Abstract
Background
Hantavirus Pulmonary Syndrome (HPS) is a rodent-borne zoonosis in the Americas, with up to 50% mortality rates. In Argentina, the Northwestern endemic area presents half of the annually notified HPS cases in the country, transmitted by at least three rodent species recognized as reservoirs of Orthohantavirus. The potential distribution of reservoir species based on ecological niche models (ENM) can be a useful tool to establish risk areas for zoonotic diseases. Our main aim was to generate an Orthohantavirus risk transmission map based on ENM of the reservoir species in northwest Argentina (NWA), to compare this map with the distribution of HPS cases; and to explore the possible effect of climatic and environmental variables on the spatial variation of the infection risk.
Methods
Using the reservoir geographic occurrence data, climatic/environmental variables, and the maximum entropy method, we created models of potential geographic distribution for each reservoir in NWA. We explored the overlap of the HPS cases with the reservoir-based risk map and a deforestation map. Then, we calculated the human population at risk using a census radius layer and a comparison of the environmental variables’ latitudinal variation with the distribution of HPS risk.
Results
We obtained a single best model for each reservoir. The temperature, rainfall, and vegetation cover contributed the most to the models. In total, 945 HPS cases were recorded, of which 97,85% were in the highest risk areas. We estimated that 18% of the NWA population was at risk and 78% of the cases occurred less than 10 km from deforestation. The highest niche overlap was between Calomys fecundus and Oligoryzomys chacoensis.
Conclusions
This study identifies potential risk areas for HPS transmission based on climatic and environmental factors that determine the distribution of the reservoirs and Orthohantavirus transmission in NWA. This can be used by public health authorities as a tool to generate preventive and control measures for HPS in NWA.
Similar content being viewed by others
Background
Hantavirus pulmonary syndrome (HPS) is a zoonotic disease endemic to the Americas caused by viruses of the Hantaviridae family, Mammantaviridae subfamily, Orthohantavirus genus, widely known as Hantaviruses [1]. These viruses are transmitted to humans through the inhalation of viral particles contained in excretions of different infected rodent species belonging to the Cricetidae family and the Sigmodontinae and Neotominae subfamilies, which act as natural reservoirs. Additionally, although not frequent, person-to-person transmission as well as through rodent bites has been reported [2, 3]. The symptomatic manifestations of HPS can be non-specific febrile syndrome, in its mildest form, or progress to the most severe manifestation with acute respiratory failure and cardiogenic shock [4]. This disease is a public health concern because of the high mortality rate (between 30 to 50%), the lack of specific treatment, and the difficult early diagnosis due to the similarity with other diseases (arboviruses, spotted fever, COVID-19, leptospirosis, hemorrhagic fever arenavirus and others non-specific infections) [3].
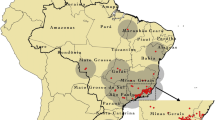
According to the Pan American Health Organization, the countries with the highest number of HPS cases in South America are Brazil and Argentina [5]. To date, at least 2 species of pathogenic Orthohantavirus that have been recognized by the International Committee of Viral Taxonomy were reported in Argentina: Andes orthohantavirus, with several viruses in the 4 endemic regions of the country (Fig. 1): Orán virus (ORNV), Bermejo virus (BERV) and Buenos Aires virus (BAV) in Northwest Argentina (NWA: including Salta, Jujuy and Tucumán provinces), Juquitiba virus and Lechiguanas virus (LECV) in Northeast Argentina, BAV, LECV and Plata virus in Central Argentina and Andes virus (ANDV) in South Argentina; and the second species Laguna Negra orthohantavirus in the Northwest endemic region, in which only Laguna Negra virus (LNV) occurs [6, 7]. The highest incidence of cases in the country is associated with Andes orthohantavirus, while Laguna Negra orthohantavirus was associated with only a few cases. Historically, the Northwestern and Central regions have reported the highest number of HPS cases, 49%, and 36% respectively, of all reported cases in the country followed by the Southern region (14%) and finally the Northeast region with only a few cases [8].
Study area. The Northwestern Argentina Hantavirus Pulmonary Syndrome (HPS) endemic area, which induces the eastern half of Jujuy, Salta, and Tucumán provinces (A). The input box shows the four endemic areas for the HPS in Argentina (a). This figure was created in QGIS V.3.20.2, using free and freely available shapefiles and images (Additional file 1)
Despite being the area with the highest HPS incidence in Argentina, several aspects related to Orthohantavirus transmission in the NWA region are still poorly investigated. Previous studies in the region have determined that Oligoryzomys chacoensis and O. flavescens occidentalis (west clade) [9], mainly captured in rural peridomestic habitats, are involved in the transmission of ORNV and BERV to humans [10,11,12]. Also, Calomys fecundus was identified as a reservoir of LNV in Yuto, Jujuy province [7]. However, in NWA there is still a nomenclature uncertainty for C. fecundus, which is currently considered a junior synonym of C. boliviae by some authors [13]. Although there is recent evidence suggesting that they are distinct species, the lack of genetic studies for C. boliviae does not allow us to make advances on this issue [13, 14].
To estimate the risk of infection for different human populations, it is necessary to understand which environmental factors shape the geographic distribution of reservoirs involved in Orthohantavirus transmission. The ecological niche models approach (ENM) provides a useful tool to identify the relationship between environmental or climatic factors and the distribution of organisms [15]. Therefore, it is possible to model the relationships between organisms associated with zoonotic diseases and the environmental setting where they live to make predictive maps of occurrences and risk of disease. Indeed, the ENM tool has been applied to estimate areas of environmental suitability for reservoirs and vectors, the effect of climate change on their distribution, and the risk of disease transmission [16]. Particularly, this approach has been useful to understand the relationships between Hantavirus and its reservoirs [17, 18]. In Argentina, the ENM approach was applied to determine the potential distribution of O. longicaudatus to explore the difference in distribution between subspecies of O. flavescens, and to determine risk areas of Orthohantavirus infection in southern Argentina [9, 19, 20].
In this context, this work aimed to generate potential distribution models for the three rodent species reservoirs of pathogenic Orthohantavirus in the NWA endemic region. We also sought to identify environmental and climatic variables that affect these potential distributions and estimate the degree of niche overlap between these three species. In addition, from these models, we sought to build a risk map of HPS occurrence, estimate the population at risk and analyze the concordance of the risk map with the historical cases of HPS in NWA. Also, we analyzed the possible spatial correlation between the distribution of HPS cases and the presence of nearby deforestation. Finally, the possible effect of the latitudinal variation of bioclimatic variables on the presence and frequency of cases was explored.
Methods
Study area
The determined study area was Northwestern Argentina in South America, an endemic region for HPS comprising the lowlands and foothills of Salta, Jujuy, and Tucumán provinces between latitudes 21.8°- 28°S and longitudes 62.32°- 68.20°O (Fig. 1). There are five ecoregions present in these provinces. The western half of the region is dominated by a desert mountain landscape with three ecoregions: The Puna, a Highland Plateau, the High Andean Steppe on the mountain tops, and the Monte Desert in the valleys. Conversely, the eastern half of the studied area is a subtropical forest with two ecoregions: The Yungas rainforest ecoregion on the eastern slopes of the Andes and the Dry Chaco ecoregion, a savanna-like plain (Fig. 1). The sharp contrast in climatic conditions due to sudden changes in altitude determine abrupt changes in rodent species distribution [21].
Dataset of rodent species presence
We obtained occurrence data from different sources, for the three rodents known to be Hantavirus reservoirs: O. chacoensis, O. f. occidentalis, and C. fecundus. The presence areas (PA; polygons) for these rodents were built based on a merge of the distribution proposals by a) Mammals of South America [22] b) the International Union for Conservation of Nature (IUCN), and c) “Sociedad Argentina para el Estudio de los Mamíferos” (SAREM) (see Additional file 1). Also, for each species, occurrences 50 km away or more from the PA were considered outliers and removed from the database to avoid including occurrences outside of the species distribution range. Rivera et al., [9] found cryptic species within the O. flavescens complex based on molecular evidence and suggested that the western Argentinean clade corresponds to O. f. occidentalis; thus, some authors followed this criterion to consider O. occidentalis as a valid species [23]. Therefore, the occurrence data of O. flavescens that were within the PA of O. f. occidentalis were included in the study.
Besides, Salazar Bravo et al. [24], based on molecular analysis of mitochondrial genes, suggested that the name C. fecundus should be applied to the Yungas ecoregion populations of NWA and Bolivia, but then without further evidence, he listed C. fecundus as a junior synonym of C. boliviae in a recent checklist of South American Mammals [22]. Furthermore, Pinotti et al. [13], based on molecular and potential distribution approaches, suggested that the Bolivian Montane Dry Forest could have acted as a barrier favoring the differentiation between C. boliviae and C. fecundus. In the same way as for O. f. occidentalis, occurrence data for C. boliviae that were within the PA of C. fecundus, were included in the study.
We searched for rodent occurrence data (O. chacoensis, C. fecundus, O. f. occidentalis, O. flavescens, and C. boliviae) in the following databases of scientific literature: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scielo (https://scielo.org/) and Google Scholar (https://scholar.google.es/schhp?hl=es) (access to data in January and February 2021). Also, we used the Global Biodiversity Information Facility (GBIF) database with RGBIF. To improve the database in our studied region we included a revised specimen based taken from the museum collection “Colección Mamíferos Lillo” (CML) at the National University of Tucumán (see Additional file 2).
We then filtered the distribution record data obtained from RGBIF to prevent problematic records using the CoordinateCleaner R package. Additionally, we eliminated all records without geographic coordinates from the database, as well as duplicated ones (i.e., same geographic coordinates), so we kept only one presence record for each species per location. Finally, we obtained records from 1818 to 2016 and constructed a database with geographic coordinates, references, sampling dates, and type of traps used (Additional file 2).
Climatic data
We obtained high-resolution raster layers of 19 climatic variables derived from spatial interpolation of temperature and precipitation, containing average values for the period 1979–2013, from the CHELSA website [25]. We also used land cover layers downloaded from the National Mapping Organizations (https://globalmaps.github.io/glcnmo.html). This geospatial information classifies the status of the land cover of the whole globe into 20 categories. Additionally, we obtained the Normalized Difference Vegetation Index (NDVI) from the R package MODIStsp, including the years 2000 to 2012. For NDVI, the maximum, minimum, median, and range were calculated by each grid cell considering the entire time period. All variables were resampled at a resolution of 2 km using the nearest neighbor and bilinear methods for land cover and the rest of the variables, respectively using the R package raster.
To eliminate overfitting and unnecessary complex relationships between climatic variables, we carried out two tests using all variables: A Pearson correlation test, to determine which were correlated, and a Jackknife analysis to determine the degree of individual contribution to a general model. From the groups of correlated variables, with values equal to or greater than 0.8 in the Pearson correlation test, we selected only those variables that presented the highest contribution values in the Jackknife analysis and discarded the remaining ones. The mean annual temperature (Bio1) and annual precipitation (Bio12) were included in almost all the sets of variables, and we eliminated those correlated with them because these two variables are considered of utmost biological importance [26,27,28]. Based on this approach, we constructed 3 sets of environmental variables of varying complexity by each rodent species (Additional file 1: supple Table A1 and Fig. A1).
Ecological niche model generation
We used the R package KUENM V.1.1.5 for the generation of the ENM. This package uses the MaxEnt modeling algorithm and largely automates the calibration, evaluation, and transfer steps of the ENM [29, 30]. For the model calibration, we defined an accessible area (M) for each rodent species. The M area represents the hypothetical historical accessible area for the species [31]. The knowledge of rodent dispersal capacity is important to define the M area, but there is no available data on this aspect of rodent biology in Northwestern Argentina. Thus, we built the M areas using the ecoregions where each species occurs based on their point presence location [32]. Then, we organized occurrence data into three data sets by species: 1) All occurrence location records (100%), 2) a training dataset that contained 80% of the randomly selected occurrence records, and 3) a test data set that contained the remaining 20% of the location records.
For each species, we created 1581 candidate models resulting from the combination of three parameters: 1) 3 sets of environmental variables, 2) 17 values of the regularization multiplier (0.1–1 at intervals of 0.1, 2–6 at intervals of 1, 8, and 10) and, 3) all the 31 possible combinations of the 5 features classes (linear = l, quadratic = q, product = p, threshold = t and hinge = h). These parameters were the same for each rodent species in order to make the models comparable. We evaluated the performance of each candidate model according to the following three criteria: a) the statistical significance of the partial Receiver Operating Characteristic curve (partial ROC, with 500 interactions and 50 percent of data for bootstrapping), b) omission rates below 5% and c) the Akaike Information Criterion corrected for small sample size (AICc) considering the difference between the best model with the remaining candidate models (delta AICc) [33]. First, we selected the models according to partial ROC; then, we reduced the number of models to include only those that met the omission rates, and finally, among these sets of remaining models, we selected those with less than 2 delta AIC values as final models [30].
For the selection of each final model, we produced 10 replicates with logistic and raw outputs to facilitate their interpretation and to avoid the effect of the other covariates in the visualization. We used the median of these outputs for visualization and further analysis. Then, we transferred these models outside area M to all of South America. To choose the type of model output (extrapolation, clamping, or no extrapolation), we assigned to each response variable the type of behavior to which it best adjusted and selected the behavior of the models based on the sum of the importance (percentage of contribution) of the response variables [34].
We developed a Movility-Oriented Parity (MOP) analysis to assess the extrapolation map in the prediction of the suitable area by each rodent species. This analysis quantifies the environmental similarity between the calibration and projection areas by highlighting areas where strict extrapolation occurs [34]. To exclude areas with the lowest analog climates, we used the MOP outputs of 10% or higher as a mask layer and cropped the raw projection outputs selected for each rodent species. Then, using QGIS V.3.20.2, we reclassified the raw maps generated into binary maps by a threshold that omits all regions with habitat suitability below the values for the lowest 10% of occurrence records. These last maps represent the potential distribution of each reservoir in the study area.
Niche overlapping
We made a niche overlap analysis for the three rodent reservoirs, using Niche Analyst 3.0 [35]. An environmental space was created from the first three main principal components that contained 90% of the variation in the 19 CHELSA climatic variables, the NDVI (maximum, minimum, mean, and range), and a land cover layer, in a polygon that contained the M areas of the rodent species. We generated simulations of occupied virtual niches from the total occurrence points for each rodent species and calculated the minimum-volume-ellipsoid. From each occupied niche, we obtained its attributes and quantified the overlap volume (niche similarity) for the three interacting species, through the Jaccard index (0 to 1).
HPS cases frequency and incidence
We obtained the HPS cases frequency by province, department, and fine geographic scale (locality, town, and farm) of the Northwestern region from the “Instituto Nacional de Enfermedades Infecciosas Malbrán—Administración Nacional de Laboratorios e Institutos de Salud Dr. Carlos Malbrán” (INEI ANLIS). We recorded all cases since the first reported case in NWA in 1997 to 2019. We considered a confirmed HPS case to be any patient who attended the health system and was diagnosed by serological and/or molecular detection techniques for Orthohantavirus. Since the population data by town or farm was not available, we had to group the mean yearly incidence at the department level (the third political level division). The population for each department was estimated by means of a linear extrapolation of the censuses of the years 1991, 2001, and 2010 from “Instituto Nacional de Estadística y Censos de la República Argentina” (INDEC) (census of the years 1991, 2001 and 2010; see Additional file 1).
Risk map, deforestation, and population at risk
To build disease transmission risk maps, we used the raw outputs of the best models cropped with the MOP data to compute an average of the three models. Then, we used the k-means unsupervised classification algorithm to classify the data into four categories of environmental suitability. In this way, the resulting map shows four categories according to the level of risk, defined based on the overlapping areas of suitability for rodent reservoir species: low risk, medium risk, high risk, and very high risk.
We estimated the population at risk using: a) the very-high and high strata of the risk map described in the previous paragraph, b) the population of the census radius of the National Institute of Statistics and Censuses (INDEC; census of the year 2010) and c) the layer of departments (third political level division) of the study area with a mean yearly incidence greater than 1/100,000 inhabitants. The departments of Yerba Buena and Burruyacu had 1 and 2 cases respectively, both in the province of Tucumán. These departments were included, given that epidemiological studies confirmed that they were autochthonous cases [7, 36].
In addition, we extracted the mean values of the risk map for each department using the QGIS zonal statistics plugin. This means that the level of risk was compared with the mean yearly incidence of HPS using Spearman’s correlation. Considering the area with a very high risk on the map, we analyzed the behavior of the bioclimatic variables according to the latitude variation and compared it with the values of the bioclimatic variables in sites with confirmed HPS cases occurrence. Additionally, we explored the question of whether the behavior of the variables fits a polynomial model.
On the other hand, we obtained a layer of deforestation areas of the study area from the Spatial Data Infrastructure of the province of Salta (http://geoportal.idesa.gob.ar/), to explore whether the sites with the presence of HPS cases are close to deforestation. In the rainforest ecoregion, most of the deforested areas are used for agricultural purposes, predominantly crops of sugar cane, bananas, and citrus [37]. Also, in the dry Chaco region, a complex process initially associated with livestock was identified. This leads from dry forests to pastures, then to monocultures, and later to monoculture and double cropping systems, where processes of expansion, substitution, and intensification occur [38]. For the analysis, we generated buffers of 5 km and 10 km from the layer of HPS cases. We only counted the sites that overlapped with the clearings and occurred between 1997 and 2019.
Results
We obtained 106 occurrence data for O. chacoensis, 99 for O. f. occidentalis, and 174 for C. fecundus. The distribution of O. chacoensis included northern Argentina, southern Bolivia, and central Paraguay. The distribution of O. f. occidentalis covers northern and central Argentina, as well as central and southern Bolivia. Finally, the distribution of C. fecundus was the most restricted one, in northern Argentina and southern Bolivia occupying the forest strip on the eastern slopes and lowlands close to the Andes (Additional file 1: supple Fig. A2, A3 and A4).
The four variables that contributed most to the models were: For O. chacoensis: temperature seasonality (Bio4), precipitation seasonality (Bio15), isothermality (Bio3), and mean monthly precipitation amount of the warmest quarter (Bio18); for O. f. occidentalis: precipitation seasonality (Bio15), mean annual temperature (Bio1), NDVI mean and annual range of temperature (Bio7); and for C. fecundus: precipitation seasonality (Bio15), the annual range of temperature (Bio7), mean annual temperature (Bio1) and annual precipitation amount (Bio12) (Additional file 1: supple Table A2). In the transfer, the response curves of the selected models suggested that their behavior outside the M area better fits the “extrapolation” type of transfer for most of the variables (Additional file 1: supple Fig. A5-A7).
The model’s evaluation allowed us to select only one best final model for each of the three reservoirs (Additional file 1: supple Table A3 and A4). For the three reservoirs, MOP analysis of the spatial projection showed that the environments represented were similar to the calibration areas to a large extent. Strict extrapolation areas for O. chacoensis were presented in southern Venezuela, eastern Colombia, western and eastern Ecuador, western, central, and southeastern Peru, central Bolivia, and northern Chile. For O. f. occidentalis, the strict extrapolation areas were in central Venezuela, western and central Ecuador, a large part of central and southern Peru, and northern and southern Chile. Finally, C. fecundus displayed strict extrapolation areas in central and southern Guyana and Venezuela, isolated areas of Colombia, western and eastern Ecuador, western and southeastern Peru, central Bolivia, northern and southern Chile, and northern Brazil (Additional file 1: supple Fig. A8). It should be noted that the MOP analysis does not suggest, for any of the species, eliminating sections of the transfer areas outside the M area, within our study area (Additional file 1: supple Fig. A8).
In general, the best models for the three reservoirs showed areas of high environmental suitability in the north and center of Salta and Tucumán, and in the southeast of Jujuy. The binary maps indicated that the potential distribution of O. chacoensis covers most of the Dry Chaco ecoregion, east and northeast of Salta, and some regions of the Yungas rainforest ecoregion extending southwards as far as northern Tucumán province; while O. f. occidentalis mainly covers the Yungas rainforest encompassing a large part of central Salta province and almost all of Tucumán province. Finally, C. fecundus potential distribution covers a large area in both ecoregions (Fig. 2).
Logistic outputs and binary maps for the three reservoirs. O. chacoensis (A, a), O. f. occidentalis (B, b) and C. fecundus (C, c). This figure was created in QGIS V.3.20.2, using free and freely available shapefiles (Additional file 1)
The Jaccard index yielded low values of niche overlap of the three rodent species analyzed. The highest niche overlap was between C. fecundus and O. chacoensis with a value of 0.26, while between O. chacoensis and O. f. occidentalis it was 0.15 and the lowest value found was between C. fecundus and O. f. occidentalis with a value of 0.11. The highest niche amplitude was reached by O. f. occidentalis, compared to O. chacoensis and C. fecundus (Fig. 3).
Minimum volume ellipsoids, in environmental three-dimensions, for the three reservoirs. O. chacoensis (orange ellipsoid), O. f. occidentalis (green ellipsoid), and C. fecundus (red ellipsoid). Gray points represent the environmental space and PC1, PC2, and PC3 represent the three principal components of the 24 environmental variables
The risk map indicated high and very-high risk of transmission of Orthohantavirus in the Yungas rainforest and part of the Dry Chaco ecoregions, which correspond to the north and center of Salta and Tucumán provinces as well as southeast of Jujuy province (Fig. 4), covering a great part of the study area. A large part of the Dry Chaco ecoregion was classified as moderate risk, which includes mostly the eastern part of Salta province. Considering the human population, it was estimated that a total of 600,791 people were at risk (18% of the total population of the 3 provinces).
Risk areas of Orthohantavirus transmission in the study area. This figure was created in QGIS V.3.20.2, using free and freely available shapefiles (Additional file 1)
A total of 945 HPS cases occurred during the study period, of which 904 had geographic information up to at least the department level. Considering the area that includes the departments with case reports, the global mean yearly incidence of HPS was 2.31/100,000 inhabitants. The HPS mean yearly incidence by department varied between 0.01 and 14.32/100,000 inhabitants and the departments with a mean yearly incidence greater than 1/10,000 inhabitants were: Orán, San Martín, Anta, Iruya, Rivadavia, and Santa Victoria in Salta province; Libertador General San Martín, Santa Bárbara, San Pedro and San Antonio in Jujuy province. Ten of the recorded cases corresponded to travelers of Bolivian origin.
Besides, a total of 744 cases could be identified at a finer scale (locality, town, or farm level). These cases were distributed in 69 sites, of which 40 belong to the Yungas rainforest (512 cases; 68.81%), 28 to the dry Chaco (231 cases; 31.04%), and 1 to the Puna (1 case; 0.13%) ecoregions. Considering the risk strata obtained by our map, we were able to observe that 47 (74.73% of cases), 15 (23.12% of cases), 4 (1.61% of cases), and 3 (0.54% of cases) of the sites corresponded to strata 3, 2, 1 and 0 respectively (Fig. 5A). Furthermore, when exploring the correlation between the HPS mean yearly incidence per 100,000 inhabitants by department with respect to the average of the risk strata (average by department of the pixels in the raster format risk map), a statistically significant positive correlation emerged (r = 0.43; p < 0.001). In addition, all the departments with mean yearly incidence values greater than 1/100,000 inhabitants are found in areas whose strata average is greater than one (Fig. 5B). Regarding deforestation, 52 sites (668 cases) and 69 sites (744 cases) were found within a radius of 5 km and 10 km from some deforestation, respectively.
Case frequency and mean yearly incidence of HPS versus risk level. A shows the frequency of cases as a function of the risk level derived from the model and B) HPS mean yearly incidence by department as a function of the average risk level obtained from the model in raster format (average of the pixels included in each department)
The variation of Bio12 against latitude showed an oscillation of the precipitation values with higher values in the north and south of the study area. The sites with the highest number of HPS cases (localities, towns, and farms) are concentrated in the northern area. In the south, the precipitation conditions in Burruyacú and Yerba Buena departments, where HPS cases were recently detected, are similar to the amount of precipitation that occurs in the north, where most cases are concentrated. Likewise, when analyzing the Bio15 variable, the lower seasonal variations in rainfall were associated with the occurrence of HPS cases. It should be noted that both the variables Bio12 (R2 = 0.29) and Bio15 (R2 = 0.54) were adjusted to third-order (cubic) polynomial models.
Discussion
Almost 30 years after the identification of HPS in Northwestern Argentina, this is the first risk map built to warn health authorities about high-risk regions for Orthohantavirus infections. This risk map predicts very well the vast majority of cases that have already occurred, despite having been generated from the information on the presence of the reservoirs, and shows us a region with apparent environmental suitability for viral transmission that should be at least less epidemiologically monitored.
As for many organisms, the rodents’ abundance can be affected by temperature and precipitation, since this can favor the growth of vegetation and access to food and shelter, thus favoring their reproduction and survival [26,27,28]. Also, these ecologically relevant climatic factors frequently impose limits to species’ physiological tolerance and thus their geographical distribution. However, the effect of the climatic variables on all rodent species is not the same [39]. For the three rodents species, the temperature-associated variables with the greatest contribution to the models were temperature seasonality, isothermality (O. chacoensis), and mean annual temperature (O. f. occidentalis and C. fecundus). We have calculated that the average annual temperatures for places with the presence of these three rodents vary between 14.7 °C to 25.7 °C, 6.85 °C to 23.2 °C and -1.4 °C to 27,7 °C respectively [25]. These value ranges in NWA are observed principally in subtropical areas that include the Yungas rainforest and Dry Chaco ecoregions, markedly different from that expected for O. longicaudatus, which is also a reservoir of Orthohantavirus in southern Argentina [19].
Regarding the rainfall, our models for the three rodent species showed that rainfall seasonality had a greater contribution to them. This is probably due to the fact that an increase in the amount of annual precipitation generates greater suitability for rodent species. If this occurs for Orthohantavirus reservoirs, the risk of transmission may be increased. Abrupt increases in population density have been reported in Argentina for the Akodon, Calomys, Mus, and Oligoryzomys genera, known as “ratadas”, associated with increases in mean annual rainfall [40]. Similar results were previously reported for reservoirs in the southern endemic region [19, 20] and for C. fecundus in the NWA endemic region [13].
The contribution of the mean NDVI to the distribution models of O. f. occidentalis and C. fecundus may be related to the productivity of the ecosystem in the M area. In fact, a previous study carried out in Mexico, in which rodent species were monitored on an altitudinal gradient, highlighted the importance of ecosystem productivity (estimated by NDVI) on the diversity and abundance of rodents [41]. However, when the vegetation cover is preserved and the community reaches its climax state, the diversity of species is greater. Therefore, in an advanced stage of ecological succession, the presence of competitors and predators may maintain reservoir populations at relatively low abundances, which may decrease the dynamics of virus transmission risk [42].
The steep elevation gradient of the Andes mountains appeared as a natural barrier limiting the western distribution of the three species [13, 43]. However, in the eastern half of the study region, we found differences between the potential geographic distributions predicted by the models for the three reservoirs. Although the three species have high environmental suitability in the Yungas ecoregion, O. chacoensis shows greater environmental suitability than the others followed by C. fecundus, then O. f. occidentalis in the dry Chaco [13]. In addition, the niche overlap was quite low between the species, being O. f. occidentalis the rodent with the largest minimum volume ellipsoid (Fig. 3), which indicates that this species has great adaptive plasticity [22, 44].
The differences found in the suitable areas between the three reservoirs could have important implications for the occurrence of HPS in the Northwestern Argentina endemic region. According to our results, the two species involved in the transmission of pathogenic Orthohantavirus, O. chacoensis, and O. f. occidentalis, for ORNV and BERV respectively, would have a similar distribution in the Yungas rainforest, but in the Dry Chaco, O. chacoensis would have a predominant role. Probably the combined abundance of the two rodent species can generate a greater number of HPS cases, compared to sites where only one of the species is present. On the other hand, C. fecundus, carrying LNV has suitable habitats in both ecoregions, but only a few infected rodents and human cases of HPS associated with LNV have been recorded in NWA [7].
Our risk map suggests that the areas with the highest risk of Orthohantavirus transmission were mainly the Yungas rainforest and the occidental zone of Dry Chaco. Particularly, our results highlight the north of Salta province and the east of Jujuy province, where indeed the HPS cases are highly clustered [6, 8]. This suggests that our risk map has a very good sensibility in detecting very high-risk areas for Orthohantavirus transmission based solely on the reservoir distribution modeling. It is important to highlight that most localities with HPS cases are geographically included in the highest stratum (very high-risk) of the risk map and that mean yearly incidence by department are positively correlated with risk levels (Fig. 5A y B) [8, 45].
The risk map also predicts areas with high risk where no cases of HPS were reported. However, HPS cases have recently been reported in the southern area of the NWA within the province of Tucumán [7, 36]. This shows that the risk zones predicted by the risk map have the conditions for the maintenance of the Orthohantavirus zoonotic transmission cycle and that the illness is probably under-reported. These places had not previously reported cases and are located in the very high-risk and high-risk strata according to the risk map, a zone that has precipitation conditions similar to the northern sector, where most of the cases are concentrated (considering the latitudinal variation; Fig. 6A and B).
Analysis of the variation of precipitation and latitude. A Annual precipitation amount (mm/year) variation versus latitude variation (Left: South; Right: North). The red dots represent the precipitation values of the sites with reported presences according to latitude. Open dots represent the precipitation values of a grid point arranged latitudinal. The blue dots represent the frequency of HPS cases according to latitude (frequency values are shown on the y-axis to the right) and B) Precipitation seasonality versus latitude variation. The red dots represent the precipitation seasonality values of the sites with cases presence according to latitude. Open dots represent the precipitation seasonality values of a grid point arranged latitudinally. The blue dots represent the frequency of HPS cases according to latitude (frequency values are shown on the y-axis to the right)
We found that localities with the highest number of cases are located in the northern part of NWA and that the HPS cases seem to be associated with high rainfall and low seasonality of rainfall (Fig. 6A and B). In a systematic review, evidence was found for the association of HPS with precipitation and habitat type, and mixed evidence for temperature and humidity [39]. Furthermore, Ferro et al., [45] showed a relationship between HPS cases and lagged rainfall and temperature with a delay of 2 to 6 months in the Argentinean Northwestern region.
Assuming there are no geographic barriers, bioclimatic conditions can affect the presence of the virus in a given place in two main ways: a) indirectly, favoring or limiting the distribution and abundance of reservoirs, and b) directly, by affecting the viability of the virus in the environment. The comparison of the risk areas predicted by our map (constructed from the potential distribution of reservoirs), with the areas where the sustained presence of HPS cases occur, might indicate which are the limiting bioclimatic variables (and the thresholds) for Orthohantavirus distribution.
The high-risk areas of Orthohantavirus transmission in NWA have a subtropical climate with high temperatures and summer rainfall, which favors the development of vegetation that serves as food and shelter for rodents. This in turn gives rise to population growth of the reservoirs and thus a greater probability of contact between them either in burrows or through fights between adult male rodents, which leads to a higher prevalence of viral infection in these reservoirs. In this context, Orthohantavirus transmission to humans commonly occurs in wild or rural places when people either work or carry out recreational activities which normally include trekking, hunting, and fishing. Meanwhile, occupational exposure can include deforestation, agriculture, transport, and military activities [6, 46]. Some HPS cases were also detected in ferrymen (“bagayeros”) that carry out cross-border trade of legal and illegal merchandise. In addition, in rural places, transmission events occur when rodents invade the sheds or warehouses indoors and their excretions are aspirated by people when cleaning the place [6].
Deforestation normally causes structural alteration in the environment that some reservoir rodents can exploit to find shelters/or even migrate to populated rural settlements or urban places. These changes in the environment can include agricultural or livestock exploitation, a combination of the two, or even the disorderly expansion of cities that can put people in contact with the reservoirs. In addition, deforestation and the subsequent change in land use usually bring with it a significant loss of biological diversity [47]. It has been previously reported that the loss of diversity in ecosystems can increase the risk of Hantavirus transmission due to the loss of the dilution effect [48]. This dilution effect consists in ecosystems with high diversity where the reservoir-virus-reservoir contact is hindered by their interaction with other organisms [42, 49, 50]. Particularly, in NWA most sites with HPS cases are at a short distance from deforestation areas. In addition, the largest area with a high risk of transmission of the virus is found in the rainforest region, where after deforestation the cleared areas are mainly destined to agricultural exploitation, which can provide a suitable environment for the maintenance of rodent populations.
In addition, when we talk about the population at risk, we are considering the areas with high risk according to the risk map and the departments with a frequent presence of cases. In our opinion, not the entire area of a department is at risk of transmission. However, due to a matter of proximity, we can think of risk as the possibility that people may be exposed due to the displacement whether for work, recreation, etc.
As limitations of this work, we can mention the difficulty to collect complete information on some cases in the region, including those reported at the beginning of the study period. Additionally, it is important to inquire about unknown hosts and reservoirs in the region, since there is increasing evidence of several animals linked to Hantavirus, such as bats or new rodent species, which may act as hosts and potential reservoirs of these and currently unknown viruses [51]. For example, C. callosus and C. laucha are reservoirs of LNV in Bolivia and Paraguay respectively. Therefore, our risk map should be updated periodically depending on whether new records and reservoirs for this zoonotic disease are found. We must also consider that the possible low accessibility of people to areas of high environmental suitability for the virus may be generating an overestimation of the population at risk.
Conclusions
In this work we have advanced in the identification of potential Orthohantavirus transmission risk zones. The area of greatest risk has been delimited in a north–south strip that covers the north, center, and south of Salta, the east of Jujuy, and the north and center of Tucumán. Most HPS cases are included in the highest-risk area of our model. The transmission events that occurred in Tucumán province, a place where HPS cases were not normally detected, may be an example that the risk model predicts areas in which transmission can be established, at least temporarily. The temperature, precipitation, and vegetation cover variables could determine the distribution of the reservoirs and the virus in NWA. Based on the model, a significant amount of the population exposed to the risk of transmission is predicted, so it is worth asking whether the number of cases detected by the health system represents only a portion of the population that has been infected with this virus. Moreover, we have found that a large part of the HPS cases have occurred in localities or places close to deforestation zones.
In addition, the niche overlap of the species is low, which leads us to consider the possibility that the differentiation of suitability zones of these rodents may expand the risk areas of virus transmission. Finally, the areas where HPS cases occur are associated with a high amount of precipitation in all seasons. These results are relevant for the local health system, as they can help to plan preventive and control interventions for HPS in northern Argentina. In addition, the potential distribution models of these rodent species that we have generated can also be used to explore areas of Orthohantavirus transmission risk in other South America countries bordering Argentina.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AICc:
-
Akaike Information Criterion Corrected
- ANDV:
-
Andes Virus
- BAV:
-
Buenos Aires Virus
- BERV:
-
Bermejo Virus
- CML:
-
Colección Mamíferos Lillo
- ENM:
-
Ecological Niche Model
- GBIF:
-
Global Biodiversity Information Facility
- HPS:
-
Hantavirus Pulmonary Syndrome
- INDEC:
-
Instituto Nacional de Estadística y Censos de la República Argentina
- INEI ANLIS:
-
Instituto Nacional de Enfermedades Infecciosas Malbrán—Administración Nacional de Laboratorios e Institutos de Salud Dr. Carlos Malbrán
- LEC:
-
Lechiguanas Virus
- LNV:
-
Laguna Negra Virus
- M:
-
Accessible Area
- MOP:
-
Mobility-Oriented Parity
- NDVI:
-
Normalized Difference Vegetation Index
- NWA:
-
Northwestern Argentina
- ORNV:
-
Orán Virus
- ROC:
-
Receiver Operating Characteristic Curve
- PA:
-
Presence areas
- Bio1:
-
Mean annual temperature
- Bio3:
-
Isothermality
- Bio4:
-
Seasonal temperature
- Bio7:
-
Annual range of temperature
- Bio12:
-
Annual precipitation
- Bio15:
-
Precipitation seasonality
- Bio18:
-
Precipitation of warmest quarter
References
Laenen L, Vergote V, Calisher CH, Klempa B, Klingström J, Kuhn JH, Maes P. Hantaviridae: current classification and future perspectives. Viruses. 2019;11(9):788. https://doi.org/10.3390/v11090788.
Martínez VP, Di Paola N, Alonso DO, Pérez-Sautu U, Bellomo CM, Iglesias AA, Coelho RM, et al. “Super-spreaders” and person-to-person transmission of Andes virus in Argentina. N Engl J Med. 2020;83(23):2230–41. https://doi.org/10.1056/nejmoa2009040.
Avšič-Županc T, Saksida A, Korva M. Hantavirus infections. Clin Microbiol Infect. 2019;21:e6–16. https://doi.org/10.1111/1469-0691.12291.
Pan American Health Organization. Hantavirus in the Americas: guidelines for diagnosis, treatment, prevention, and control. https://iris.paho.org/handle/10665.2/40176. Accessed 2 Nov 2021.
Pan American Health Organization. Hantavirus. https://www.paho.org/hq/index.php?option=com_content&view=article&id=14911:hantavirus&Itemid=40721&lang=es. Accessed 2 Nov 2 2021.
Martínez VP, Bellomo CM, Cacace ML, Suárez P, Bogni L, Padula PJ. Hantavirus pulmonary syndrome in Argentina, 1995–2008. Emerg Infect Dis. 2010;16(12):1853–60. https://doi.org/10.3201/eid1612.091170.
Calderón GE, Brignone J, Martin ML, Calleri F, Sen C, Casas N, Calli R, et al. Brote de Síndrome Pulmonar por Hantavirus, Tucumán, Argentina. Medicina (B Aires). 2018;78(3):151–7.
Alonso DO, Iglesias A, Coelho R, Periolo N, Bruno A, Córdoba MT, Filomarino N, et al. Epidemiological description, case-fatality rate, and trends of Hantavirus Pulmonary Syndrome: 9 years of surveillance in Argentina. J Med Virol. 2019;91(7):1173–81. https://doi.org/10.1002/jmv.25446.
Rivera PC, González-Ittig RE, Robainas Barcia A, Trimarchi LI, Levis S, Calderón GE, Gardenal CN. Molecular phylogenetics and environmental niche modeling reveal a cryptic species in the Oligoryzomys flavescens complex (Rodentia, Cricetidae). J Mammal. 2018;99(2):363–76. https://doi.org/10.1093/jmammal/gyx186.
Calderón G, Pini N, Bolpe J, Levis S, Mills J, Segura E, Guthmann N, et al. Hantavirus reservoir hosts associated with peridomestic habitats in Argentina. Emerg Infect Dis. 1999;5(6):792–7. https://doi.org/10.3201/eid0506.990608.
Gonzalez Della Valle M, Edelstein A, Miguel S, Martínez V, Cortez J, Cacace ML, Jurgelenas G, et al. Andes virus associated with Hantavirus Pulmonary Syndrome in northern Argentina and determination of the precise site of infection. Am J Trop Med Hyg. 2002;66(6):713–20. https://doi.org/10.4269/ajtmh.2002.66.713.
Sosa-Estani S, Martinez VP, Gonzalez Della Valle M, Eldestein A, Miguel S, Padula PJ, Cacace ML, et al. Hantavirus en población humana y de roedores de un área endémica para el Sindrome Pulmonar por Hantavirus en la Argentina. Medicina (B Aires). 2002;62(1):1–8.
Pinotti JD, Ferreiro AM, Martin ML, Levis S, Chiappero M, Andreo V, González-Ittig RE. Multiple refugia and glacial expansions in the Tucumane-Bolivian Yungas: The phylogeography and potential distribution modeling of Calomys fecundus (Thomas, 1926) (Rodentia: Cricetidae). J Zool Syst Evol Res. 2020;00(4):1–15. https://doi.org/10.1111/jzs.12375.
Gonzalez Ittig RE, Pinotti JD, Carballo J, Martín ML, Levis S, Calderón G, Gómez-Villafañe I, et al. Molecular systematics and biogeographic insights of the Calomys callosus complex (Rodentia, Cricetidae). Zoolog Scr. 2022;00:1–24. https://doi.org/10.1111/zsc.12556.
Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Mayer E, Nakamura M, Araújo MB. Ecological Niches and Geographic Distributions. Princeton: Princeton University Press; 2011. https://doi.org/10.1515/9781400840670.
Peterson A. Ecologic niche modeling and spatial patterns of disease transmission. Emerg Infect Dis. 2006;12(12):1822–6. https://doi.org/10.3201/eid1212.060373.
Astorga F, Escobar LE, Poo-Muñoz D, Escobar-Dodero J, Rojas-Hucks S, Alvarado-Rybak M, Duclos M, et al. Distributional ecology of Andes hantavirus: a macroecological approach. Int J Health Geogr. 2018;17(1):1–12. https://doi.org/10.1186/s12942-018-0142-z.
Donalisio MR, Peterson AT. Environmental factors affecting transmission risk for hantaviruses in forested portions of southern Brazil. Acta Trop. 2011;119(2–3):125–30. https://doi.org/10.1016/j.actatropica.2011.04.019.
Andreo V, Neteler M, Rocchini D, Provensal C, Levis S, Porcasi X, Rizzoli M, et al. Estimating Hantavirus Risk in Southern Argentina: A GIS-Based Approach Combining Human Cases and Host Distribution. Viruses. 2014;6(1):201–22. https://doi.org/10.3390/v6010201.
Andreo V, Glass G, Shields T, Provensal C, Polop J. Modeling Potential Distribution of Oligoryzomys longicaudatus, the Andes Virus (Genus: Hantavirus) Reservoir. Argentina Ecohealth. 2011;8(3):332–48. https://doi.org/10.1007/s10393-011-0719-5.
Sandoval M, Ferro I. Biogeographical analysis of rodent endemism and distributional congruence in the southern-central Andes (north-western Argentina). Biol J Lin Soc. 2014;112(1):163–79. https://doi.org/10.1111/bij.12233.
Patton JL, Pardiñas UFJ, D’Elía G. Mammals of South America, vol. 2. Chicago: University of Chicago Press; 2015. https://doi.org/10.5710/AMGH.v53i4.1.
Trimarchi LI, Gonzalez-Ittig RE, d’ Hiriart S. Oligoryzomys occidentalis. Categorización de los mamíferos de Argentina. 2019. https://cma.sarem.org.ar/es/especie-nativa/oligoryzomys-occidentalis. Accessed 8 Jun 2021.
Salazar-Bravo J, Gragoo JW, Tinnin DS, Yates TL. Phylogeny and Evolution of the Neotropical Rodent Genus Calomys: Inferences from Mitochondrial DNA Sequence Data. Mol Phylogenet Evol. 2001;20(2):173–84. https://doi.org/10.1006/mpev.2001.0965.
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, et al. Climatologies at high resolution for the earth’s land surface areas. Sci Data. 2017;4(1):1–20. https://doi.org/10.1038/sdata.2017.122.
Carbajo AE, Vera C, González PLM. Hantavirus reservoir Oligoryzomys longicaudatus spatial distribution sensitivity to climate change scenarios in Argentine Patagonia. Int J Health Geogr. 2009;8(1):44. https://doi.org/10.1186/1476-072X-8-44.
Murúa R, González LA, Lima M. Population dynamics of rice rats (a Hantavirus reservoir) in southern Chile: feedback structure and non-linear effects of climatic oscillations. Oikos. 2003;102(1):137–45. https://doi.org/10.1034/j.1600-0706.2003.12226.x.
Pearson OP. A Perplexing Outbreak of Mice in Patagonia, Argentina. Studies on Neotropical Fauna and Environment. 2002;37(3):187–200. https://doi.org/10.1076/snfe.37.3.187.8563.
Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190(3–4):231–59. https://doi.org/10.1016/j.ecolmodel.2005.03.026.
Cobos ME, Peterson AT, Barve N, Osorio-Olvera L. kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ. 2019;7(2):1–15. https://doi.org/10.7717/peerj.6281.
Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, Soberón J, et al. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Model. 2011;222(11):1810–9. https://doi.org/10.1016/j.ecolmodel.2011.02.011.
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’amico JA, et al. Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience. 2001;51(11):933–8. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2.
Warren DL, Seifert SN. Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecol Appl. 2011;21(2):335–42. https://doi.org/10.1890/10-1171.1.
Owens HL, Campbell LP, Dornak LL, Saupe EE, Barve N, Soberón J, Ingenloff K, et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol Modell. 2013;263:10–8. https://doi.org/10.1016/j.ecolmodel.2013.04.011.
Qiao H, Peterson AT, Campbell LP, Soberón J, Ji L, Escobar LE. NicheA: creating virtual species and ecological niches in multivariate environmental scenarios. Ecography. 2016;39(8):805–13. https://doi.org/10.1111/ecog.01961.
Ciancaglini M, Bellomo CM, Cabreros CLT, Alonso D, Bassi SC, Iglesias AA, Martínez VP. Hantavirus Pulmonary Syndrome in Tucumán Province Associated to an Unexpected Viral Genotype. Medicina (B Aires). 2017;77(2):81–4.
Brown AD, Grau HR, Malizia LR, Grau A. Bosques nublados del neotrópico: Argentina. In: Kapelle M, Brown AD, eds. INBio: Costa Rica. 2001:623-698.
Mosciaro MJ, Seghezzo L, Texeira M, Paruelo J, Volante J. Where did the forest go? Post-deforestation land use dynamics in the Dry Chaco region in Northwestern Argentina. Land Use Police. 2023;106650(129):1–13. https://doi.org/10.1016/j.landusepol.2023.106650.
Douglas KO, Paine K, Sabino-Santos G Jr, Agard J. Influence of climatic factors on human hantavirus infections in Latin America and the Caribbean: a systematic review. Pathogens. 2021;11(1):1–27. https://doi.org/10.3390/pathogens11010015.
Jaksic FM, Lima M. Myths and facts on ratadas: bamboo blooms, rainfall peaks and rodent outbreaks in South America. Austral Ecol. 2003;28(3):237–51. https://doi.org/10.1046/j.1442-9993.2003.01271.x.
Ramírez-Bautista A, Williams JN. The importance of productivity and seasonality for structuring small rodent diversity across a tropical elevation gradient. Oecologia. 2019;190(2):275–86. https://doi.org/10.1007/s00442-018-4287-z.
Prist PR, Prado A, Tambosi LR, Umetsu F, de Arruda Bueno A, Pardini R, Metzger JP. Moving to healthier landscapes: forest restoration decreases the abundance of Hantavirus reservoir rodents in tropical forests. Sci Total Environ. 2021;752:141967. https://doi.org/10.1016/j.scitotenv.2020.141967.
Muñoz-Valencia V, Montoya-Lerma J, Seppa P, Diaz F. Landscape genetics across the Andes Mountains: environmental variation drivers genetics divergence in the leaf-cutting ant Atta cephalotes. Mol Ecol. 2021;00:1–15. https://doi.org/10.1111/mec.16742.
Maroli M, Crosignani B, Piña CI, Coelho R, Martínez VP, Gómez Villafañe IE. New data about home range and movements of Oligoryzomys flavescens (Rodentia: Cricetidae) help to understand the spread and transmission of Andes virus that causes Hantavirus Pulmonary Syndrome. Zoonoses Public Health. 2020;00:1–10. https://doi.org/10.1111/zph.12690.
Ferro I, Bellomo CM, López W, Coelho R, Alonso D, Bruno A, Córdoba FE, Martinez VP. Hantavirus pulmonary syndrome outbreaks associated with climate variability in Northwestern Argentina, 1997–2017. PLoS Negl Trop Dis. 2020;14(11):e0008786. https://doi.org/10.1371/journal.pntd.0008786.
Sosa-Estani S, Salomón OD, Gómez AO, Esquivel ML, Segura EL. Diferencias regionales y Síndrome Pulmonar por Hantavirus (enfermedad emergente y tropical en Argentina). Cad Saude Publica. 2001;17(suppl):S47–57. https://doi.org/10.1590/s0102-311x2001000700013.
Giam X. Global biodiversity loss from tropical deforestation. PNAS. 2017;114(23):5775–7. https://doi.org/10.1073/pnas.1706264114.
Keesing F, Ostfeld RS. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc Natl Acad Sci. 2021;118(17):e2023540118. https://doi.org/10.1073/pnas.2023540118.
Min KD, Kim H, Hwang SS, Cho S, Schneider MC, Hwang J, Cho SI. Protective effect of predator species richness on human hantavirus infection incidence. Sci Rep. 2020;10(1):21744. https://doi.org/10.1038/s41598-020-78765-6.
Epstein PR, Chikwenhere GP. Environmental factors in disease surveillance. Lancet. 1994;343(8910):1440–1. https://doi.org/10.1016/s0140-6736(94)92570-4.
Sabino-Santos G Jr, Maia FGM, Martins RB, Gagliardi TB, Souza WM, Muylaert RL, Luna LKS, et al. Natural infection of Neotropical bats with hantavirus in Brazil. Sci Rep. 2018;8(1):9018. https://doi.org/10.1038/s41598-018-27442-w.
Acknowledgements
Not applicable.
Funding
This research was funded by Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, under grant PICT 2016–4213 and Ministerio de Ciencia, Tecnología e Innovación Productiva—Consejo Nacional de Investigaciones Científicas y Técnicas, under grant PIP 2022–2024 code 11220210100393CO.
Author information
Authors and Affiliations
Contributions
WRL, MA, and JFG participated in the conceptualization, study design and data interpretation; WRL, MA, CB, VPM, SDK, IF and JFG carried out the data collection and curation; WRL, MA, JFG and MIS performed the statistical analyses; WRL, MA, SDK, IF and JFG wrote and prepared the original draft; WRL, MA, SDK, IF, CB, VPM, MIS and JFG wrote, revised, and edited the final version of the manuscript. All authors have read and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Tables and Figures. Supple Table A1.
Sets of environmental variables used for ecological niche models for three reservoirs. Supple Table A2. Values and range of values of maximum environmental suitability of the variables of the best models for the three reservoirs. Supple Table A3. Number of models that passed the different evaluation criteria applied in the calibration for the three reservoirs. Supple Table A4. Selected models according to the three defined criteria of selection: Partial ROC, Omission Rates, and Akaike Informative Criteria corrected (AICc). Supple Fig. A1. Jackknife analysis and Pearson test in M area for the three reservoirs. Supple Fig. A2. Points of occurrence of O. chacoensis in South America. Supple Fig. A3. Points of occurrence of O. f. occidentalis in South America. Supple Fig. A4. Points of occurrence of C. fecundus in South America. Supple Fig. A5. Response curve for O. chacoensis. Supple Fig. A6. Response curve for O. f. occidentalis. Supple Fig. A7. Response curve for C. fecundus. Supple Fig. A8. Logistic outputs of best models extrapolated in South America, and MOP analysis for the three reservoirs.
Additional file 2:
Excel A1. Rodents occurrence records.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
López, W.R., Altamiranda-Saavedra, M., Kehl, S.D. et al. Modeling potential risk areas of Orthohantavirus transmission in Northwestern Argentina using an ecological niche approach. BMC Public Health 23, 1236 (2023). https://doi.org/10.1186/s12889-023-16071-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16071-2