Abstract
Background
Childhood overweight/obesity has been associated with an elevated risk of insulin resistance and cardiometabolic disorders. Waist-to-height ratio (WHtR) may be a simple screening tool to quickly identify children at elevated risk for cardiometabolic disorders. The primary objective of the present study was to create sex-specific tertile cut points of WHtR and assess its association with Insulin resistance and elevated liver enzyme concentrations in children, factors using cross-sectional data from the randomized, controlled Family Weight Management Study.
Methods
Baseline data from 360 children (7–12 years, mean Body Mass Index (BMI) ≥ 85th percentile for age and sex) were used to calculate WHtR tertiles by sex, male: ≤ 0.55 (T1), > 0.55- ≤ 0.59 (T2), > 0.59 (T3); female: ≤ 0.56 (T1), > 0.56- ≤ 0.6 (T2), > 0.6 (T3). The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was used to categorize participants as insulin-resistant (HOMA-IR ≥ 2.6) and insulin-sensitive (HOMA-IR < 2.6). Liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were categorized as normal vs. elevated (AST of < 36.0 µkat/L or ≥ 36.0 µkat/L; ALT of < 30.0 µkat/L or ≥ 30.0 µkat/L; ALT > 26 µkat/L males, > 22 µkat/L females). We examined differences in baseline cardiometabolic risk factors by WHtR tertiles and sex-specific multivariable logistic regression models to predict HOMA-IR and elevation of liver enzymes.
Results
Study participants had a mean WHtR of 0.59 ([SD: 0.06]). Irrespective of sex, children in WHtR T3 had higher BMIz scores, blood pressure, triglycerides, 2-h glucose, fasting 2-h insulin, and lower high-density lipoprotein cholesterol (HDL-C) concentrations than those in T2 and T1. After adjusting for covariates, the odds of elevated HOMA-IR (> 2.6) were over five-fold higher among males in T3 versus T1 [OR, 95%CI: 5.83, 2.34–14.52] and T2 [OR, 95%CI: 4.81, 1.94–11.92] and females in T3 [OR, 95%CI: 5.06, 2.10–12.20] versus T1. The odds of elevated ALT values (≥ 30) were 2.9 [95%CI: 1.01–8.41] fold higher among females in T3 compared to T1.
Conclusion
In public health settings, WHtR may be a practical screening tool in pediatric populations to identify children at risk of metabolic syndrome.
Similar content being viewed by others
Background
Obesity, characterized by the excessive accumulation of adipose tissue, increases cardiometabolic risk factors such as hyperglycemia, dyslipidemia, and insulin resistance (IR), has increased dramatically over the past few decades [1, 2]. Many of these adverse metabolic factors are strongly associated with their prevalence later in life. Although various anthropometric and biochemical measures are viable markers for cardiometabolic risk detection in adults, there is a lack of substantial research examining the accuracy of these measures as predictors in children [3]. Childhood obesity is also associated with an increased risk of developing non-alcoholic fatty liver disease (NAFLD) later in life, with one of the histological stages being liver fibrosis [4]. Hepatic ectopic lipid deposition can result in inflammation and liver fibrosis [5,6,7]. Obesity and IR increase liver fibrosis risk [8, 9]. Measures of liver function, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), are indicators of cellular liver injury [10]. The lack of research on the pathogenesis of liver fibrosis in children and the unavailability of current assessment methods further emphasizes the need for simple, noninvasive methods to assess this condition [11, 12].
Waist-to-height ratio (WHtR), derived as a ratio of waist circumference in centimeters (cms) by height (cms), a simple screening tool [13], has recently been proposed as a diagnostic measure of early cardiometabolic risk in both children [3, 14,15,16] and adults [16,17,18,19]. WHtR is more strongly associated with cardiovascular and cardiometabolic risk factors than individual anthropometric measures, such as waist circumference, Body Mass Index (BMI), and waist-hip ratio [15, 17, 20,21,22,23,24]. [21, 22]. The results of systematic reviews and meta-analyses of WHtR in adults have suggested values above 0.5 represents increased cardiometabolic risk [14, 25]. A recent meta-analysis [18] examining WHtR values in children and adolescents suggested a single cutpoint of 0.49 for both boys and girls. In contrast, a second meta-analysis [16] did not support this conclusion.
The aim of the study was to establish sex-specific tertile cut points of WHtR and assess their association with Insulin Resistance (IR), cardiometabolic risk factors, and elevated liver enzyme concentrations in children 7–12 years. Our analyses explore the potential to identify the risk phenotype using the waist-to-height ratio (WHtR) [25].
Methods
Setting
The study utilized baseline data from the Family Weight Management Study (also known as the Fun Healthy Families study), a randomized controlled trial [26] conducted from 2009 to 2013 in a pediatric ambulatory program of an urban hospital that provides safety-net primary care services in the Bronx, New York, United States.
Participants
Study participants (N = 360) included children aged 7–12 years with a BMI ≥ 85th percentile for age and sex [27]. Exclusion criteria for the participants included any chronic illnesses, a physical, cognitive, or emotional impairment that would impact the safety of participants during study procedures, medical treatment causing fluctuations in body weight, inconvenient transportation distances, involvement in a separate weight management program, and unwillingness or inability of the parents or child to provide consent and assent, respectively. The trial design with the CONSORT diagram and study process is described elsewhere [26]. The Albert Einstein College of Medicine Institutional Review Board (IRB) approved all study protocols; all study participants provided written consent (parent or guardian) or assent (children).
Anthropometric measures
Height and weight were measured in light clothing and without shoes. A stadiometer and a digital scale were used to obtain height and weight, respectively. The waist circumference was measured using an elastic tape at the iliac crest, and the hip circumference at the point of maximal protrusion of the gluteal muscles in the lateral position. Both were recorded to the nearest centimeter. Scales and stadiometer were calibrated, and anthropometry tapes were examined for signs of wear weekly using standardized protocols.
Cardiometabolic parameters
As previously reported in Wylie-Rosett et al. [26], systolic and diastolic blood pressures were measured three times according to traditional pediatric standards using appropriate cuff size with a manual sphygmomanometer after sitting for 2 min. Blood specimens were obtained after a minimum of an 8-h fast. Fasting glucose, triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol concentrations were measured spectrophotometrically using a Beckman-Coulter LX-20 auto-analyzer (Brea, CA). A glucose amount of 1.75 g/kg body weight (GlucolaTM) was administered for the 2-h Oral Glucose Tolerance Test. The liver enzymes, alanine transaminase (ALT/SGPT) and aspartate aminotransferase (AST/SGOT), concentrations were measured using an Immulite 2000 analyzer (Bio-DPC; Siemens Medical, Gwynedd, UK).
Intermediate parameters
The following variables were used as markers for increased cardiometabolic risk:
-
WHtR parameters: There is no consensus on the appropriate cut points of WHtR in pediatric populations [15,16,17,18, 20, 28]. Previous reviews and analyses have indicated that dichotomized WHtR cut points at ≥ 0.5 [18] or 0.55 [29] are surrogates of increased risk in children; however, they may be insignificant when assessed for sensitivity and specificity to certain variables [30,31,32]. Therefore, the following cut points by sex were used based on the sample data to group children into three categories: females WHtR ≤ 0.56 (T1), WHtR > 0.56—≤ 0.60 (T2), and WHtR > 0.60 (T3); males WHtR ≤ 0.55 (T1), WHtR > 0.55—≤ 0.59 (T2), and WHtR > 0.59 (T3).
-
Insulin Resistance (IR): IR is a critical component of cardiovascular disease and metabolic syndrome (MetS) [1, 33]. Though increased HOMA-IR values are associated with higher risk, no clear cut point is used to assess IR in pediatric clinical studies [33]. We used HOMA-IR values < 2.6 and ≥ 2.6 to evaluate increased cardiometabolic risk based on prior published research from the Family Weight Management Study [34, 35].
-
Liver enzymes: Measures of serum AST(SGOT) and ALT(SGPT) levels have been used extensively in studies to assess liver damage [10]. There are different cut points proposed [36,37,38]. AST values of < 36.0 µkat/L and ≥ 36.0 µkat/L and ALT values of < 30.0 µkat/L and ≥ 30.0 µkat/L are proposed as cut points associated with increased risk of liver injury in children [39] and adopted for this study. In addition to these cut points, we also examined the following cut points 22 µkat/L for girls and 26 µkat/L for boys based on the North American Society For Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) Clinical Practice Guideline review [36, 38].
Statistical analysis
A sex-specific demographic, anthropometric, and cardiometabolic biomarker distribution was summarized using descriptive statistics. Normally distributed continuous variables were numerically summarized using mean (standard deviation), while non-normally distributed were presented with median (interquartile range). The categorical variables were presented as frequency counts and percentages. The difference in child characteristics among the WHtR tertile categories (sex-specific) was assessed using analysis of variance, the Kruskal–Wallis test, or the Pearson chi-square test. We modeled HOMA-IR, AST, and ALT values as binary variables for association models. The association between WHtR tertile categories (sex-specific) and outcome variables HOMA-IR, AST, and ALT, adjusting for the other covariates, was examined using a multivariable logistic regression model. Firth's bias-corrected logistic regression was used to associate WHtR and outcomes with a small number of events or when the issue of quasi or complete separation arose. We also modeled binary WHtR cut points (> 0.5 v ≤ 0.5; > 0.55 v ≤ 0.55; M: > 0.59 v ≤ 0.59, F: > 0.60 v ≤ 0.60) for comparison. Covariates (child’s age, race, ethnicity, household income, parent’s education, occupation, Tanner stage) that were significantly different at the 20% level at the univariable model as were demographic confounders were considered for the multivariable model. Final multivariable models were adjusted for the child’s age, race, ethnicity, parent’s education, and occupation; in addition, HOMA-IR models were adjusted for the tanner stage. The Tanner stage variable had 13.6% missing data, which was addressed using a fully conditional specification multiple imputations approach. Ten imputation data sets were generated, and estimates were pooled using Rubin’s rules [40].
Results
Participant Characteristics
Three hundred and sixty children participated in the study, of which 52% (n = 185) were females and 48% (n = 175) were males. Seventy-four percent (n = 267) self-identified as Hispanic, 17.5% (n = 63) as non-Hispanic African American or Black, and 8.3% as non-Hispanic origin, others including Caucasian or White, Asian, Hawaiian, and multiracial. The average age of children was 9.3 (SD: 1.7). A detailed summary of participant characteristics has been previously reported [26]. The average WHtR among the participants was 0.59 (SD: 0.06). The average HOMA-IR, AST(SGOT), and ALT (SGPT) values were 3.68(SD: 2.58), 25.83 (SD: 17.57), and 30.96(SD: 9.40), respectively (Table 1). The demographic, anthropometric, and cardiometabolic characteristics distribution between the sex-specific WHtR tertile were similar to different WHtR categories. For comparisons with our proposed WHtR tertile categories, we also categorized WHtR by commonly used cut points and their distribution by sex is presented in Supplemental Table 1
Differences in cardiometabolic risk parameters
In both sexes, cardiometabolic risk markers, including BMI- z score, SBP, DBP, and 2-h glucose, fasting, and 2-h insulin concentrations, were lowest in children in WHtR T1, intermediate in WHtR T2 and highest in WHtR T3 among categories (Table 1), with markers showing linear relation (either a monotonic increase or decrease) among the WHtR tertile categories in both sexes. HDL-cholesterol concentration was lowest in the WHtR T3 category in both males and females, consistent with the previously reported observation of an inverse relationship between adiposity and HDL-cholesterol concentrations [41]. The liver function biomarker (SGPT/ALT) was positively associated with concentrations that were higher with increasing WHtR categories in males (p = 0.03) but did not reach statistical significance in females (p = 0.08).
Association of WHtR with insulin resistance and liver biomarkers
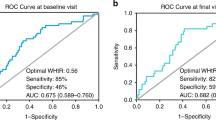
The complete case and multiple imputation model estimated for the HOMA-IR are presented in Table 2. Based on a multiple imputation analysis, female children in T3 (WHtR > 0.60) had a 5.06 (95% CI: 2.10–12.20) fold higher odds of being insulin resistant (HOMA-IR > 2.6) than those in T1 (WHtR ≤ 0.56) (Table 2). The odds of insulin resistance were 4.81 (95%CI: 1.94–11.92) fold higher among T2 than T1. Similarly, the odds of insulin resistance were 5.83 (95%CI: 2.34–14.52) fold higher among T3 (WHtR > 0.59) category than T1 (WHtR ≤ 0.55) among the males. The effect size was not statistically significant and was half for those in T2. We also compared established WHtR binary cut points of > 0.5 [18], > 0.55 [29] and > 0.6 [25, 42, 43]. The gender-specific adjusted OR for binary cut points (males > 0.59 and females > 0.60) were 4.54 (95%CI: 2.17–9.50) and 2.54 (95%CI: 1.22–5.26) for males and females, respectively. The established conservative binary cut points showed elevated risk, but the strength of association was smaller.
ALT as a marker for liver injury in children was assessed using two cut-off criteria: (i) ≥ 30 vs. < 30) and NASPGHAN sex-specific criteria of > 26 vs. ≤ 26 for males and > 22 vs. ≤ 22 for females. Among the females, the odds of elevated ALT (≥ 30) were 2.9-fold higher among T3 compared to the T1 WHtR category (aOR, 2.92; 95% CI: 1.01, 8.41). Although an elevated association was observed in the T2 WHtR category (aOR = 1.77; 95%CI: 0.59, 5.35), the difference did not reach statistical significance (Table 3). Among males, there was a non-significant elevated association between T3 WHtR (aOR = 1.87) and T2 WHtR (aOR = 1.13). When assessed using the NASPGHAN criteria, among both female and male children, the odds of elevated ALT (males > 26; female > 22) showed stronger associations in theT3 WHtR than the T1 category. A non-significant positive association was observed between AST and WHtR among males but not females (Table 4).
As the WHtR tertile cut points were similar in both sexes, we also examined the association between common WHtR tertile cut points and HOMA-IR and liver enzymes. The results suggested a similar pattern to sex-specific results. (Supplemental Table 2).
Discussion
The study's primary finding confirmed our hypothesis and suggested that higher WHtR is associated with an unfavorable cardiometabolic profile, specifically IR and elevated liver biomarkers. The odds ratio magnitude with WHTR tertiles was more substantial in female than male children except for NASPGHAN ALT and AST.
Within this cohort of 7–12-year-old children with a BMI ≥ 85th percentile for age and sex, children in the upper tertile for WHtR had almost 1.83 -5.68-fold higher odds of elevated liver enzyme levels and IR than children in the lowest tertile in both sexes. These results are consistent with previous studies in adults [21, 23, 33] analyzing various WHtR thresholds predictive of higher cardiometabolic risk, adding to the research on anthropometric predictor value and cardiometabolic risk in pediatric populations. Ashwell and Hsieh [13] suggested dichotomized optimal WHtR cut point of 0.5 for both children and adults among different ethnic groups between both sexes. While Khoury et al. [25, 44] used arbitrary cut points < 0.5, > 0.5 to < 0.6, ≥ 0.6 in combination with BMI, showed higher WHtR categories were significant risk factors for lipid and cardiometabolic markers in children with obesity. Another study [45] used 0.512 as the WHtR cut point, ignoring the child’s sex, and concluded there is little difference between BMI and WHtR but preferred WHtR in identifying children with adverse cardiovascular disease (CVD) risk factors. A recent meta-analysis [18] of diagnostic studies assessing the WHtR cut-off value suggested an optimal practical cut point of 0.5; however, this was not replicated in our cohort. A difference between the study cohorts may be their makeup; ours is composed predominantly of Hispanic and Black children with a BMI ≥ 85th percentile for age and sex. This cut-off > 0.6 has been suggested by other studies [25, 42, 43] that showed a similar robust association with cardiometabolic risk and metabolic syndrome in children with obesity.
ALT and AST are widely used as noninvasive screening tools for NAFLD and non-alcoholic steatohepatitis (NASH) in the pediatric population [46]. Although ALT is suggested as currently the best inexpensive screener of NAFLD in children [36], it has limitations such that there is no consensus on ALT normal values, and not all ALT-positive screening will have liver disease, leading to inconsistencies [47]. While proportional relationships between the liver enzymes and varying WHtR thresholds have been established [13, 21, 33], this was not replicated in our cohort, except for ALT (≥ 30). ALT) was nearly threefold higher in female children in WHtR T3 compared to T1. When ALT was assessed using sex-specific NASPGHAN ALT criteria, there was a stronger association with WHtR in both sexes; T3 WHtR compared to the T1 WHtR category.
Obesity screening programs could be incorporated into pediatric settings such as schools and conducted with protocols similar to those used in school FitnessGram and other obesity evaluations [48, 49]. Using the WHtR as a screening tool in schools and public health settings could quickly identify high-risk children who should be referred for further assessment. A population-based screening should be conducted in safe, confidential spaces to minimize stigmatizing children with overweight and obesity. Our study suggests that WHtR could be helpful in identifying children with an unhealthy phenotype of obesity.
Strengths of this study include the scientific rigor of data collection, the availability of a database with an adequate sample size to test the hypotheses, the interface with safety-net primary care, the availability of relevant cardiometabolic biomarkers, and diverse ethnic/racial distributions of the participants.
Study limitations include a single measure of anthropometric and biochemical measures obtained at baseline, hence, the cross-sectional design. Consistent with the parent study protocol criteria, all the children in the trial were overweight or obese, so we did not have a normal weight control group to perform a comparative analysis. Although we had a relatively large sample size, we may not have had adequate statistical power to assess sex-specific differences. The population's demographic characteristics (majority of parents/guardians identified as Hispanic and were born outside of the continental United States) and setting (pediatric safety-net primary care) potentially limit the generalizability of our results.
Conclusion
Assessing WHtR may prove to be an efficient and quick screening method to identify children with overweight and obesity who are at elevated risk for cardiometabolic disorders, particularly those who have IR and elevated liver biomarkers. The approach minimizes the stigma or social disparities associated with obesity. This screening method is feasible for use in schools and other pediatric environments, such as fitness grams and evaluations.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available. Questions about access can be directed to the Study PI’s Drs. Wylie-Rosett (5R18DK075981) and Lichtenstein (5R01HL101236).
Abbreviations
- WHtR:
-
Waist-to-height ratio
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TG:
-
Triglyceride,
- TC:
-
Total cholesterol
- LDL:
-
Low-Density Lipoprotein
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- SD:
-
Standard Deviation
- IR:
-
Insulin Resistance
- NAFLD:
-
Non-Alcoholic Fatty Liver Disease
- NASH:
-
Non-Alcoholic Steatohepatitis
- SGOT:
-
Serum Glutamic-Oxaloacetic Transaminase
- SGPT:
-
Serum Glutamate Pyruvate Transaminase
- NASPGHAN:
-
North American Society For Pediatric Gastroenterology, Hepatology & Nutrition
- aOR:
-
Adjusted Odds Ratio
- BMI:
-
Body Mass Index
- CVD:
-
Cardiovascular disease
- IRB:
-
Institutional Review Board
References
Kostovski M, Simeonovski V, Mironska K, Tasic V, Gucev Z. Metabolic Profiles in Obese Children and Adolescents with Insulin Resistance. Open Access Maced J Med Sci. 2018;6(3):511–8.
WHO: Report of the commission on ending childhood obesity. In. Edited by Organization WH. Geneva, Switzerland; 2016.
Alvim RdO. Zaniqueli D, Neves FS, Pani VO, Martins CR, Peçanha MAdS, Barbosa MCR, Faria ERd, Mill JG: Waist-to-height ratio is as reliable as biochemical markers to discriminate pediatric insulin resistance. Jornal de Pediatria. 2019;95(4):428–34.
Marietti M, Bugianesi E. Obesity: Childhood obesity: time bomb for future burden of chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13(9):506–7.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31.
Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9(1):1–10.
Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–47.
Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99(6):1160–74.
Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol. 1999;5(4):301–4.
Huang X-J, Choi Y-K, Im H-S, Yarimaga O, Yoon E, Kim H-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors. 2006;6(7):756–82.
Berumen J, Baglieri J, Kisseleva T, Mekeel K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mechanisms of Disease. 2021;13(1): e1499.
Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123(5):1887–901.
Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56(5):303–7.
Aguilar-Morales I, Colin-Ramirez E, Rivera-Mancia S, Vallejo M, Vazquez-Antona C: Performance of Waist-To-Height Ratio, Waist Circumference, and Body Mass Index in Discriminating Cardio-Metabolic Risk Factors in a Sample of School-Aged Mexican Children. Nutrients 2018, 10(12).
Daneshzad E, Rostami S, Aghamahdi F, Mahdavi-Gorabi A, Qorbani M. Association of cardiometabolic risk factors with insulin resistance in overweight and obese children. BMC Endocr Disord. 2022;22(1):320.
Ezzatvar Y, Izquierdo M, Ramírez-Vélez R, Del Pozo CB, García-Hermoso A. Accuracy of different cutoffs of the waist-to-height ratio as a screening tool for cardiometabolic risk in children and adolescents: A systematic review and meta-analysis of diagnostic test accuracy studies. Obes Rev. 2022;23(2): e13375.
Maffeis C, Banzato C, Talamini G. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr. 2008;152(2):207–13.
Eslami M, Pourghazi F, Khazdouz M, Tian J, Pourrostami K, Esmaeili-Abdar Z, Ejtahed HS, Qorbani M. Optimal cut-off value of waist circumference-to-height ratio to predict central obesity in children and adolescents: A systematic review and meta-analysis of diagnostic studies. Front Nutr. 2022;9: 985319.
Ashwell M, Gibson S. Waist-to-height ratio as an indicator of “early health risk”: simpler and more predictive than using a “matrix” based on BMI and waist circumference. BMJ Open. 2016;6(3): e010159.
Umano GR, Di Sessa A, Cirillo G, Ursi D, Marzuillo P, Miraglia Del Giudice E. Waist-to-height ratio is more strongly associated than other weight-related anthropometric measures with metabolic variables. Acta Paediatr. 2019;108(12):2296–7.
Yoo EG. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J Pediatr. 2016;59(11):425–31.
Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–53.
Hsieh SD, Yoshinaga H, Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int J Obes Relat Metab Disord. 2003;27(5):610–6.
Hsieh SD, Yoshinaga H. Waist/height ratio as a simple and useful predictor of coronary heart disease risk factors in women. Intern Med. 1995;34(12):1147–52.
Khoury M, Manlhiot C, McCrindle BW. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62(8):742–51.
Wylie-Rosett J, Groisman-Perelstein AE, Diamantis PM, Jimenez CC, Shankar V, Conlon BA, Mossavar-Rahmani Y, Isasi CR, Martin SN, Ginsberg M, et al. Embedding weight management into safety-net pediatric primary care: randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15(1):12.
CDC: Defining Childhood Weight Status. In., 12/03/2021 edn. Atlanta, GA: Center for Disease Control and Prevention (CDC); 2021.
Arellano-Ruiz P, García-Hermoso A, García-Prieto JC, Sánchez-López M, Vizcaíno VM, Solera-Martínez M: Predictive Ability of Waist Circumference and Waist-to-Height Ratio for Cardiometabolic Risk Screening among Spanish Children. Nutrients 2020, 12(2).
Muñoz-Hernando J, Escribano J, Ferré N, Closa-Monasterolo R, Grote V, Koletzko B, Gruszfeld D, ReDionigi A, Verduci E, Xhonneux A, et al. Usefulness of the waist-to-height ratio for predicting cardiometabolic risk in children and its suggested boundary values. Clin Nutr. 2022;41(2):508–16.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–86.
Bohr AD, Laurson K, McQueen MB. A novel cutoff for the waist-to-height ratio predicting metabolic syndrome in young American adults. BMC Public Health. 2016;16(1):295.
Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U, Dörr M, Felix S, Lehnert H, Pittrow D, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95(4):1777–85.
Jamar G, Almeida FR, Gagliardi A, Sobral MR, Ping CT, Sperandio E, Romiti M, Arantes R, Dourado VZ: Evaluation of waist-to-height ratio as a predictor of insulin resistance in non-diabetic obese individuals. A cross-sectional study. Sao Paulo Med J 2017, 135(5):462–468.
Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Investig. 2000;106(4):473–81.
Kruger HS, Faber M, Schutte AE, Ellis SM. A proposed cutoff point of waist-to-height ratio for metabolic risk in African township adolescents. Nutrition. 2013;29(3):502–7.
Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–34.
Di Sessa A, Umano GR, Cirillo G, Passaro AP, Verde V, Cozzolino D, Guarino S, Marzuillo P, Miraglia Del Giudice E. Pediatric non-alcoholic fatty liver disease and kidney function: Effect of HSD17B13 variant. World J Gastroenterol. 2020;26(36):5474–83.
Furthner D, Weghuber D, Dalus C, Lukas A, Stundner-Ladenhauf HN, Mangge H, Pixner T. Nonalcoholic Fatty Liver Disease in Children with Obesity: Narrative Review and Research Gaps. Horm Res Paediatr. 2022;95(2):167–76.
Yu Y, Cai J, She Z, Li H. Insights into the Epidemiology, Pathogenesis, and Therapeutics of Nonalcoholic Fatty Liver Diseases. Adv Sci (Weinh). 2019;6(4):1801585.
van Buuren S. Flexible Imputation of Missing Data, Second. Edition. Boca Raton, FL: Chapman & Hall/CRC; 2018.
Rashid S, Genest J. Effect of obesity on high-density lipoprotein metabolism. Obesity (Silver Spring). 2007;15(12):2875–88.
Rodea-Montero ER, Evia-Viscarra ML, Apolinar-Jiménez E. Waist-to-Height Ratio Is a Better Anthropometric Index than Waist Circumference and BMI in Predicting Metabolic Syndrome among Obese Mexican Adolescents. Int J Endocrinol. 2014;2014: 195407.
Saydah S, Bullard KM, Imperatore G, Geiss L, Gregg EW. Cardiometabolic risk factors among US adolescents and young adults and risk of early mortality. Pediatrics. 2013;131(3):e679-686.
Khoury M, Manlhiot C, Dobbin S, Gibson D, Chahal N, Wong H, Davies J, Stearne K, Fisher A, McCrindle BW. Role of waist measures in characterizing the lipid and blood pressure assessment of adolescents classified by body mass index. Arch Pediatr Adolesc Med. 2012;166(8):719–24.
Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr. 2007;86(1):33–40.
Yang HR. Noninvasive diagnosis of pediatric nonalcoholic fatty liver disease. Korean J Pediatr. 2013;56(2):45–51.
Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, Ellis LL, Vanderwall K, Fontanesi J. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(10):1267–77.
Walker JL: The Association Between Waist Circumference and FITNESSGRAM® Aerobic Capacity Classification in Sixth-Grade Children. Pediatric Exercise Science, 27(4):488–493.
Y B: School fitness assessment and promotion: State and national evaluations with FITNESSGRAM. Graduate Theses and Dissertations. In. Ames, IA: Iowa State University; 2016.
Acknowledgements
Not applicable.
Funding
This study was made possible with funding from the National Institute of Diabetes Digestive and Kidney Diseases (5R18DK075981 and P30DK111022) and the National Heart Lung and Blood Institute (5R01HL101236); the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.W-R., A.E.G-P, P.M.D., and M.G., contributed to designing and conducting the parent study. T.E.U conducted the current examination under the supervision of J. W.-R and V.S. The manuscript was written by T.E.U under the supervision of J.W-.R and V.S. The manuscript was reviewed and edited by J.W-R., V.S, A.E.G-P, P.M.D., J.R., N.R.M., and A.H.L. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki guidelines, and the Albert Einstein College of Medicine (IRB# 2005–582) Institutional Review Board approved all study procedures. Written informed consent was obtained from all parents/guardians, and assent was obtained from children who entered the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental Table 1. Distribution of Different Waist-to-Height Ratio (WHtR) categories by Sex.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ukegbu, T.E., Wylie-Rosett, J., Groisman-Perelstein, A.E. et al. Waist-to-height ratio associated cardiometabolic risk phenotype in children with overweight/obesity. BMC Public Health 23, 1549 (2023). https://doi.org/10.1186/s12889-023-16418-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16418-9