longitudinal weight trajectory groupings might inform comprehensive efforts targeted at improving healthy maternal weight and infant outcomes.
Similar content being viewed by others
Introduction
In recent years, low-middle-income countries are increasingly facing a dual burden of malnutrition characterised by both undernutrition and overnutrition [1]. This trend is particularly prominent among women in rural settings, with some continuing to experience underweight while others are overweight and obese [2]. Undernutrition is partly attributed to persistent poverty and household food insecurity while overnutrition is partly attributed to increased consumption of inexpensive high calorie dense foods accompanied by a sedentary lifestyle [3, 4]. In sub-Saharan Africa (SSA), undernutrition is mainly observed among people living with HIV due to HIV infection itself and the risk of opportunistic infections [5]. However, obesity is also highly common in women living with HIV (WLHIV) due to exposure to the ‘obesogenic’ environment faced by the general public [6, 7]. In addition, some classes of antiretroviral therapy (ART) are implicated in contributing to weight gain among the HIV group [8, 9]. Therefore, we compared weight trajectories in WLHIV and without HIV.
Weight status in women of reproductive age influences maternal and infant health outcomes. Elevated pre-pregnancy weight and excessive gestational weight gain (GWG) increase the risk of gestational diabetes and hypertension, including pre-eclampsia and eclampsia, as well as complicated delivery and caesarean Sects [10,11,12,13]. Further, pre-pregnancy obesity and excessive GWG is linked to postpartum weight (PPW) retention, which is recognised as an important predictor of non-communicable diseases (NCDs) in mid-life including cardiovascular diseases (CVDs) [14,15,16,17]. Pregnant women with elevated weight are also at risk of having stillbirths, preterm as well as large for gestational age (LGA) infants [18, 19]. On the other hand, underweight weight in pregnancy is linked to anaemia which is highly prevalent in South Africa and associated with hypertensive disorders [20, 21]. In addition, underweight increases the risk of having preterm and small size for gestational age (SGA) infants [22, 23]. Although some preterm and SGA infants remain small throughout their childhood [24, 25], others experience rapid ‘catch-up’ growth; both scenarios are associated with childhood obesity and increased lifetime risk of CVDs [26,27,28,29]. Likewise, most data report that LGA infants experience obesity and related-metabolic complications later in life [30, 31], while some report a compensatory slower growth trajectory [32, 33].
Despite the evident contribution of not only pre-pregnancy BMI but also GWG and PPW to poor maternal health outcomes, there are no studies in SSA that have applied a trajectory approach to understand weight changes over the perinatal period and implications for infant growth. Therefore, the objective of this study was to examine maternal weight trajectories from pregnancy through to 12 months postpartum, factors associated with higher weight trajectory class membership and associations of weight trajectories with infant growth at 12 months.
Methods
Study design and population
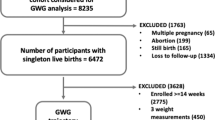
For this prospective cohort study, consecutive pregnant women attending antenatal care (ANC) services at the Gugulethu Community Health Centre in Cape Town, South Africa were enrolled between January 2017 and July 2018. Eligible women were those who were ≥ 18 years of age and presenting for the first time for ANC regardless of gestational age. Women living with HIV had either initiated efavirenz-based ART before conception or intended to initiate ART during pregnancy following their first ANC visit. Gugulethu is a peri-urban community with a population of ~ 300 000, with predominantly low socioeconomic status (SES) and HIV prevalence of 30% among pregnant women [34,35,36]. Pregnant women were prospectively followed through face-to-face interviews with intensive anthropometry measurements that took place during the first, second and third visit conducted in pregnancy; and were followed-up with their infants at < 7 days, 10 weeks, 6 months and 12 months postpartum. All 989 women enrolled in the cohort were included for the weight trajectory analysis, however 613 of 907 (68%) live singleton infants were included for infant growth analysis. Excluded infants (n = 376) were twins (n = 14), miscarriage/stillbirths/terminations (n = 58), neonatal/infant/maternal death (n = 10), maternal/infant seroconversion to HIV (n = 11), no longer interested (n = 36), relocated (n = 50), lost to follow-up (n = 129) and mom attended 12 months visit without baby (n = 68) (Figure S1).
Outcome assessment
The primary outcome of interest was maternal weight trajectory from first ANC visit through 12 months postpartum. Weight measurements from enrolment in pregnancy through to 12 months postpartum were conducted by a trained study nurse using a calibrated scale (Charder, Taichung City, Taiwan) accurate to within 0.5 kg. A study nutritionist repeated anthropometric training for the study nurse at regular intervals, with structured and supervised competency. A total of up to 7 weight assessments (first, second and third visits in pregnancy; and at < 7 days, 10 weeks, 6 and 12 months postpartum periods) were used to examine weight trajectories. Missing weights at the first visit were 0.01%, second visit were 36% and third visit were 51% in pregnancy; and at < 7 days were n = 42%, 10 weeks were 38%, 6 months were 35% and 12 months were 30% postpartum. Participants with missing datapoints were included in the model, the model created a trajectory using timepoints with available data and excluding timepoints with missing data [37, 38]. The secondary outcome of interest was infant anthropometry Z-scores (WAZ, LAZ and WLZ) at 12 months calculated using WHO child growth standards [39]. Child anthropometry assessments (weight and length) at 12 months of age were performed by the same trained study nurse who conducted maternal anthropometry measurements following a standardised protocol based on WHO guidelines [39].
Other characteristics
At enrolment, height measurements were taken to the nearest 0.1 cm using a stadiometer (Seca, Birmingham, United Kingdom). Using standard GWG charts developed using estimated gestational age (GA) at measurement and related BMI categories, we applied a correction factor on weight measured at first ANC visit to estimate pre-pregnancy BMI using a method described by Santos et al. [40]. Briefly, using international standards for GWG in pregnancy [40], the median weight gained for each week of gestation was subtracted from the weight measured at first ANC visit based on BMI category as GWG differs according to pre-pregnancy BMI. Estimated pre-pregnancy BMI was then calculated as GA-corrected weight divided by squared height; and was categorised as underweight (< 18.5), normal (18.5–24.9), overweight (25–29.9) and obese (≥ 30) in kg/m2. Weekly maternal GWG was calculated by dividing the weight change between enrolment and second or third visits by the number of weeks lapsed between the two intervals [41], and expressed as kg/week. Rate of GWG was categorised as slow, normal and fast based on the Institute of Medicine (IOM) recommended median GWG (IQR) which vary by BMI category [42]. Postpartum weight change was calculated as the difference between measured 12 months postpartum weight and estimated pre-pregnancy weight, and was categorised as loss (< 0 kg), stable (0–4.9kg) and gain (≥ 5 kg) [43].
Maternal socio-demographic and clinical data were collected via interviewer-administered questionnaires. SES was a composite score based on level of education, employment status, type of housing, and presence of a toilet, running water, electricity, fridge, telephone, and television in the house [44]; participants were categorised into tertiles corresponding to lowest, middle and highest SES group. Baseline blood pressure (BP) and haemoglobin (Hb) measurements were performed by healthcare providers as part of routine care at the first ANC visit. BP was categorised as: normal (< 120/80 mmHg), elevated (systolic 120–129 and diastolic < 80 mmHg), stage 1 hypertension (systolic 130–139 or diastolic 80–89 mmHg) or stage 2 hypertension (systolic ≥ 140 or diastolic ≥ 90 mmHg) [45]. Hb was categorised as normal (≥ 11.0), mild anaemia (10–10.9), moderate anaemia (7–9.9) or severe anaemia (< 7) in g/dL [46]. Alcohol use was assessed using AUDIT questionnaire and was defined as ‘yes’ if indicated alcohol use since finding out about the current pregnancy. Breastfeeding duration was obtained through self-report and categorised as ‘never’, ‘ever’; those who ‘ever’ breastfed were further categorised into < 6 months and ≥ 6 months duration of any intensity. Food intake was assessed at first ANC via a 7-day recall food frequency questionnaire with responses categorised as ‘never’, ‘1–3’ and ‘4–7’ days. Parity, HIV status (with HIV and without HIV), ART initiation timing (before pregnancy and during pregnancy) and CD4 count were abstracted from medical records. Relationship status was self-reported and categorised as ‘not in a relationship’, ‘not cohabiting/married but not living together’ and ‘cohabiting/married and living together’.
Gestational age (GA) was determined by ultrasound operated by an experienced research sonographer at the first study visit. Delivery GA was calculated by adding the number of weeks elapsed between enrolment and delivery dates to the GA measured at first ANC visit. Delivery GA was categorised as term (≥ 37 weeks) or preterm delivery (PTD, < 37 weeks) [47]. Preterm delivery was further categorised into spontaneous and medically-indicated PTD based on delivery mode information abstracted from medical records. Spontaneous PTD was defined as onset of labour and spontaneous vaginal delivery at less than 37 weeks GA, while medically-indicated PTD was defined as delivery at less than 37 weeks after induction of labour or by caesarean section. Neonatal data at delivery including birth weight and gender were obtained from medical records. Infant birth weight was categorised as low (< 2500), normal (2500 – 4000) and high (> 4000) birthweight in grams [48]. Size for GA was calculated based on infant GA, birthweight and sex using INTERGROWTH-21st standards and categorised as small (< 10th), appropriate (10 – 90th) and large (> 90th) for GA in percentiles [49].
Statistical analysis
Latent-class growth modelling was used to create maternal weight trajectory classes from pre-pregnancy through to 12 months postpartum period. This method allows longitudinal data to inform groupings of individuals following distinct patterns over time, enabling those who are LTFU to also contribute available data [37, 38]. Latent-class growth models were fit with random effects for each participant and fixed effects for time period. A minimum of three classes was considered a priori. However, model fit based on Bayesian Information Criterion (BIC) values was used to guide the final number of classes included. We evaluated baseline factors associated with maternal weight trajectory class using univariate logistic regression. These baseline factors are typically collected at the first ANC visit in this setting. The factors included maternal age (continuous and categorical), estimated pre-pregnancy BMI (continuous), BP (continuous and categorical), Hb (continuous and categorical), education (categorical), SES (categorical), relationship status (categorical), alcohol use (categorical), GA at first ANC (continuous and categorical), parity (continuous and categorical), HIV status (categorical), ART initiation timing (categorical) and past 7-day food frequency (categorical). Because pre-pregnancy BMI is a known predictor of weight progression in pregnancy and postpartum, we performed BMI-adjusted analyses to assess weight trajectory predictors that are independent of BMI. To examine the association between maternal weight trajectory class and infant WAZ, LAZ and WLZ at 12 months, univariable and multivariable linear regression were used. Multivariable models for infant growth were adjusted for maternal (estimated pre-pregnancy BMI, parity, SES and alcohol use) and infant (sex, delivery GA and feeding duration) factors based on the basis of existing literature, theoretical and conceptual reasoning. Having observed high obesity in WLHIV in our previous studies [50, 51], we tested the hypothesis that weight trajectories in WLHIV will not differ from those of women without HIV, hence we stratified the analysis by HIV status. In all analyses, a p-value < 0.05 was considered statistically significant. With the exception of maternal weight trajectory class analyses which were created using R studio, all other analyses were performed using STATA version 15.0 (Stata Corporation, College Station, TX, USA).
Results
A total of 989 women (48% WLHIV) were included in the analyses for maternal weight trajectories. At first ANC entry, the median GA was 20 weeks (IQR, 14–25), maternal median age was 29 years (IQR, 25–34) and 25% were primiparous. Median BMI was 29 kg/m2 (IQR, 24–34): 26 (2%) underweight, 273 (28%) normal, 244 (25%) overweight and 428 (43%) obese BMI. Median GWG rate was 0.36 kg/week (IQR, 0.14–0.54): 264 (27%) slow, 121 (12%) normal, 361 (37%) fast GWG rate. Median PPW at 12 months was 3.87 kg (IQR, -1.10–9.04): 209 (21%) weight loss, 178 (18%) stable weight, 300 (30%) high weight retention (Table 1).
Maternal weight trajectories
Four distinct maternal weight trajectory classes were identified and included in the analysis. This decision was based on BIC values which did not change substantially beyond the 4th class. To assign individuals into a particular class, the model used the class with the highest predicted probability out of the 4 classes for that individual [37, 38]. The probability cut-off for assigning a class to an individual was high at 0.90. The trajectories were named based on initial weight in pregnancy and the pattern observed at the postpartum period; class 1 (consistent low), class 2 (consistent medium), class 3 (medium–high) and class 4 (consistent high). The 4 trajectories started off at different mean weights in the first visit (consistent low [59kg], consistent medium [74kg], medium–high [91kg], consistent high [114kg], p-value 0.01). However, all trajectories displayed the same pattern of steady increase in weight in second and third visits, followed by a decline at < 7 days after delivery as expected (Fig. 1A). From 10 weeks through 12 months postpartum, the weight remained stable for consistent low and consistent medium; but it increased for medium–high and consistent high.
Four distinct maternal weight trajectory classes in the overall sample, A. average weights and B. individual weights by estimated pre-pregnancy BMI. Notably, normal BMI women in consistent low class had significantly lower mean GWG (0.41 vs 0.58 kg/week, p = 0.01) and PPW change (3.54 vs 8.94 kg, p = 0.01) compared to normal BMI women in consistent medium class (Fig. 2). Overweight BMI women in consistent medium class had significantly lower mean GWG (0.37 vs 0.84 kg/week, p = 0.01) and PPW (3.94 vs 13.58 kg, p = 0.01) compared to overweight BMI women in medium–high class. Although non-significant, obese BMI women in medium–high class had lower mean GWG (0.31 vs 0.42 kg/week, p = 0.46) compared to obese BMI women in consistent high class
Consistent low (29%) was composed of women who had underweight (7%), normal (72%), overweight (15%), obese (2%) and unknown (4%) estimated pre-pregnancy BMI at baseline, the majority of these women gained weight at either low/normal rate (per GWG [kg/week] IOM guidelines) during pregnancy and either lost/retained a stable (< 0 to 4.9kg) amount of weight postpartum (Fig. 1B). Consistent medium (37%) was composed of women who had normal (19%), overweight (49%), obese (30%) and unknown (2%) estimated pre-pregnancy BMI, the majority of these women gained weight at a low/normal rate (per GWG [kg/week] IOM guidelines) during pregnancy and either lost/retained a stable (< 0 to 4.9kg) amount of weight postpartum. Medium–high (24%) was composed of women who had normal (0.5%), overweight (9%), obese (90%) and unknown (0.5%) estimated pre-pregnancy BMI, the majority of these women gained weight at a normal/elevated rate (per GWG [kg/week] IOM guidelines) during pregnancy and retained either a stable/excess (0 to > 5.00 kg) amount of weight postpartum. Consistent high (10%) was composed of women who had obese (97%) and unknown (3%) estimated pre-pregnancy BMI, the majority of these women gained weight at a faster rate (per GWG [kg/week] IOM guidelines) during pregnancy and retained an excess (≥ 5.00 kg) amount of weight postpartum. The weight trajectory pattern for all 4 membership classes was similar in both women living with and without HIV (Figure S2).
Predictors of maternal weight trajectory class
In anadjusted analysis (Table 2), continuous baseline factors positively associated with consistent high class membership included age (OR 1.05, 95% CI 1.01–1.09), estimated pre-pregnancy BMI (OR 2.24, 95% CI 1.97–2.56), systolic BP (OR 1.04, 95% CI 1.02–1.06), diastolic BP (OR 1.05, 95% CI 1.05–1.08), Hb levels (OR 1.39, 95% CI 1.11–1.74) and parity (OR 1.39, 95% CI 1.15–1.67). Categorical baseline factors associated with consistent high class membership included BP, Hb, parity and living with HIV. In particular, compared to normal BP, elevated BP (OR 2.06, 95% CI 1.16–3.67), stage 1 hypertension (OR 3.28, 95% CI 1.68–6.41) and stage 2 hypertension (OR 2.12, 95% CI 1.09–4.10) were positively associated; compared to normal Hb, moderate anaemia (OR 0.44, 95% CI 0.19–0.99) was negatively associated; compared to primiparity, multiparity (OR 2.30, 95% CI 1.20–4.41) was positively associated; compared to not living with HIV, living with HIV (OR 0.47, 95% CI 0.30–0.74) was negatively associated.
To account for the contribution of estimated pre-pregnancy BMI to the observed associations, we included it in multivariable models (Table 2). In estimated pre-pregnancy BMI-adjusted analyses, continous Hb levels (aOR 1.58, 95% CI 1.10–2.25) remained positively associated with consistent high weight trajectory. Compared to normal Hb, severe anaemia (OR 1.14–7, 95% CI 2.05–8-6.32–7) was positively associated and living with HIV (aOR 0.45, 95% CI 0.21–0.95) remained inversely associated with consistent high weight trajectory compared to not living with HIV. Although the trend for continous Hb and categorical severe anaemia are in the same direction, this is not the case for moderate anaemia which shows a negative relationship with consistent high class membership.
Maternal weight trajectory class and infant growth
At delivery, the proportion of infants born LBW was 10% and 4% had high birth weight (Table S2). Overall, 11% of infants were born SGA and 10% were born LGA. The proportion of spontaneous (11%) and medically indicated (10%) preterm births were similar. Overall, a high proportion (96%) of infants were breastfed from birth. However, HIV-exposed uninfected (HEU) infants were less likely to be breastfed for 6 months or longer compared to HIV-unexposed uninfected (HUU) infants (36% vs 64%, p-value 0.01). In descriptive analysis of z-scores at 12 months, overall mean WAZ was 0.52 (SD = 1.36), LAZ was -0.61 (SD = 1.28) and WLZ was 1.09 (SD = 1.54). HEU infants had significantly lower mean WAZ (0.39 SD = 1.35 vs 0.64 SD = 1.36, p-value 0.02) and LAZ (-0.77 SD = 1.23 vs -0.46 SD = 1.31, p-value 0.01), but there were no apparent differences in mean WLZ (1.01 SD = 1.51 vs 1.15 SD = 1.58, p-value 0.21) compared to HUU infants. Infants born from women with consistent high weight trajectory were more likely to be LGA (20% vs 7%, p-value 0.03) compared to those born from mothers with consistent medium weight trajectory (Table S3).
Variables included in adjusted models were maternal age, pre-pregnancy BMI, SES, parity, alcohol use, infant gender, gestation at birth and breastfeeding duration. In adjusted analysis, overall, only associations with infant WAZ remained statistically significant (Table 3). Compared to consistent medium weight trajectory, infants born from mothers with consistent low weight trajectory had decreased (mean difference -0.41, 95% CI -0.71;-0.12) WAZ at 12 months of age; and those born from mothers with consistent high weight trajectory had increased (mean difference 0.59, 95% CI 0.09;1.09) WAZ at 12 months of age. Associations between consistent low weight trajectory and decreased infant WAZ remained significant in both WLHIV (mean difference -0.30, 95% CI -0.70;-0.10) and those without HIV (mean difference -0.53, 95% CI -0.99;-0.08).
Discussion
In this cohort of women in an urban setting in South Africa, we identified four distinct patterns of weight trajectories from pregnancy through to 12 months postpartum, ranging from consistently low to consistently high. Infants born from women with consistent low weight trajectory had decreased WAZ; while those born from women with consistent high weight trajectory had increased WAZ at 12 months of age. These findings demonstrate the relationship between longitudinal maternal weight and infant growth, and could help inform the design of adaptive weight management interventions.
Pre-pregnancy BMI is traditionally used to categorise individuals in order to guide weight management interventions. While this is useful, in the context of pregnancy increased GWG and PPW are also of public health concern in addition to pre-pregnancy BMI. This study used a weight trajectory approach to detect women with ‘abnormal’ weight progression over time. We found that women with normal estimated pre-pregnancy BMI were assigned to consistent low and some to consistent medium trajectories due to different weight progression trends over time. In agreement, using a trajectory approach, Bogaerts et al. [52] found that some lean women experienced excessive GWG and retained the highest amount of weight after delivery compared to those who started off with obese BMI. This trajectory approach allowed these lean women with excessive GWG, who would otherwise be excluded from targeted weight management interventions, to be identified as high risk for adverse health outcomes. Considering the high fertility and multiple pregnancies with short birth intervals in sub-Saharan Africa [53], identification of high risk women using a comprehensive trajectory method might be useful for planning inclusive weight management interventions.
Maternal exposures during pregnancy influence fetal development, thereby setting life course health outcomes for offspring. Despite the evidence showing the association between pre-pregnancy BMI or GWG and adverse infant outcomes [19, 54] there are limited data on the associations with maternal weight patterns over time. Catov et al., [55] highlighted the usefulness of studying weight as a trajectory over time by showing that the influence of GWG on infant weight is dependent on the timing in which the weight change occurred. The authors found that women who experienced excessive weight gain before 20 weeks of pregnancy were more likely to have LGA infants, with a recommendation that weight gain pattern throughout the course of pregnancy might be a useful marker of abnormal fetal growth [55]. In this cohort we found that consistent low trajectory was associated with decreased, while consistent high trajectory was associated with increased infant WAZ at 12 months, regardless of maternal HIV status. Notably, the majority (72%) of women with consistent low weight trajectory had normal pre-pregnancy BMI. Using cross-sectional BMI as an exposure, these women would not be identified as being at risk of having infants with non-optimal growth patterns. Poor infant growth during the first 1000 days lays the foundation for risk of reduced human capital and economic productivity due to poor brain development [56]; as well as risk of chronic metabolic diseases such as obesity and diabetes over life-course [57]. Therefore, inclusive detection of women at risk of adverse weight trajectories is necessary for inclusive interventions directed at reducing adverse infant outcomes.
Indeed, identification of risks factors contributing to ‘abnormal’ weight patterns was carried out in this study. We found that women with medium high and consistent high weight trajectories were more likely to be older and to have increased BMI, blood pressure, haemoglobin levels and parity. These results highlight the type of women in which weight management programs should be targeted to before and during pregnancy in low-resource settings to minimise adverse infant growth outcomes. Fortunately, all these factors are examined routinely at the first ANC visit in South African public sector antenatal clinics. This means that the applicability of these findings in this setting would be feasible. For example, this could involve communication of risk to patient by the healthcare practitioners to raise early awareness, followed by close monitoring of weight gain throughout pregnancy. Further, counselling regarding the importance of healthy postpartum weight retention would be essential to minimise the long-term risk of adverse maternal health outcomes.
Caution should be exercised when interpreting these study findings due to certain limitations. Women were enrolled at first ANC which was mainly in the second trimester of pregnancy and hence pre-pregnancy weight was not known. Although pre-pregnancy weight was estimated, there is still potential for misclassification bias in the variables that include this corrected weight such as pre-pregnancy BMI and PPW variables. The GWG charts used to apply a correction factor on measured first ANC weight to estimate pre-pregnancy BMI were developed using international standards which do not include WLHIV and no data exist for this group. However, considering the similar weight trajectories observed among both women living with and those without HIV, substantial differences in the GWG trends are unlikely. Other important weight trajectory predictors such as mental health and physical activity were not measured. Not all infants could be included in the study, this missing data may have led to selection bias. Despite the intuitive nature of weight trajectory analytical approach compared to cross-sectional BMI, some subtypes of weight trajectory classes may not have been identified due to missing weight assessments at some visits and due to a limited sample size, and large-scale studies are needed. However, longitudinal follow-up of mother-infant pairs throughout pregnancy to 12 months postpartum is a unique strength.
In conclusion, this study is among the first to identify distinct maternal weight trajectories in African women. Consistent low weight trajectory was associated with decreased, while consistent high was associated with increased infant WAZ at 12 months of age. A longitudinal weight trajectory approach might inform comprehensive efforts targeted at improving healthy maternal weight and infant outcomes.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its supplementary information files). In addition, the datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- GWG:
-
Gestational weight gain
- PPW:
-
Postpartum weight
- WAZ:
-
Weight-for-age
- LAZ:
-
Length-for-age
- WLZ:
-
Weight-for-length
- OR:
-
Odds ratio
- WLHIV:
-
Women living with HIV
- ART:
-
Antiretroviral therapy
- NCDs:
-
Non-communicable diseases
- CVDs:
-
Cardiovascular diseases
- LGA:
-
Large for gestational age
- SGA:
-
Small for gestational age
- ANC:
-
Antenatal care
- SES:
-
Socioeconomic status
- WHO:
-
World Health Organisation
- IOM:
-
Institute of Medicine
- BP:
-
Blood pressure
- Hb:
-
Haemoglobin
- AUDIT:
-
Alcohol Use Disorders Identification Test
- GA:
-
Gestational age
- PTD:
-
Preterm delivery
- BIC:
-
Bayesian Information Criterion
References
World Health Organization. Double burden of malnutrition policy brief. Geneva, Switzerland, 2017. https://www.who.int/publications/i/item/WHO-NMH-NHD-17.3
Jaacks LM, Slining MM, Popkin BM. Recent underweight and overweight trends by rural-urban residence among women in low- and middle-income countries. J Nutr. 2015;145(2):352–7.
Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84(2):289–98.
Steyn NP, Mchiza ZJ. Obesity and the nutrition transition in Sub-Saharan Africa. Ann N Y Acad Sci. 2014;1311:88–101.
Fuseini H, Gyan BA, Kyei GB, Heimburger DC, Koethe JR. Undernutrition and HIV infection in sub-Saharan Africa: health outcomes and therapeutic interventions. Curr HIV/AIDS Rep. 2021;18(2):87–97.
Caniglia EC, Shapiro R, Diseko M, Wylie BJ, Zera C, Davey S, et al. Weight gain during pregnancy among women initiating dolutegravir in Botswana. EClin Med. 2020;29–30:100615.
Guehi C, Badjé A, Gabillard D, Ouattara E, Koulé SO, Moh R, et al. High prevalence of being Overweight and Obese HIV-infected persons, before and after 24 months on early ART in the ANRS 12136 Temprano Trial. AIDS Res Ther. 2016;13(1):12–24.
Esber AL, Chang D, Iroezindu M, Bahemana E, Kibuuka H, Owuoth J, et al. Weight gain during the dolutegravir transition in the African Cohort Study. J Int AIDS Soc. 2022;25(4):e25899.
Bengtson AM, Myer L, Abrams EJ, Jao J, Cu-Uvin S. INSTIs and weight gain in pregnancy. Lancet HIV. 2020;7(10):e663–5.
Ren M, Li H, Cai W, Niu X, Ji W, Zhang Z, et al. Excessive gestational weight gain in accordance with the IOM criteria and the risk of hypertensive disorders of pregnancy: a meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):281.
Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2013;209(4):327 e1-.e17.
O'Brien TE, Ray JG, Chan W-S. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiol 2003:368–74.
Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597–604.
Kew S, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care. 2014;37(7):1998–2006.
McDermott MM, Brubaker L. Prepregnancy Body Mass Index, Weight Gain During Pregnancy, and Health Outcomes. JAMA. 2019;321(17):1715.
Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol. 2015;125(1):144–52.
Nicklas JM, Rosner BA, Zera CA, Seely EW. Association Between Changes in Postpartum Weight and Waist Circumference and Changes in Cardiometabolic Risk Factors Among Women With Recent Gestational Diabetes. Prev Chronic Dis. 2019;16:E47-58.
Liu B, Xu G, Sun Y, Du Y, Gao R, Snetselaar LG, et al. Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: a population-based study. Lancet Diabetes Endocrinol. 2019;7(9):707–14.
Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. BioMed Res Int 2014;2014.
Dorsamy V, Bagwandeen C, Moodley J. The prevalence, risk factors and outcomes of anaemia in South African pregnant women: a systematic review and meta-analysis. Syst Rev. 2020;09(1):209–13.
Mocking M, Savitri AI, Uiterwaal CSPM, Amelia D, Antwi E, Baharuddin M, et al. Does body mass index early in pregnancy influence the risk of maternal anaemia? An observational study in Indonesian and Ghanaian women. BMC Public Health. 2018;18(1):873–81.
Xiao P-L, Zhou Y-B, Chen Y, Yang M-X, Song X-X, Shi Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth. 2015;15(1):246–57.
Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16(1):153–66.
Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW. Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 1998;152(12):1225–31.
Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatr. 2003;111(5):986–90.
Vasylyeva TL, Barche A, Chennasamudram SP, Sheehan C, Singh R, Okogbo ME. Obesity in prematurely born children and adolescents: follow up in pediatric clinic. Nutr. 2013;12(1):150.
Cho WK, Suh B-K. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. 2016;59(1):1.
Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–8.
Kwon EJ, Kim YJ. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet Gynecol Sci. 2017;60(6):506–19.
Derraik JG, Maessen SE, Gibbins JD, Cutfield WS, Lundgren M, Ahlsson F. Large-for-gestational-age phenotypes and obesity risk in adulthood: a study of 195,936 women. Sci Rep. 2020;10(1):1–9.
Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10(2):77–83.
Chiavaroli V, Cutfield WS, Derraik JG, Pan Z, Ngo S, Sheppard A, et al. Infants born large-for-gestational-age display slower growth in early infancy, but no epigenetic changes at birth. Sci Rep. 2015;5:14540.
Tanner JM. Growth as a target-seeking function. Human growth: Springer; 1986. p. 167–79.
City of Cape Town. City of Cape Town – 2011 Census Suburb Gugulethu. data supplied by Statistics South Africa. 2011;Compiled by Strategic Development Information and GIS Department (SDI&GIS).
Gadama LA. Adverse perinatal events observed in obese pregnant women in the Metro West Region: University of Cape Town; 2014.
Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N-Y, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Med. 2018;15(3): e1002547.
Jones B, Nagin D. A note on a Stata plugin for estimating group-based trajectory models. Sociol Methods Res. 2013;42:608–13.
Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol. 2009;5:11–24.
World Health Organization, United Nations Children's Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children. A Joint Statement. Switzerland, Geneva 2009.
Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles M-A, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. 2018;16(1):201–16.
Gilmore LA, Redman LM. Weight gain in pregnancy and application of the 2009 IOM guidelines: toward a uniform approach. Obes. 2015;23(3):507–11.
Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington DC: National Academies Press; 2009.
Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin. 2009;36(2):317–32.
Myer L, Stein DJ, Grimsrud A, Seedat S, Williams DR. Social determinants of psychological distress in a nationally-representative sample of South African adults. Soc Sci Med. 2008;66(8):1828–40.
American College of Obstetricians and Gynecologists. Preeclampsia and High Blood Pressure During Pregnancy, FAQ 034. . American College of Obstetricians and Gynecologists. 2018.
Kariyeva G.K. MA, Sharman, A. Anemia: the DHS program. Medicine 2002:141–47.
World Health Organization. Preterm birth. Geneva, Switzerland 2018. http://www.who.int/news-room/fact-sheets/detail/preterm-birth.
Ota E, Haruna M, Suzuki M, Anh DD, Tho LH, Tam NTT, et al. Maternal body mass index and gestational weight gain and their association with perinatal outcomes in Viet Nam. Bull World Health Organ. 2011;89:127–36.
Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68.
Madlala HP, Malaba TR, Newell ML, Myer L. Elevated body mass index during pregnancy and gestational weight gain in HIV-infected and HIV-uninfected women in Cape Town, South Africa: association with adverse birth outcomes. Trop Med Int Health. 2020;25(6):702–13.
Madlala HP, Steyn NP, Kalk E, Davies MA, Nyemba D, Malaba TR, et al. Association between food intake and obesity in pregnant women living with and without HIV in Cape Town, South Africa: a prospective cohort study. BMC Public Health. 2021;21(1):1504.
Bogaerts A, De Baetselier E, Ameye L, Dilles T, Van Rompaey B, Devlieger R. Postpartum weight trajectories in overweight and lean women. Midwifery. 2017;49:134–41.
Norton M, MacDonald T, Mwebesa W, Pearson L. Care for girls and women before pregnancy. Switzerland: WHO; 2006. https://www.academia.edu/18413239/Care_for_girls_and_women_before_pregnancy.
Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019;16(6):e1002817.
Catov JM, Abatemarco D, Althouse A, Davis EM, Hubel C. Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obes. 2015;23(5):1071–8.
Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol. 2017;29(2):https://doi.org/10.1002/ajhb.22952.
Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21(4):199–205.
Acknowledgements
The authors thank the participants, clinic staff at Gugulethu Community Health Clinic and staff members of “B positive” study.
Funding
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number 1R01HD080465-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
L.M., A.B., E.K. conceptualised and designed the study. H.P.M., D.N., T.R.M. collected the data and conceptualised analysis. H.P.M., L.H. performed data analysis and interpreted the outputs. H.P.M. searched the literature and wrote the manuscript. All authors reviewed, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Faculty of Health Sciences Human Research Ethics Committee of the University of Cape Town (REF 541/2015) and the Western Cape Department of Health (REF WC_2016RP6_286). All women provided informed consent to participate and all procedures were carried out in accordance with relevant guidelines and regulations of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Madlala, H.P., Bengtson, A.M., Hannan, L. et al. Maternal weight trajectories and associations with infant growth in South African women. BMC Public Health 23, 2055 (2023). https://doi.org/10.1186/s12889-023-16963-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16963-3