Abstract
Background
This study aims to investigate the association between sleep quality and infertility among women and to explore the mediating effects of DNA methylation in this association.
Methods
This study is a population-based case–control study. The relationship between sleep quality and infertility was investigated in women with anovulatory infertility (n = 43) and healthy controls (n = 43). Genome-wide DNA methylation was profiled from peripheral blood samples using the Illumina Infinium Human Methylation 850k BeadChip. Differentially methylated CpGs between cases and controls were identified using the ChAMP R package. The mediating effect of DNA methylation between sleep quality and infertility among women was investigated using the Bayesian estimation method provided by the R package “mediation”.
Results
The survey included 86 women of reproductive age, with 43 participants each in the case and control groups. The average age of the women was 27.6 ± 2.8 years (case group: 27.8 ± 3.0 years, control group: 27.4 ± 2.7 years). A total of 262 differentially methylated CpGs corresponding to 185 genes were identified. Difficulty falling asleep was a risk factor for infertility in women (OR = 3.69, 95%CI = 1.14, 11.99), and a causal mediation effect of DNA methylation CpGs was found. The mediating effect coefficient for cg08298632 was 0.10 (95%CI = 0.01–0.22), and the proportion of the total effect mediated by this methylation site increased to 64.3%.
Conclusion
These results suggest that DNA methylation CpGs (cg08298632) play a significant role in the relationship between difficulty falling asleep and infertility in females. These findings contribute to our understanding of the underlying mechanisms that connect difficulty falling asleep and infertility in women. Further studies are necessary to fully understand the biological significance and potential therapeutic applications of these findings. The identified DNA methylation sites provide new and valuable insights and potential targets for future studies aiming to prevent and treat female infertility.
Similar content being viewed by others
Background
Reproductive health plays an important role in human survival, reproduction, and social development. Infertility is one of the most important components of reproductive health. Infertility is diagnosed when a couple is unable to conceive after engaging in unprotected sexual intercourse for one year [1]. In recent years, there has been an increase in the number of individuals seeking infertility treatment [2]. Infertility is a global medical and social concern, with approximately half of the cases attributed to female factors [3]. It not only raises the risk of mental illness, pregnancy-related complications, and chronic diseases but also increases the chances of offspring developing conditions such as mental retardation [4,5,6]. Moreover, infertility can cause discrimination, strain in marital relationships, domestic violence, and even marital breakdowns [7,8,9].
Advancements in science and technology have improved the accessibility and effectiveness of infertility treatments. However, these treatments are often expensive, yield unsatisfactory outcomes, and can have side effects such as ovarian hyperstimulation syndrome [10]. Thus, it is necessary to strengthen the surveillance of infertility issues, identify preventable causes of infertility, and tailor effective prevention interventions to reduce the prevalence of this condition.
Infertility is a complex condition influenced by various factors, such as genetics, epigenetics, environmental factors, and lifestyle choices [11,12,13]. Recently, increased attention has been paid to the adverse effects of sleep behavior and circadian disturbances on fertility. Studies have shown that sleep patterns, such as staying up late and sleeping in on weekends, can lead to social jet lag and sleep disorders, which may adversely affect follicle development and hormone production and increase the risk of pregnancy-related difficulties [14, 15]. Both the US National Health Interview Survey consisting of 9 137 women of childbearing age and a study conducted in China with 2 687 women of childbearing age showed a U-shaped association between sleep duration and the probability of achieving pregnancy [16]. Furthermore, a prospective cohort study found that women with medically diagnosed non-apneic sleep disorders had a 3.7-fold increased risk of infertility compared to those without sleep disorders [17]. The North American pre-pregnancy cohort study also reported reduced fertility among women who experienced nighttime sleep difficulties or insufficient sleep [18].
Although the adverse effects of sleep quality on female reproductive health, such as irregular menstruation, low fertility, infertility, and adverse pregnancy and birth outcomes, have been widely reported, the underlying mechanisms behind these effects have not been fully elucidated. With advances in epigenetics, the association between epigenetic modification and reproductive health has attracted increasing attention [19, 20]. Epigenetic modifications are influenced by a combination of genetic and environmental factors, and DNA methylation is one of the most common and important epigenetic modifications regulating gene expression [21, 22]. Numerous studies have shown that DNA methylation is significantly affected by sleep, and specific genomic regions undergo changes in DNA methylation profiles following sleep deprivation [23,24,25,26]. Previous studies have elucidated that aberrant DNA methylation may play an important role in the pathogenesis of conditions such as polycystic ovary syndrome (PCOS) and endometriosis (EMS), and that these defects in DNA methylation can promote the dysregulation of genes involved in inflammation, immunity, and hormone synthesis [27, 28]. Therefore, epigenetic mechanisms, such as DNA methylation, may play an important role in the relationship between sleep quality and infertility in women.
Therefore, this study aimed to clarify the association between sleep quality and infertility among women, and to use causal mediation analysis to further explore the potential mediating effect of DNA methylation on the relationship between sleep quality and infertility. By elucidating the relationship between sleep quality, DNA methylation, and infertility, this study aimed to provide relevant strategies to support the prevention of female infertility.
Methods
Study design and population
This study utilized a prospective pre-pregnancy cohort known as the Reproductive Health of Childbearing Couples—Anhui Cohort (RHCC-AC). The RHCC-AC was divided into two subcohorts: newlyweds cohort and infertility-specific cohort. The details of the newlyweds and infertility-specific cohort are described in Table S1. Written informed consent was obtained from each participant prior to enrolment in the study.
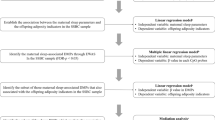
The present study is a population-based, case‒control study involving women with infertility from the infertility-specific cohort as the case group and individually matched women with normal fertility from the newlyweds cohort as the control group. The cases were filtered based on the following exclusion and inclusion criteria. The inclusion criteria were as follows: 1) women aged < 35 years; 2) availability of complete questionnaire data; 3) eligible DNA samples extracted from whole blood samples; 4) failure to achieve a clinical pregnancy after at least 12 months of regular unprotected intercourse; and 5) ovulation disorders identified as the cause of infertility. The exclusion criteria were as follows: 1) other causes of infertility, such as chromosomal anomalies; blocked, damaged, or absent fallopian tubes; EMS, and others and 2) failure to meet the quality control standards for the Illumina Human Methylation 850K BeadChip Genome-wide methylation experiment. Cases and controls were matched in a 1:1 ratio based on age less than or equal to 3 years, sex, and region of residence. A total of 43 cases and 43 controls were included in the final analysis. The flowchart depicting the selection process is shown in Fig. 1.
The Ethics Committee of Anhui Medical University (number: 20189999) approved all study protocols, and informed consent was obtained from all participants before enrolment in the study.
Information and sample collection
Under the guidance of trained staff, participants completed a self-reported questionnaire that solicited information regarding age (21–25, 26–29, and 30–32 years), education (technical secondary school or below or college degree or above), income (less than 60,000 Yuan or at least 60,000 Yuan), employment (employed or unemployed), menarche age (< 13, 13–15, or ≥ 15 years), history of adverse pregnancy (spontaneous abortion, embryo arrest, stillbirth, etc.), cigarette smoking (yes or no), alcohol consumption (yes or no), consumption of cured/grilled/fried food (≤ 3 times a week or > 3 times a week), consumption of sugar-sweetened beverages (SSBs) (≤ 3 times a week or > 3 times a week), consumption of takeaway foods (≤ 3 times a week or > 3 times a week), average sitting time ([sitting time on weekdays × 5) + (sitting time on rest days × 2])/7, categorized as < 8 h or ≥ 8 h], physical activity (based on the International Physical Activity Questionnaire [29], categorized as moderate or high level of physical activity or low level of physical activity), duration of electronic device usage before bedtime (< 30 min and ≥ 30 min), height and weight, and depressive symptoms.
The study utilized a comprehensive approach to construct two composite variables, namely “socioeconomic status (SES)” and “unhealthy lifestyle.” “SES” was constructed by amalgamating education, income, and employment factors [30], whereas “unhealthy lifestyle” was constructed by amalgamating cigarette smoking; consumption of alcohol, cured/grilled/fried food, SSBs, and takeaway foods; average sitting time; physical activity; and electronic device usage before bedtime [30].
Body mass index (BMI) was calculated using the participants’ weight in kilograms divided by the height in meters squared using the Chinese criteria. The BMI values were then classified into two groups: “underweight and normal weight” (< 23.9 kg/m2) and “overweight and obesity” (≥ 24 kg/m2). Depressive symptoms were evaluated using the Patient Health Questionnaire 9 (PHQ-9). Further details regarding the evaluation of depressive symptoms are described elsewhere [31].
Clinical information of the study participants, including the type of infertility and the duration for which they were trying to become pregnant, was obtained from their medical records.
Sleep behaviors
Difficulty falling asleep was assessed using a single question, “How long do you need to fall asleep?” Participants who reported a time of 30 min or longer to fall asleep were classified as experiencing difficulty falling asleep.
Daytime sleepiness among couples of childbearing age was measured using the Epworth Sleepiness Scale (ESS), which was designed at Epworth Hospital in Melbourne, Australia. It serves as a tool to assess the degree of daytime sleepiness easily and accurately among study participants [32]. The ESS consists of eight items, and participants rate individual items on a 4-point scale, with 0 (never doze), 1 (rarely doze), 2 (sometimes doze), and 3 (often doze) points. The scores for the eight items are added up to generate a total score ranging from 0 to 24, with higher scores indicating a more obvious tendency toward daytime sleepiness. According to the scores, participants can be divided into two groups: nondaytime sleepiness (0–10 points) and daytime sleepiness (11–24 points). In this study, the Cronbach’s α coefficient of the ESS was 0.76, indicating good internal consistency.
Whole blood DNA extraction and BeadChip DNA methylation assay
At the time of participant recruitment, whole blood specimens were collected by the laboratory physician. The samples were stored at room temperature, transferred to the laboratory, and stored in the laboratory at − 80°C until further analysis. Whole blood DNA was extracted and purified from the blood samples of patients and controls using the MagNA Pure nucleic acid purification platform (Roche Diagnostics GmbH, Germany) along with the MagNA Pure 24 nucleic acid purification kit. DNA samples were selected based on an OD260/280 ratio ranging from 1.7 to 2.0 for further experiments. The integrity of all DNA samples was assessed by agarose gel electrophoresis. During electrophoresis, the DNA samples were separated based on size and observed for the presence of a clear main band. The main band was required to be intact and not less than 10 kb in size, and without any obvious degradation.
DNA isolation and analysis of DNA methylation patterns were performed using the Infinium Human Methylation 850 K BeadChips (Illumina Inc., San Diego, CA, USA), which enable the assessment of methylation levels at 853 307 CpG sites across the genome. To prepare the DNA samples for analysis, they were subjected to bisulfite treatment using the EZ DNA Methylation Kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s protocol. The processed DNA samples were then hybridized to the BeadChip (Illumina) according to the Illumina Infinium HD Methylation Protocol. The resulting Illumina intensity data (IDAT) files from the chip were further processed by the R/Bioconductor (version 3.3.3) package ChAMP. Batch effect were adjusted for DNA methylation. To mitigate the potential influence of varying proportions of cell types on DNA methylation patterns [33], methylation data were corrected for cell type heterogeneity between samples [34]. Additionally, we identified differently methylated probes (DMPs) at significance of BH-adjusted p-value < 0.05 and absolute value of △β > 0.05. The DNA methylation level at each CpG site was reported as a β value, ranging from 0–1, where 0 represents a nonmethylated probe and 1 represents a fully methylated probe.
Statistical analysis
All statistical analyses were performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA), and R 4.1.2 software. A two-sided P value < 0.05 was considered statistically significant.
Descriptive statistics, such as mean (standard deviation) or percentage, were used to describe the demographic characteristics of the participants. The chi-square test was used to compare various basic characteristics (e.g., age, SES, lifestyle, depressive symptoms) between the cases and controls. Conditional logistic regression analysis was performed to investigate the effect of sleep characteristics of women on infertility.
The mediator variable, DNA methylation sites, underwent initial screening prior to conducting the causal mediation analysis. The screening process involved three steps. Firstly, the DMP function of the R package ChAMP was utilized to identify methylation sites that exhibited significant differences between the infertility group and the control group. Secondly, the DMP function was employed to identify methylation sites that showed statistical associations with difficulty falling asleep. Thirdly, the candidates identified from the previous two steps, which demonstrated co-associations with both difficulty falling asleep and infertility, were further screened. Finally, the causal mediation effects of the candidate methylation sites were analyzed using the Bayesian estimation method provided by the R package “mediation”. Specifically, the average direct effect, average causal mediation effect, and total effect were estimated.
Results
Characteristics of participants
A total of 86 women of reproductive age were included in the survey, with 43 women each in the case and control groups. The average age of the participants was 27.6 ± 2.8 years, and the average ages of the women in the case group and the control group were 27.8 ± 3.0 years and 27.4 ± 2.7 years, respectively. No significant differences were observed in the average age between the two groups (t = − 0.69, P > 0.05). The results of the χ2 goodness-of-fit test indicated that the rates of overweight and obesity in the case group were significantly different from those in the control group (P < 0.05; Table 1).
Overall changes in blood genomic DNA methylation among women of reproductive age
After data processing and quality control, DNA methylation data generated using the Illumina Methylation EPIC array were available for analysis in 43 cases and 43 controls, and a total of 753 354 eligible CpG sites were selected for further analysis. Figure 2A and B depict the methylation density distribution plots for the original and normalized data, respectively. These plots provide an overview of the distribution of methylation levels across all loci in each sample. The x-axis represents the β-value, which ranges from 0 to 1, whereas the y-axis represents the frequency of loci occurrence. Based on the observations presented in Fig. 2, it is evident that the implementation of standardization treatment effectively mitigated the disparities among the samples within each group. Moreover, the overall magnitude of methylation differences between the samples was relatively modest. Furthermore, the density curves exhibited a bimodal distribution, suggesting that the majority of methylated loci were either hypomethylated or hypermethylated. The distribution of average methylation levels for the case and control groups is shown in Fig. 3. The β-value, ranging from 0 to 1, represents the methylation level at each CpG site. The evenly distributed boxes in the figure indicate the symmetry of the methylation data in this assay.
Box plot for case and control samples. The horizontal coordinates in the box-and-line plot represent each sample, the vertical coordinates represent the β-value (a β-value of 0 indicates that no methylation has occurred at the locus, a β-value < 0.2 indicates hypomethylation, a β-value between 0.2 and 0.8 indicates partial methylation, a β-value > 0.8 indicates hypermethylation, and a β-value equal to 1 indicates complete methylation), and the black line in the box represents the median (i.e., the average level of methylation) methylation level. The upper and lower limits of the boxes represent the quartiles of methylation
Of the 753 354 probes analyzed on the EPIC BeadChips, a total of 262 differentially methylated CpGs corresponding to 185 differential methylation genes were identified after adjusting for batch effect and cell type effects. Among these, there were 180 hypermethylated CpGs and 82 hypomethylated CpGs (Table S1). Probes that showed a greater than 5% change in β-value (BH-adjusted P < 0.05) between the case and control groups were classified as differentially methylated sites (Fig. 4).
Volcano plot for differential DNA methylation analysis between the case and control groups. The x-axis shows the DNA methylation difference (△β), and the y-axis shows the − log10 p-value of each CpG site. Red color represents hypermethylation sites, blue color represents hypomethylation sites, and black color represents nonsignificant sites
Effects of sleep quality on female infertility
Among the 86 women of childbearing age included in this study, 26 (30.2%) reported experiencing difficulty falling asleep. The results showed that there were significant differences between the case and control groups in terms of difficulty falling asleep (χ2 = 5.51, P < 0.01). Moreover, after controlling for confounding factors, logistic regression analysis revealed that difficulty falling asleep was a significant risk factor for infertility in women (OR = 3.69, 95%CI = 1.14, 11.99; Table 2).
Causal mediation analysis of difficulty falling asleep, DNA methylation, and infertility among women
The mediator variable, DNA methylation sites, underwent initial screening prior to conducting causal mediation analysis. The DMP function of the R package ChAMP was utilized to identify a total of 262 CpG methylation sites that exhibited significant differences between the infertility group and the control group. Eight methylation sites were selected based on their co-association with difficulty falling asleep and infertility. These eight selected CpG sites were included in the causal mediation analysis (Table 3). Model 1, which did not control for covariates, identified only one methylation site, cg08298632, that played a positive mediating role in the relationship between difficulty falling asleep and infertility among women. The mediating effect coefficient for cg08298632 was 0.10 (95%CI = 0.01–0.21), and the proportion of the total effect mediated by this methylation site was 37.3% (Table 3). After adjusting for covariates in Model 2, the positive mediating role of cg08298632 in the relationship between difficulty falling asleep and infertility remained. The mediating effect coefficient for cg08298632 was 0.10 (95%CI = 0.01–0.22), and the proportion of the total effect mediated by this methylation site increased to 64.3% (Fig. 5).
Discussion
Infertility is a significant public health concern, and it is necessary to identify factors that contribute to infertility and tailor effective prevention interventions. To the best of our knowledge, this study is the first to report altered DNA methylation patterns in the whole blood of infertile women. We identified a total of 262 differential DNA methylation sites, with 180 sites showing significant hypermethylation and 82 sites showing hypomethylation in women with ovulation disorders compared to the normal fertility control group. The study revealed that difficulty falling asleep is associated with an increased risk of infertility in women of childbearing age. The causal mediation analysis identified specific DNA methylation sites (cg08298632) that mediate the relationship between difficulty falling asleep and infertility.
We found that difficulty falling asleep was a risk factor for infertility in women. This is consistent with previous studies. A study conducted on 1,176 couples in North America also reported reduced fertility in couples where women experienced difficulty falling asleep compared to those with normal sleep patterns [18]. Another study conducted at Tangshan Maternal and Child Health Hospital found that poor sleep quality, late sleeping time, and insufficient sleep were all associated with an increased risk of infertility [35]. Previous studies have also shown that circadian disturbances affect various aspects of reproductive function, such as follicular development, hormone secretion, and regulation of the menstrual cycle; they have also been associated with an increased risk of spontaneous abortion and preterm birth [14, 36]. The sleep characteristics of women with infertility may vary depending on the underlying etiology, and studies have found that compared to women with unexplained infertility, women with PCOS report more daytime sleepiness [37, 38]. However, in the present study, daytime sleepiness was not found to be a risk factor for female infertility, despite its association with difficulty falling asleep. The most common sleep issues are difficulty falling asleep and excessive daytime sleepiness [39], difficulty in falling asleep commonly leads to daytime sleepiness, lethargy, and a general feeling of being unwell [40]. However, difficulty falling asleep and excessive daytime sleepiness are two different sleep issues with distinct symptoms, causes, and mechanisms [41]. Further research and in-depth analysis are necessary to explore these relationships and gain a deeper understanding of the mechanisms and factors influencing sleep and its impact on female infertility.
Studies investigating the mediating role of DNA methylation in the relationship between sleep and infertility are relatively limited. However, previous research has demonstrated that DNA methylation could potentially function as an intermediary mechanism linking environmental exposure and the onset of diseases, thereby influencing the relationship between these two factors through the regulation of gene expression [42, 43]. A prospective cohort study showed the mediating effect of DNA methylation in the association between maternal sleep during pregnancy and offspring adiposity status. Specific DNA methylation sites, such as cg04351668, cg12225226, and cg12232388 were found to significantly mediate the relationship between sleep midpoint and offspring’s subcutaneous fat and BMI [44]. Additionally, a survey outcome revealed that sperm DNA methylation mediates the association between male age and reproductive outcomes among couples undergoing infertility treatment [45].
In this study, the causal mediation analysis revealed that a DNA methylation site (cg08298632) played a positive mediating role in the relationship between difficulty falling asleep and infertility in women. This site is located on the KCNC2 gene, which is a protein-coding gene that encodes components of voltage-gated potassium channels that regulate voltage-dependent potassium ion permeability in excitable membranes and is mainly expressed in the brain. Studies have shown that overexpression of the KCNC2 gene in hippocampal neurons may lead to higher amplitude and faster dynamics of potassium currents, which are linked to neuronal hyperexcitability in patients with bipolar disorder [46]. Other studies have shown that Kcnc1 is closely related to circadian rhythm. Knockout mice lacking Kcnc1 and Kcnc2 are unable to express Kv 3.1 and Kv 3.2 potassium channels in the suprachiasmatic nucleus, and the fast delayed rectifying potassium channel current is greatly reduced, showing an extremely disordered daily rhythm [47]. Furthermore, the Kcnc2 polymorphism is also associated with reproductive traits in animals, such as litter size in sows [48]. In conclusion, we suggest that difficulty falling asleep among women may influence the occurrence of infertility through DNA methylation of the KCNC2 gene. However, further research is needed to fully understand the underlying mechanisms and pathways involved in this association.
To the best of our knowledge, this study is the first to utilize patients with anovulatory infertility as the case group, while normal fertility females were used as the control group. The use of a DNA methylation 850 K chip for genome-wide DNA methylation analysis allowed for a comprehensive examination of the association between DNA methylation and infertility, shedding light on the epigenetic pathogenesis and mechanisms underlying female infertility. Causal mediation analysis was used to further explore the mediation effect of DNA methylation in the relationship between sleep quality and infertility in women, providing more robust evidence for the underlying biological mechanisms linking sleep quality and infertility, and providing a theoretical basis to develop strategies for the prevention and treatment of infertility.
This study has some limitations that should be considered. First, the assessment of sleep quality relied on self-report measures, which might lead to self-reporting bias. Second, due to the limited funding, the sample size of this study was small, and no comparative analysis of different types and causes of infertility was conducted. Increasing the sample size and conducting in-depth stratification analysis in future studies would provide more robust and comprehensive findings. Third, this study did not include in-depth functional studies of differentially methylated sites, methylated genes, and the selected genes with mediation effects identified from the methylated 850 K chips. Finally, while the causal mediation analysis provides valuable insights, it only suggests possible causal associations and cannot fully confirm the causal associations between difficulty falling asleep, DNA methylation, and infertility, which should be further verified by experimental studies in the future.
Conclusion
In conclusion, the findings of this study suggest that DNA methylation sites play a significant role in mediating the relationship between difficulty falling asleep and infertility in women. Specifically, the DNA methylation site cg08298632 was found to be a mediating variable. These findings contribute to our understanding of the underlying mechanisms that connect difficulty falling asleep and infertility in women. Further studies are necessary to fully understand the biological significance and potential therapeutic applications of these findings. The identified DNA methylation sites provide new and valuable insights and potential targets for future studies aiming to prevent and treat female infertility.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76.
Sun H, Gong T-T, Jiang Y-T, Zhang S, Zhao Y-H, Wu Q-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: results from a global burden of disease study, 2017. Aging (Albany NY). 2019;11(23):10952–91.
Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–26.
Zurlo MC, Cattaneo Della Volta MF, Vallone F. Infertility-related stress and psychological health outcomes in infertile couples undergoing medical treatments: testing a multi-dimensional model. J Clin Psychol Med Settings. 2020;27(4):662–76.
Kasman AM, Del Giudice F, Eisenberg ML. New insights to guide patient care: the bidirectional relationship between male infertility and male health. Fertil Steril. 2020;113(3):469–77.
Palomba S, Falbo A, Daolio J, Battaglia FA, La Sala GB. Pregnancy complications in infertile patients with polycystic ovary syndrome: updated evidence. Minerva Ginecol. 2018;70(6):754–60.
Onat G, Kizilkaya Beji N. Effects of infertility on gender differences in marital relationship and quality of life: a case-control study of Turkish couples. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):243–8.
Alipour Z, Kazemi A, Kheirabadi G, Eslami A-A. Relationship between marital quality, social support and mental health during pregnancy. Community Ment Health J. 2019;55(6):1064–70.
Sharifi F, Jamali J, Larki M, Roudsari RL. Domestic violence against infertile women: a systematic review and meta-analysis. Sultan Qaboos Univ Med J. 2022;22(1):14–27.
Kawwass JF, Badell ML. Maternal and fetal risk associated with assisted reproductive technology. Obstet Gynecol. 2018;132(3):763–72.
Hart RJ. Physiological aspects of female fertility: role of the environment, modern lifestyle, and genetics. Physiol Rev. 2016;96(3):873–909.
Mumford SL, Johnstone E, Kim K, Ahmad M, Salmon S, Summers K, Chaney K, Ryan G, Hotaling JM, Purdue-Smithe AC, et al. A prospective cohort study to evaluate the impact of diet, exercise, and lifestyle on fertility: design and baseline characteristics. Am J Epidemiol. 2020;189(11):1254–65.
Pisarska MD, Chan JL, Lawrenson K, Gonzalez TL, Wang ET. Genetics and epigenetics of infertility and treatments on outcomes. J Clin Endocrinol Metab. 2019;104(6):1871–86.
Fernandez RC, Marino JL, Varcoe TJ, Davis S, Moran LJ, Rumbold AR, Brown HM, Whitrow MJ, Davies MJ, Moore VM. Fixed or rotating night shift work undertaken by women: implications for fertility and miscarriage. Semin Reprod Med. 2016;34(2):74–82.
Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:813764.
Shi F, Liu C, Liu K, Sun L, Yang H, Cao J, Chen Q. Female and male sleep duration in association with the probability of conception in two representative populations of reproductive age in US and China. Sleep Med. 2020;74:9–17.
Wang ID, Liu Y-L, Peng C-K, Chung C-H, Chang S-Y, Tsao C-H, Chien PhD W-C. Non-Apnea Sleep Disorder Increases the Risk of Subsequent Female Infertility-A Nationwide Population-Based Cohort Study. Sleep. 2018;41(1):zxs186.
Willis SK, Hatch EE, Wesselink AK, Rothman KJ, Mikkelsen EM, Wise LA. Female sleep patterns, shift work, and fecundability in a North American preconception cohort study. Fertil Steril. 2019;111(6):1201–10.
Ilie IR, Georgescu CE. Polycystic ovary syndrome-epigenetic mechanisms and aberrant MicroRNA. Adv Clin Chem. 2015;71:25–45.
Grimstad FW, Decherney A. A review of the epigenetic contributions to endometriosis. Clin Obstet Gynecol. 2017;60(3):467–76.
Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21.
Ziller MJ, Gu H, Müller F, Donaghey J, Tsai LTY, Kohlbacher O, De Jager PL, Rosen ED, Bennett DA, Bernstein BE, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500(7463):477–81.
Gaine ME, Chatterjee S, Abel T. Sleep Deprivation and the Epigenome. Front Neural Circuits. 2018;12:14.
Koopman-Verhoeff ME, Mulder RH, Saletin JM, Reiss I, van der Horst GTJ, Felix JF, Carskadon MA, Tiemeier H, Cecil CAM. Genome-wide DNA methylation patterns associated with sleep and mental health in children: a population-based study. J Child Psychol Psychiatry. 2020;61(10):1061–9.
Carskadon MA, Chappell KR, Barker DH, Hart AC, Dwyer K, Gredvig-Ardito C, Starr C, McGeary JE. A pilot prospective study of sleep patterns and DNA methylation-characterized epigenetic aging in young adults. BMC Res Notes. 2019;12(1):583.
Lahtinen A, Puttonen S, Vanttola P, Viitasalo K, Sulkava S, Pervjakova N, Joensuu A, Salo P, Toivola A, Härmä M, et al. A distinctive DNA methylation pattern in insufficient sleep. Sci Rep. 2019;9(1):1193.
Ding L, Yang L, Ren C, Zhang H, Lu J, Wang S, Wu Z, Yang Y. A review of aberrant DNA methylation and epigenetic agents targeting DNA methyltransferases in endometriosis. Curr Drug Targets. 2020;21(11):1047–55.
Vázquez-Martínez ER, Gómez-Viais YI, García-Gómez E, Reyes-Mayoral C, Reyes-Muñoz E, Camacho-Arroyo I, Cerbón M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction. 2019;158(1):R27–40.
van Poppel MNM, Chinapaw MJM, Mokkink LB, van Mechelen W, Terwee CB. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med. 2010;40(7):565–600.
Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, Liu G, Pan A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. 2021;373:n604.
Gan H, Li M, Wang X, Yang Q, Tang Y, Wang B, Liu K, Zhu P, Shao S, Tao F. Low and mismatched socioeconomic status between newlyweds increased their risk of depressive symptoms: A multi-center study. Front Psychiatry. 2022;13:1038061.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5.
Jaffe AE, Irizarry RA. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol. 2014;15(2):R31.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86.
Shang G, Ju-Xiang Y, Bao-Sheng H. A case-control study on related risk factors of female infertility. Maternal and Child Health Care of China. 2015;30(18):3014–6.
Begtrup LM, Specht IO, Hammer PEC, Flachs EM, Garde AH, Hansen J, Hansen ÅM, Kolstad HA, Larsen AD, Bonde JP. Night work and miscarriage: a Danish nationwide register-based cohort study. Occup Environ Med. 2019;76(5):302–8.
Chatterjee B, Suri J, Suri JC, Mittal P, Adhikari T. Impact of sleep-disordered breathing on metabolic dysfunctions in patients with polycystic ovary syndrome. Sleep Med. 2014;15(12):1547–53.
Eisenberg E, Legro RS, Diamond MP, Huang H, O’Brien LM, Smith YR, Coutifaris C, Hansen KR, Santoro N, Zhang H. Sleep Habits of Women With Infertility. J Clin Endocrinol Metab. 2021;106(11):e4414–26.
Gauld C, Lopez R, Morin C, Geoffroy PA, Maquet J, Desvergnes P, McGonigal A, Dauvilliers Y, Philip P, Dumas G, et al. Symptom network analysis of the sleep disorders diagnostic criteria based on the clinical text of the ICSD-3. J Sleep Res. 2022;31(1):e13435.
Djokic G, Vojvodić P, Korcok D, Agic A, Rankovic A, Djordjevic V, Vojvodic A, Vlaskovic-Jovicevic T, Peric-Hajzler Z, Matovic D, et al. The effects of magnesium - Melatonin - Vit B complex supplementation in treatment of insomnia. Open Access Maced J Med Sci. 2019;7(18):3101–5.
Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–94.
Schübeler D. Function and information content of DNA methylation. Nature. 2015;517(7534):321–6.
Fujii R, Sato S, Tsuboi Y, Cardenas A, Suzuki K. DNA methylation as a mediator of associations between the environment and chronic diseases: A scoping review on application of mediation analysis. Epigenetics. 2022;17(7):759–85.
Meng M, Jiang Y, Lin J, Zhang J, Wang G, Zhu Q, Lin Q, Jiang F. The mediating effect of DNA methylation in the association between maternal sleep during pregnancy and offspring adiposity status: a prospective cohort study. Clin Epigenetics. 2022;14(1):66.
Oluwayiose OA, Wu H, Saddiki H, Whitcomb BW, Balzer LB, Brandon N, Suvorov A, Tayyab R, Sites CK, Hill L, et al. Sperm DNA methylation mediates the association of male age on reproductive outcomes among couples undergoing infertility treatment. Sci Rep. 2021;11(1):3216.
Stern S, Sarkar A, Stern T, Mei A, Mendes APD, Stern Y, Goldberg G, Galor D, Nguyen T, Randolph-Moore L, et al. Mechanisms underlying the hyperexcitability of CA3 and dentate gyrus hippocampal neurons derived from patients with bipolar disorder. Biol Psychiatry. 2020;88(2):139–49.
Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci. 2011;31(8):2746–55.
Sato S, Kikuchi T, Uemoto Y, Mikawa S, Suzuki K. Effect of candidate gene polymorphisms on reproductive traits in a Large White pig population. Anim Sci J. 2016;87(12):1455–63.
Acknowledgements
The authors thank the newlyweds who participated in our study and the doctors, nurses and other clinic staff who supported this study.
Funding
This study was supported by the National Key Reasearch and Development Program of China (Grant No. 2018YFC1004201), Key Program of Natural Science Research of Higher Education of Anhui Province (No. 2022AH050672), 2022 Anhui Provincial Key Laboratory of Population Health and Aristogenics open topics (JKYS20224) and the Research Fund of Anhui Institute of Translational Medicine (2022zhyx-C05). The funder was not involved in any of the processes including the design of the study, the data collection, analysis, data interpretation and manuscript writing.
Author information
Authors and Affiliations
Contributions
YT, HG: Conceptualization, Investigation, Data curation and analysis, Writing-Original draft preparation and revision. BW, XW, ML, QY: Conceptualization, Investigation, Testing. MG: Conceptualization, Investigation supervision, Writing- Reviewing and Editing. Conceptualization, Investigation supervision, Funding acquisition, Writing- Reviewing and Editing. PZ, SS: Critical revision, Writing-Reviewing and Editing. FT: Project administration and supervision, Funding acquisition, Critical revision, Writing-Reviewing and Editing. Final approval of the version to be published: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Ethics Committee of Anhui Medical University (Number: 20189999). Additionally, all methods in this study were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The details of the Reproductive Health of Childbearing Couples—Anhui Cohort (RHCC-AC). Table S2. Comparison of differential methylation sites between case and control group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, Y., Gan, H., Wang, B. et al. Mediating effects of DNA methylation in the association between sleep quality and infertility among women of childbearing age. BMC Public Health 23, 1802 (2023). https://doi.org/10.1186/s12889-023-16681-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16681-w