Abstract
Background
A high COVID-19 vaccine uptake is essential to achieve herd immunity to combat the current strain of COVID-19 and potential future variants. This review aimed to identify factors associated with public intention to receive COVID-19 vaccines until February 2021 to provide accessible data to policymakers to inform framing and targeting of messages designed to optimise vaccine uptake.
Methods
Medline, Embase, CINAHL, PsycINFO, PsycARTICLES, Sociological Abstracts and Applied Social Sciences Index and Abstracts were searched for cross-sectional studies reporting data regarding COVID-19 vaccine intentions, published between 01/01/2020 and 12/02/2021. Title/abstract and full-text screening were performed independently by two authors. The Appraisal Tool for Cross-sectional Studies (AXIS) was used to assess bias and quality. Both random-effects meta-analysis and narrative synthesis were used to describe vaccine intentions and associated factors. A subgroup analysis assessing the impact of sex, sampling method and time of survey on COVID-19 vaccine intention was performed.
Results
Searches identified 4739 studies, and 23 cross-sectional studies were deemed eligible for the review; 22 used online surveys and one used a mixed-methods study design. Eighteen surveys were conducted in the first half of 2020 and five were conducted in the latter half of 2020. Fifteen countries were represented, with the most common being the United States (n = 4) and the United Kingdom (n = 4) sampling 41,403 participants across all surveys. Most studies employed convenience sampling and 11 non-responder rates raised concerns over non-response bias. From the 18 studies included in the meta-analysis, the pooled proportion of survey participants willing to receive the COVID-19 vaccine was 73.3% (n = 18, 95% Confidence Interval 64.2 to 81.5%, I2 = 99.7%). Factors associated with a higher COVID-19 vaccine acceptance included greater perceived risk of COVID-19, lower level of perceived vaccine harm, higher educational attainment and household income, older age, being of White ethnicity and male sex.
Conclusions
There was a high willingness to receive the COVID-19 vaccine which was influenced by sociodemographic factors and risk perceptions. The findings suggest future research should explore reasoning behind vaccine intentions for different sociodemographic groups to allow targeted communication strategies to be formulated by public health agencies.
Registration
PROSPERO Registration Number: CRD42021239134.
Similar content being viewed by others
Background
Since coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China in December 2019 [1], there have been numerous coronavirus case surges around the globe [2]. The development of effective COVID-19 vaccines has given hope to the global community, with the vaccine rollout marking a ‘turning point’ in the battle against coronavirus [3].
Mass vaccination programmes aim to vaccinate a large proportion of the population so that disease transmission is slowed and vulnerable individuals who cannot be vaccinated are still protected [4]. This phenomenon is known as herd immunity and can only be achieved when a substantial proportion of the population is vaccinated [5]. The threshold to achieve herd immunity against COVID-19 is estimated at between 60 and 70% [4]. However, due to the viral nature of COVID-19, mutations are inevitable and the main ‘Delta’ variant in India has taken over as the dominant strain in many countries [6]. New variants may be more transmissible which will require a higher herd immunity threshold, and/or more likely to cause severe infection [7]. The situation is constantly evolving hence a high vaccine uptake is essential to combat the current dominant strain of COVID-19 and any potential future strains [8].
Global public trust in governments has rapidly declined throughout the pandemic, with the Edelman Trust Barometer reporting a significant decline in trust between both the Chinese and American government and their own citizens between May 2020 and January 2021 [9]. Together with the increasingly prominent role of social media, pandemics are a breeding ground for fearmongering and rumours to circulate [10]. Online misinformation circulating on social media regarding COVID-19 is a growing problem [11]. In the case of COVID-19 (as in past pandemics), the dissemination of vaccine misinformation has been particularly prevalent in fuelling a growing anti-vaccination movement [12, 13]. A recent analysis of social media identified that 39% of online rumours regarding the COVID-19 pandemic were about the COVID-19 vaccine, with 76% of such rumours reported to be false [14].
The term ‘vaccine hesitancy’ refers to a delayed acceptance or complete refusal of a vaccine [15]. The effects of vaccine hesitancy can be devasting. The diphtheria-tetanus-pertussis (DTP) vaccine was routinely used in the UK for over 20 years [16]. However, following the publication of case-series linking the vaccine to a rare neurological side-effect, there was a dramatic fall in immunisation rates against DTP from 77 to 33% [16, 17]. This was followed by three whooping-cough epidemics in the UK [17]. Therefore, historic evidence suggests that uncertain times can increase individual and societal vaccine hesitancy.
Many factors may contribute to vaccine hesitancy [15]. A systematic review of adults aged 65 years and older in the United States of America (USA) identified female sex, older age, higher education, higher household income and White ethnicity all increase the likelihood of seasonal influenza vaccination uptake [18]. It is currently unclear whether COVID-19 vaccine intentions are influenced by the same trend in socio-demographic factors. Public Health England (PHE) reported large disparities in mortality and morbidity risks of COVID-19 infection between different sociodemographic groups, with more deprived areas and ethnic minority individuals (particularly Black ethnic groups) shown to be at a higher risk [19]. A rapid national assessment in 2021 in the United States highlighted that less educated, minority groups had a higher rate of COVID-19 vaccine hesitancy [20]. Arguably, these groups would benefit the most from the COVID-19 vaccine. A theory-based analysis of COVID-19 vaccine hesitancy among African Americans in the United States in July-August 2021 discovered that 48.6% of African-Americans had not been COVID-19 vaccinated and expressed vaccine hesitancy and the rates of vaccine hesitancy were significantly higher amongst younger individuals [21]. This highlights both similarities and differences between traditional socio-demographic trends in vaccine hesitancy and COVID-19 vaccine hesitancy.
Since the beginning of the COVID-19 pandemic, several systematic reviews have been conducted into COVID-19 vaccine uptake and adherence [22,23,24,25,26].
Lin et al. (December 2020) evaluated 126 cross-sectional surveys of vaccine intentions dating from February to October 2020 [22]. A narrative synthesis of results described a declining trend in vaccine intention and highlighted socioeconomic and ethnic issues pertaining to vaccine availability. Due to the dynamic nature of public opinion during the pandemic, the review recommends continuous monitoring of vaccine intentions, especially following the introduction of mass-vaccination programmes. A recent study has suggested that traditional sociodemographic factors may not be effective predictors of COVID-19 vaccine uptake [27]. Therefore, it is not unreasonable to suggest that traditional public health campaigns may be ineffective at boosting COVID-19 vaccine intentions. The continuous monitoring of COVID-19 vaccine intentions among different socioeconomic groups is essential in order to design effective public health campaigns to tackle hesitancy towards the COVID-19 vaccine specifically.
Robinson et al. (December 2020) published a smaller review of 28 international cross-sectional studies and survey dates ranged from March-October [23]. The review reported a high rate of vaccine intention across survey participants (72.9% of total participants were prepared to have a COVID-19 vaccine, 95% confidence interval (CI) 66.6 to 78.4%, I2 = 99.6%) and found a declining trend in vaccine willingness over time. It searched only two online databases and only one author conducted all stages of screening and data extraction. Limited database searching can introduce selection bias [28] and the absence of conventional double screening can result in the omission of key studies [29]. This review included surveys with a large sample size only (n ≥ 100) [23], compared to Lin et al.’s review which included surveys with smaller sample sizes [22]. Overall, the review conducted by Robinson et al. provides a systematic summary of the global populations’ acceptance towards the COVID-19 vaccine up until October 2020 [23]. Since the rollout of COVID-19 vaccine programmes, booster doses of the vaccine have emerged to provide continuous long-term protection from the virus. A cross-sectional study among the American population suggested a strong predictor of booster hesitancy was primary COVID-19 vaccine status; the vaccine-booster-hesitant groups were almost 5 times more likely to be unvaccinated [30]. This highlights the importance of tackling hesitancy towards the primary vaccine dose, due to the apparent domino effect this may have on subsequent booster doses. Given the emergence of breakthrough infections and new variants, it is currently unclear how long the population will be required to receive booster doses to maintain immunity and control the virus. Therefore, it is essential that vaccine hesitancy towards the COVID-19 vaccine is understood, before we can begin to improve booster vaccine acceptance.
Given the rapidly evolving course of the pandemic, ongoing research is needed to reflect the changing evidence base and summarise public opinion later in the pandemic. Therefore, this systematic review will provide an updated summary of public opinion towards the COVID-19 vaccine around the globe up until February 2021, with the intention of providing readily accessible data to policymakers.
Aims
This review aimed to assess: (1) general population intention to receive the COVID-19 vaccine around the world up until February 2021 and changes over time; (2) factors associated with COVID-19 vaccine acceptance; (3) reasons behind individuals’ vaccination intention.
Methods
Eligibility criteria
As recommended by the Cochrane Collaboration, the research question and eligibility criteria were framed using the SPIDER search tool, to maintain a focused review (Additional file 1) [31].
Search methods
This review was developed and structured in line with the 2020 Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines and the protocol was registered in PROSPERO (Registration Number: CRD42021239134) [32].
Information sources
A literature search of Medline (Ovid) [33], Embase (Ovid) [34] and CINAHL (EBSCO) [35] was undertaken by one author (ET). Specialist social sciences databases were also searched: APA PsycINFO (Ovid) [36], APA PsycARTICLES (Ovid) [37], Sociological Abstracts (ProQuest) [38] and Applied Social Sciences Index and Abstracts (ASSIA, ProQuest) [39].
Search strategy
Search strategy development was guided by a librarian specialist. Several scoping searches were conducted on Medline [33] and Embase [34] to identify relevant literature and understand any differences in standardised subject terms across the databases. Development of search terms were guided by the results of the scoping review. The search strategy was subsequently piloted using Medline [33] and refined until all key papers identified in the scoping review were retrieved from the first 100 search results. A combination of text words and standardised subject terms were used, adjusted for each database, to avoid missing key literature.
For the purpose of this review, studies investigating vaccine intentions were included, with vaccine hesitancy defined as “a delay in acceptance or refusal of vaccination despite availability of vaccination services” [40] and vaccine acceptance defined as “outcome behaviour resulting from a complex decision-making process that can be potentially influenced by a wide range of factors” [40]. We searched explicitly for papers that included data on these terms, using the search terms ‘COVID-19’, ‘Pandemics’, ‘Intention’, ‘Attitude to Health’, ‘Mass Vaccination’, ‘Vaccination Refusal’, ‘Anti-Vaccination Movement’ and ‘Vaccination’. Search terms were combined with the Boolean operators ‘AND’ or ‘OR’, the explosion function was used where possible, and truncation was utilised to capture all alternative spellings of the terms. Searches were conducted on 12/02/2021. Full detailed searches, tailored to each database can be found in Additional file 2.
Limits
Date of publication was limited from 1st January 2020 to the day the search was undertaken. The study-type was limited to cross-sectional studies. No geographical limits were applied, but only studies published in English were eligible.
Data management
Endnote was used to store references and remove duplicates automatically [41]. The web-based reviewing platform Rayann was used for title/abstract and full-text screening [42].
Selection process
Title and abstract screening were performed independently by two authors (ET and SC), who both performed full-text assessment of potentially eligible studies (Additional file 1). All discrepancies in inclusion/exclusion decisions at both stages of screening were discussed by ET and SC over the online video platform Zoom initially and with SD as a third reviewer when a decision could not be made [43]. As screening was undertaken by two novice reviewers, inter-rater reliability was measured using Cohen’s Kappa coefficient at both stages, with a Kappa value of > 0.6 deemed to represent substantial agreement [44].
Data extraction and synthesis
Data collection process
A data extraction form (Additional file 6) was developed and piloted prior to use. Data were extracted by ET and a random sample of 10% of studies was co-assessed by SD, to minimise data extraction errors. Any differences of opinions were discussed, to ensure that all relevant data were extracted. For each study, study characteristics were extracted including study design, sample size, location, survey timescale, method of recruitment, participant demographics, validation and standardisation of the survey instruments, the specific survey questions used to capture attitudes towards the COVID-19 vaccine, response scales, recorded proportions of vaccine intentions and any other relevant information.
Risk of Bias and quality assessment
Study quality and risk of bias were assessed using the Appraisal Tool for Cross-Sectional Studies (AXIS) (Additional file 7) [45] which was piloted for suitability on two studies. ET assessed all studies, with 10% co-assessed independently by a second author (SC). Again, Cohen’s Kappa coefficient was calculated to test inter-rater reliability.
Data synthesis
Data were summarised using narrative syntheses and meta-analyses as appropriate. Where response proportions were represented as raw numbers, data were converted to percentages of total survey participants in each included study.
All studies were assessed to determine whether it was appropriate to statistically combine the survey findings. Guided by Lin at al., surveys were excluded from analysis if questions included persuasive or influencing language; if they included phrases similar to ‘if a safe and effective vaccine was available’ [22]. The remaining surveys were included in a random-effects meta-analysis using the ‘metaprop’ command [46], to estimate the proportion of total survey participants reporting vaccine acceptance (including 95% confidence intervals). For studies that included a 5-point Likert scale, vaccine acceptance represented the proportions of both ‘strongly agree’ and ‘agree’ responses. Percentage proportions were presented using forest plots, including statistical heterogeneity. Substantial heterogeneity (I2 > 85%) was expected due to the nature of the survey outcome; an individual’s decision on vaccine uptake may be influenced by multiple and potentially overlapping factors simultaneously. Publication bias was not assessed; instead, sub-group analyses of the meta-regression by sample size (n < 1000 vs n ≥ 1000) and sampling method (non-probability vs probability sampling) were performed.
Across studies that included four response categories (variations of ‘strongly agree’, ‘agree’, ‘disagree’ and ‘strongly disagree’), the mean proportion of responses in each category were compared. Across studies that included a hesitant response category (variations of ‘maybe’), the overall proportion of survey participants reporting COVID-19 vaccine hesitant and improbable/very improbable were compared.
For all studies, the influence of health beliefs and sociodemographic variables (age, gender, ethnicity, education, and income level) on vaccine acceptance, and reasons for vaccine hesitancy were summarised narratively. Of the studies included in the meta-regression, further sub-group analyses of participants reporting vaccine acceptance by gender and time of survey were performed on studies that reported the relevant data.
Statistical significance for all analyses was set at the 5% level (p = 0.05) and all statistical analyses were conducted using STATA16 [46].
Results
Study selection
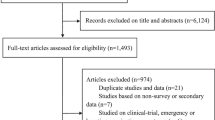
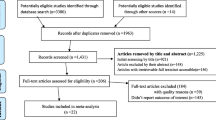
The literature search returned 5447 studies and following removal of duplicates, 4739 studies were considered for title and abstract screening. Following title and abstract screening, 55 full-text articles were assessed for eligibility. Of these, 23 studies met the inclusion criteria and were deemed eligible for the review [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Reasons for exclusion at full-text screening included a lack of specific focus on the COVID-19 vaccine and the absence of extractable raw data (Fig. 1). Cohen’s Kappa was 0.7 at title and abstract screening and 0.8 at full-text screening.
PRISMA Flow Diagram illustrating the summary of search strategy results from initial search to included studies [32]
Summary of included studies
All studies had a cross-sectional study design: 22 used online surveys [47,48,49,50, 52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] and one study used a mixed-methods approach, including both an online survey and semi-structured interviews [51]. Eighteen surveys were conducted in the first half of 2020 (January-June) [47,48,49,50, 52,53,54,55,56,57, 59,60,61,62, 66,67,68,69] and five were conducted in the latter half of 2020 (July-December) [49, 58, 63,64,65]. Fifteen countries were represented in the review, the commonest being the USA (n = 4) [54, 58, 62, 63] and the UK (n = 4) [51, 60, 65, 67]. In total, 41,403 participants were sampled across all surveys, with sample sizes ranging from 525 [58] to 5677 [48] participants. The majority of participants were aged between 25 and 50 years old, and all surveys reported a higher proportion of female participants with one exception [58]. Ethnicity data were only reported in nine studies [51, 54, 58, 60,61,62,63, 65, 68], with Black, Asian and Minority Ethnic (BAME) representation ranging from 3.6% [68] to 36.7% [54] (Additional file 3).
Survey questions used to assess vaccination intentions could largely be categorised into two; 18 studies used neutral questions such as ‘Will you get the coronavirus vaccine when available?’ [47, 50,51,52,53,54, 56,57,58,59, 61, 62, 64,65,66,67,68]; five studies used persuasive language that may have potentially influenced self-reported vaccine acceptance, for example ‘If a new vaccine for COVID-19 was released that was proven to be safe and effective, I would get vaccinated immediately’ [48, 49, 60, 63, 69]. Fifteen studies recorded responses using a Likert-scale, adopting variations of the terms ‘Strongly Agree to Disagree’ [47,48,49, 51, 53, 57, 59, 62, 63, 65,66,67,68,69], seven studies utilised a simple ‘Yes’, ‘No’ and/or ‘Maybe’ response scale [50, 52, 54,55,56, 60, 61, 64] and one study used a best-fit statement response [58] (Additional file 4).
Quality assessment and risk of Bias
Of the 23 studies included in the review, 17 studies used piloted, trialled or previously published survey instruments (Additional file 5) [47, 48, 51, 52, 54,55,56,57,58,59,60,61,62,63,64,65, 68, 69]. Only 11 studies used an adequate sampling frame to achieve a representative sample [49, 50, 54, 55, 58,59,60, 62, 63, 65, 66] and 10 studies were deemed to use an adequate selection process [51, 54, 55, 59,60,61, 63, 65, 66]. Of the studies that used adequate sampling frames, eight used existing online research panels [50, 54, 58, 60, 62, 63, 65, 66] (two most common being Qualtrics, n = 2 [58, 60] and the AmeriSpeak panel, n = 2 [54, 63]). Sixteen studies did not categorise non-responder rates [47, 49, 51,52,53, 55, 56, 58, 60, 62, 64,65,66,67,68] and 11 non-responder rates raised concerns over non-response bias [48,49,50, 53, 57, 59, 63, 64, 66, 67, 69]. Non-responder bias could not be determined for six studies due to lack of adequate information [47, 52, 56, 60, 65, 66].
Vaccine intentions
Five studies were removed from the meta-analysis due to the use of persuasive questions to assess vaccine intentions [48, 49, 60, 63, 69]. From the 18 studies included in the meta-analysis, the pooled proportion of survey participants willing to receive the COVID-19 vaccine was 73.3% (n = 18, 95%CI 64.2 to 81.5%, I2 = 99.7%, p = 0.00 Fig. 2) [47, 50,51,52,53,54, 56,57,58,59, 61, 62, 64,65,66,67,68]. Only two studies included in the meta-analysis reported a higher proportion of participants unwilling to receive the vaccine (71.3% in Sallam et al. [64] and 51.9% in Mouchtouri et al) [59].
Random-effects meta-analysis of 18 cross-sectional studies [47, 50,51,52,53,54, 56,57,58,59, 61, 62, 64,65,66,67,68]: Estimated proportion of survey participants reporting vaccine acceptance (% of total sample). Vaccine ‘acceptance’ is defined as either definite (responding ‘yes/ accepting/ strongly agree/ very likely’) or possible (responding ‘agree/ probably yes/ somewhat likely/ unsure but leaning towards yes’). Vaccine ‘unwillingness’ is defined as hesitant (responding ‘not sure/ neither agree nor disagree/ neutral/ I don’t know/ Maybe/ Hesitant), improbable (responding ‘somewhat unlikely/ disagree/ unsure but leaning towards no/ probably no) or very improbable (responding ‘no/ very unlikely/ strongly disagree/ resistant). CI = confidence interval
Across the 10 studies that included four response categories (variations of ‘strongly agree’, ‘agree’, ‘disagree’ and ‘strongly disagree’), individuals were more confident in accepting the vaccine than rejecting the vaccine [47,48,49, 51, 53, 57, 59, 62, 67, 68]. A mean proportion of 51.3% participants were definitely willing, compared to only 30.7% participants possibly willing to receive the COVID-19 vaccine. Contrastingly, a higher proportion of participants reported improbable rather than very improbable intentions to receive the COVID-19 vaccine, a mean proportion of 6.0 and 4.9% respectively.
Across the 15 studies that included a hesitant response choice (variations of ‘maybe’), participants were more likely to be vaccine hesitant than either improbable/very improbable, with a mean proportion of 22.2 and 9.4% respectively [47,48,49, 53, 54, 59,60,61,62,63, 65, 67,68,69].
Factors associated with Vaccine intentions
Health beliefs
A lower perceived individual risk and perceived severity of COVID-19, lower levels of worry regarding the pandemic and lower perceived likelihood of becoming infected with COVID-19 were all found to be major variables reducing vaccine acceptance in all eight studies investigating these factors [50, 53, 57, 61, 62, 66, 68, 69]. One survey reported that personal fear about COVID-19 meant the individual was almost 2.5 times significantly more likely to accept the vaccine (Odds Ratio (OR) 2.5, 95%CI 2.0-3.0, p < 0.001) compared to individuals with no fear [53]. Additionally, positive attitudes towards past influenza vaccines significantly increased the likelihood of COVID-19 vaccine acceptance [50, 54, 65]. Higher levels of perceived vaccine harm, concerns about side-effects and vaccine efficacy significantly contributed to a reduced vaccine acceptance in four out of four studies [57, 62, 65, 68]. One survey reported a significant increase in the likelihood of vaccine acceptances if individuals perceived the vaccine to reduce the risk of COVID-19 infection (OR 3.1, 95%CI 2.1 to 4.8, p < 0.001) [57].
Sociodemographic variables
Sex
Males were significantly more willing to receive the COVID-19 vaccine than females in all seven studies investigating this variable [50, 54, 57, 62, 64, 66, 68]. One survey reported that males were almost twice as likely as females to receive the COVID-19 vaccine (OR 1.9, 95%CI 1.5-2.3, p < 0.001) [53]. A subgroup analysis by gender across the seven studies reporting gender proportions revealed a similar trend; the pooled proportion willing to vaccinate for males was 71.9% (95%CI 59.4 to 83.0%) and 58.0% (95%CI 37.1 to 77.4%) for females, but this was not statistically significant (p = 0.247, Fig. 3) [50, 54, 57, 62, 64, 66, 68]. Similarly, females were consistently recorded as more likely to be vaccine hesitant than their male counterparts [49, 50, 60] with an Australian survey recording females as almost twice as likely to be vaccine hesitant than males (Relative Risk Ratio (RRR) = 2.0, 95%CI 1.5 to 2.6, p < 0.001) [50].
Subgroup analysis by gender of 7 studies [50, 54, 57, 62, 64, 66, 68]: Estimated proportion of survey participants reporting COVID-19 vaccine acceptance (% of total sample in each gender group). Group 1 = Female Participants, Group 2 = Male Participants, CI = confidence interval. Seven studies presented proportions of total survey participants willing to receive the COVID-19 vaccine by gender and hence data was collated in a subgroup analysis. Response proportions were combined into 2 categories; vaccine ‘acceptance’ (responding with either strongly agree or agree to receiving the COVID-19 vaccine) and vaccine ‘unwillingness’ (responding with either maybe, disagree, strongly disagree to receiving the COVID-19 vaccine)
Ethnicity
BAME individuals reported lower vaccination intentions than White individuals in all four studies that assessed acceptance by ethnicity [51, 54, 60, 62]. Specifically, individuals of Black ethnicity were reported to be less accepting than White ethnic individuals in both studies investigating specific ethnicities [54, 62] and less accepting than both Hispanic and White ethnic individuals in one survey [62]. One study reported Black individuals to be up to 6.4 times more likely to be either hesitant or resistant (RRR 6.4, 95%CI 3.2 to 13.0, no p-value reported) than their White counterparts [54].
Household income
Individuals with a lower household income were significantly less willing to receive the vaccine in three out of four studies [51, 58, 62]. One study reported that lower income households were over two times more likely to reject the vaccine than higher income households (OR 2.1, 95%CI 1.3-3.3, p < 0.001) [51]. However, one survey appeared to contradict this trend, suggesting that individuals in the lowest income band were significantly more likely to express vaccine hesitancy than rejection compared to individuals in higher income brackets [60].
Educational attainment
In all three studies investigating education, lower education was associated with lower vaccine acceptance [49, 54, 64]. In one study, the risk of individuals with no high school diploma rejecting and/or hesitating over the vaccine was almost eight times higher than those with a diploma or higher (RRR 7.8, 95%CI 3.1 to 19.6, no p-value reported) [54].
Age
Four out of seven studies reported younger individuals to be less vaccine willing [53, 58, 65, 66]. However, there were substantial variations in the age groupings used by included studies. Two studies reported individuals aged < 30 years [53] and < 35 years old [66] to be the least willing age group to receive the vaccine (OR 1.5, 95%CI 1.3-1.9, p < 0.001 and OR 1.2, 95%CI 1.1-1.5, p < 0.001 respectively). One study opposed this trend, reporting individuals aged 35-44 years as most likely to reject (OR 3.3, 95%CI 1.2-9.5, p < 0.05) [60]. Another conflicting study drew conclusions from proportions alone, suggesting individuals aged < 35 years old were more vaccine willing than those in older age groups [48].
Time of survey
Across the 11 studies adopting a large sample size (n ≥ 1000), the proportion reporting vaccine acceptance reduced significantly over time [50, 51, 53, 56, 57, 59, 62, 64,65,66, 68]. The nine surveys conducted between March-June had a pooled mean proportion of 76.8% survey participants reporting vaccine acceptance (n = 9, 95%CI 68.5 to 84.1%, p = 0.0) [50, 51, 53, 56, 57, 59, 62, 66, 68] compared to 39.1% survey participants reporting vaccine acceptance (n = 2, 95%CI 39.1 to 40.5%, p = 0.0) across the two studies conducted between July-December (Fig. 4) [64, 65]. Smaller studies (n < 1000) were more likely to increase the heterogeneity of the results, so following the example of Robinson et al. [23], the authors chose to restrict the subgroup analysis to larger studies which may have had more robust estimates of vaccine acceptance.
Subgroup analysis by time of survey of 11 studies [50, 51, 53, 56, 57, 59, 62, 64,65,66, 68]: Estimated proportion of survey participants reporting COVID-19 vaccine acceptance (% of total sample). Only the 11 studies with a sample size ≥1000 were included in the analysis. Group 1 = March-June 2020, Group 2 = July-December 2020, CI = Confidence Interval
Sampling type
A subgroup analysis assessing study methodology used for recruitment reveals that survey participants recruited via probability sampling [54, 58, 59, 64, 66] were significantly less willing to receive the COVID-19 vaccine than survey participants recruited via non-probability sampling [47, 50,51,52,53, 56, 57, 61, 62, 65, 67,68,69] (p = 0.029, Fig. 5), 55.6% (95%CI 34.0 to 76.1%) compared to 79.3% (95%CI 73.0 to 85.1%) respectively.
Subgroup analysis of vaccine acceptance by method of recruitment of 18 studies [47, 50,51,52,53,54, 56,57,58,59, 61, 62, 64,65,66,67,68]: (ES) estimated proportion of total survey participants reporting COVID-19 vaccine acceptance (% of total sample). 1 = Non-Probability Sampling, 2 = Probability Sampling, CI = Confidence Interval
Reasons for Vaccine hesitancy
Concern over vaccine safety was the most common reason reported for both vaccine hesitancy and rejection cited in all six studies investigating vaccine reasoning [51, 54, 57, 58, 62, 67]. Three studies explicitly stated that fears of potential side-effects were the main cause for concern [57, 58, 62]. Other reasons include concern over vaccine efficacy [57, 62], speed of vaccine production and lack of evidence [51, 66], a lack of trust in both scientific and governmental bodies [69], and general anti-vaccination attitudes [51, 66]. For all studies investigating reasons for vaccine willingness, the main justification for vaccine acceptance was for the protection of both the individual and others [51, 57, 67].
Discussion
Comparison to existing literature
Guidance on how to achieve high vaccine uptake could be based on existing evidence regarding uptake of previous vaccines and specific research on COVID-19 vaccine intention. This systematic review investigated intentions to receive the COVID-19 vaccination across the global population and the relevant influencing factors, as reported in eligible international cross-sectional studies published between March and December 2020. A total of 4739 journal articles were screened and 23 cross-sectional surveys were selected for inclusion in the review [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Twenty-one out of the twenty-three studies reported high intentions to receive the vaccine across survey participants [47,48,49,50,51,52,53,54,55,56,57,58, 60,61,62,63, 65,66,67,68,69], with a significant trend towards a declining willingness to vaccinate over time; concordant with the findings of existing reviews investigating COVID-19 vaccine willingness [22,23,24,25,26]. Consistent with similar reviews, the main reasons behind COVID-19 vaccine acceptance reported in this review are for the protection of oneself and others, suggesting that receiving the vaccine is regarded as a social responsibility [22,23,24,25,26].
Compared to self-reported acceptance to receive past-vaccines, the rate of COVID-19 vaccine acceptance was generally higher [70,71,72]. It is important to note the significant effect of sampling bias on self-reported vaccine acceptance in surveys included in the review. The inclusion of cross-sectional studies that used non-probability recruitment methods may have further limited the generalisability of the review findings to the general population.
This review confirms the majority of findings reflected in existing literature investigating the sociodemographic trends in COVID-19 vaccine acceptance with higher educational attainment and household income, older age and being of White ethnicity to be associated with a higher acceptance [22,23,24,25,26]. Furthermore, this pattern is consistent with literature investigating attitudes towards past-vaccines [73,74,75,76]. Literature investigating past-vaccines consistently reports that females are likely to express higher vaccine acceptance in general compared to males [77, 78]. However, the findings of this review investigating attitudes towards the COVID-19 vaccine report the opposite. Research has suggested that females are more likely to lower their intention to vaccinate following exposure to vaccine misinformation than males. The widespread conspiracy that the COVID-19 vaccine causes infertility may have contributed to this significant difference [79, 80]. This same misconception posed an obstacle to uptake of the Polio vaccine in Nigeria, India and Pakistan [80]. Females are more likely to express greater levels of concern towards their personal health than males which could explain why females are more inclined to believe COVID-19 vaccine conspiracies [81]. Social media is reported to be the main source of vaccine misinformation [82]. The narrative of social media is rapidly changing, suggesting that the influence of social media on vaccine intentions is subject to change. The findings of this review are supported by two recent systematic reviews conducted in 2021 [22, 23]. Interestingly, Lin et al.’s narrative review reported inconsistent findings towards the influence of sex on COVID-19 vaccine acceptance [22]. Similarly, Robinson et al. stated that whilst seven out of fourteen studies reported that females have significantly lower COVID-19 vaccine intentions than males, five studies reported no significant association between COVID-19 vaccine acceptance and sex [23].
The association between household income and willingness to receive the COVID-19 vaccine has been echoed throughout the literature [22,23,24,25,26, 73,74,75,76]. Unwillingness to receive the COVID-19 vaccine can be described as a negative health behaviour. In the literature, there is an established correlation between low income level and negative health behaviours, which may provide an explanation for the association between income and vaccine willingness [83]. However, the included studies did not control for confounding factors that may have influenced the strength of the association between household income and vaccine willingness including education level and health literacy; ethnicity; access to vaccines and healthcare services; and urban vs rural living to name a few [84,85,86,87]. A Finnish study suggests it may be more appropriate to use the term ‘low socio-economic status’ rather than ‘low household income’ to account for the confounding variables that may be contributing to this association [88, 89]. Going forward, it would be interesting to further examine the impact that these confounding variables may have had on the association between income and willingness. This may identify areas to target in an attempt to encourage vaccine uptake in this population group.
A recent study published in January 2022 echoed the findings of this review, discovering that COVID-19 vaccine acceptance was lower amongst minority groups and less educated individuals [90]. Another study discovered that 48% of unvaccinated African-Americans were reported to be vaccine-hesitant, and of such individuals, rates of hesitancy were highest among the lesser educated [21]. These disparities may be due to concerns regarding the side-effect profile of the vaccine and found a higher acceptance for a combination vaccine (COVID-19 and influenza) than for COVID-19 alone among minorities [90]. This suggests that novelty of the vaccine may be contributing to the risk-profile, highlighting areas that need to be addressed to combat low vaccine uptake among minority groups.
Risk perception is a well-established determinant in vaccine decision-making [91, 92]. This pattern of behaviour is reported as being no different for the COVID-19 vaccine and can be explained by the Health Belief Model; individuals are more likely to engage in health-protective behaviours if they perceive themselves to be at a higher risk from the disease in question [93, 94]. The high level of uncertainty towards the threat of COVID-19 and rapid rate of transmission of the virus substantially increased individuals’ perceived risk of ill-health and state of anxiety during the pandemic, motivating individuals to perform health-protective behaviours [95,96,97].
Further research
This review has contributed to the literature in providing the most recent representation of the public’s views towards the COVID-19 vaccine around the globe. The findings of this review suggest that global policymakers cannot rely on the findings of existing literature about past-vaccines to formulate public health campaigns regarding COVID-19 vaccine uptake. Despite similar social pressures and the influence of risk perception on the uptake of both existing vaccines and the COVID-19 vaccine, there are several sociodemographic aspects specific to the COVID-19 vaccine that need to be considered and further researched, particularly in terms of sex and age. Public attitudes towards the COVID-19 vaccine around the globe need to be continuously explored as there appears to be evidence that attitudes can change rapidly, for example, the influence of social media on differences in vaccine acceptance between males and females. Consequently, we cannot rely solely on existing findings of past-vaccines and early COVID-19 vaccine research to guide government advice.
Since the rollout of COVID-19 vaccine programmes, booster doses of the vaccine have emerged, as the duration of protection provided by the vaccine is currently unknown [98, 99]. A cross-sectional study among the American population suggested a strong predictor of booster hesitancy is primary COVID-19 vaccine status [30]. The sociodemographic trends in vaccine hesitancy towards booster doses must be investigated, as it is currently unclear whether the sociodemographic trends in vaccine hesitancy towards primary doses of the COVID-19 vaccine that were highlighted in this review, can be directly applicable to further booster doses. If there are other influencing factors at play, these must be identified and closely monitored by public health officials to guarantee the success of the vaccine and achieve herd immunity.
Translation into practice
Following the subsequent roll-out of mass-vaccination programmes across the globe, uptake of the COVID-19 vaccine has been higher than anticipated. As of August 2021, Israel has successfully vaccinated over 70% of all adults over the age of 16 [100] and the UK has almost achieved 80% of all adults over the age of 16 double vaccinated [101]. The literature suggests there are considerable discrepancies between decision-making in real-life and hypothetical situations, with individuals more likely to focus on the outcome of decisions in real-life situations [102]. As evidenced in this review, a major reason behind an individual’s intention to receive the COVID-19 vaccine may be the protection of others as well as themselves. This suggests that receiving the vaccine may be seen as a social responsibility [103]. The UK no longer recommends the use of AstraZeneca in under-40s with no underlying health conditions following reports that the AstraZeneca vaccine may have a higher risk of blood clots than other vaccines [104,105,106]. However, this association was later disproved following a review by the European Medicines Agency [107]. Nevertheless, the Pfizer vaccine is the only vaccine authorised for young adults aged 12-17 years old in the UK [108] and numerous European countries have stopped administering the AstraZeneca vaccine across all age-groups [109]. It is important to acknowledge the political implications that Brexit may have had on the decision of the European Union to discontinue the use of AstraZeneca, a UK-made vaccine [110, 111]. This decision may have fuelled vaccine hesitancy, with several European polls reporting a substantial drop in perceived vaccine safety following the AstraZeneca blood clot scares [110]. Following the development of several licenced vaccines, vaccine acceptability and personal risk perceptions may be further affected by the type of vaccine offered to individuals. Thus, the reporting of vaccine risk assessments must be carefully navigated and the prevention of vaccine misinformation across social media is imperative if a high vaccine uptake is to be achieved across the globe.
This review has identified sub-groups of the population that are at a higher risk of vaccine hesitancy and low vaccine uptake. There is therefore a continued risk of pockets of local outbreaks across sub-groups of the population despite the vaccine now being available [112]. The findings of this review can guide local policy-makers towards the close monitoring of vaccine uptake amongst sub-groups of the population at risk of vaccine hesitancy, now vaccines are available. It is also important to acknowledge the impact that COVID-19 vaccine accessibility may have had on vaccine uptake, especially in low-economically developed countries where there are well-documented issues pertaining to vaccine inequity [113, 114]. This review has highlighted the dangerous impact that vaccine misinformation can have on vaccine hesitancy, and thus can be used by local policy-makers to control the spread of COVID-19 misinformation on social media, focusing particularly on debunking COVID-19 vaccine myths targeted towards individuals at a higher risk of vaccine hesitancy. This review contributes to the growing evidence base suggesting that males are more likely to receive the COVID-19 vaccine than females [24, 25], opposing the trend in past-vaccine hesitancy across the sexes reported in existing literature [71, 72].
Strengths and limitations
This systematic review has several strengths. Multiple databases were searched and there was a high level of agreement between screeners. Thorough quality and risk of bias assessments were also undertaken using validated tools that were piloted before use. However, searches were limited to English language studies and grey literature was explicitly excluded to ensure a manageable volume of literature was retrieved. This may have led to the exclusion of relevant literature. In a similar vein, we acknowledge that we were unable to search every possible database and that by omitting searches of databases such as Web of Science and Scopus, we may have missed a small number of potentially eligible studies that were only indexed in those databases.
It is important to acknowledge the fast-moving nature of the COVID-19 pandemic; four licenced vaccines are now available whereas there were either no/limited vaccines available at the time when eligible studies were conducted [115]. The availability of vaccines may have a role in vaccine intentions. Due to the nature of systematic reviews, we were only able to focus on factors contributing to vaccine hesitancy that were cited within the papers eligible for inclusion in the review. It is important to acknowledge that multiple factors may contribute to vaccine hesitancy and this review does not provide an exhaustive list, there may be other factors at play that contribute to both vaccine hesitancy and willingness. We were unable to explore the impact of issues such as access to the COVID-19 vaccine as this was outside the scope of the review, but it is nevertheless important to acknowledge.
Conclusion
Overall, the review discovered positive attitudes towards the COVID-19 vaccine before February 2021, with 73% of the total survey participants reporting a high intention to receive the COVID-19 vaccine. COVID-19 vaccine acceptance can be influenced by many sociodemographic factors and individual risk perception towards COVID-19. The findings of this review imply that future research should explore the reasoning behind vaccine intentions for different sociodemographic groups, to allow targeted communication strategies to be formulated by governments and public health agencies. The impact of both vaccine availability and reported adverse effects must be monitored so public health policies can address these concerns. A high vaccine uptake to current mass-vaccination programmes and potential booster vaccinations is essential to achieve the end goal of herd immunity and combat any potential future variants.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- COVID-19 :
-
Coronavirus Disease 2019
- WHO:
-
World Health Organisation
- UK:
-
United Kingdom
- DTP:
-
Diphtheria-tetanus-pertussis Vaccine
- A/H1N1:
-
Influenza A Virus subtype H1N1
- PHE:
-
Public Health England
- CI:
-
Confidence Interval
- SPIDER:
-
Sample, Phenomenon of Interest, Design, Evaluation, Research Type
- PICO:
-
Population, Intervention, Comparison, Outcomes
- AXIS:
-
Appraisal Tool for Cross-Sectional Studies
- USA:
-
United States of America
- BAME:
-
Black, Asian and minority ethnic
- OR:
-
Odds Ratio
- RRR:
-
Relative Risk Ratio
- NHS:
-
National Health System
References
World Health Organisation. Archived: WHO Timeline - COVID-19. 2021. Available from: https://www.who.int/news/item/27-04-2020-who-timeline%2D%2D-covid-19 [cited 20 Apr 2021].
Bhowmick N. How India’s second wave became the worst COVID-19 surge in the world. 2021. Available from: https://www.nationalgeographic.com/science/article/how-indias-second-wave-became-the-worst-covid-19-surge-in-the-world [cited 20 Apr 2021].
NHS. News NHS vaccine programme ‘turning point’ in battle against the pandemic. 2020. Available from: https://www.england.nhs.uk/2020/12/nhs-vaccine-programme-turning-point-in-battle-against-the-pandemic/ [cited 20 Apr 2021].
Randolph HE, Barreiro LB. Herd Immunity: Understanding COVID-19. Immunity. 2020;52(5):737–41 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7236739/ [cited 27 Apr 2021].
World Health Organisation. Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19. 2020. Available from: https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19 [cited 27 Apr 2021].
Walensky RP, Walke HT, Fauci AS. SARS-CoV-2 variants of concern in the United States. JAMA. 2021;325(11):1037–8 Available from: https://jamanetwork.com/journals/jama/fullarticle/2776739 [cited 10 Aug 2021].
Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nat Med. 2021;591(7851):520–2 Available from: https://pubmed.ncbi.nlm.nih.gov/33737753/ [cited 5 May 2021].
Darby AC, Hiscox JA. Covid-19: variants and vaccination. BMJ. 2021;372:n771 Available from: https://www.bmj.com/content/372/bmj.n771 [cited 5 May 2021].
Pew Research Center U. S Politics & Policy. Public Trust in Government: 1958-2021. 2021. Available from: https://www.pewresearch.org/politics/2021/05/17/public-trust-in-government-1958-2021/ [cited 10 May 2021].
Evans RJ. Why pandemic create conspiracy theories. NewStatesman: Coronavirus. 2020. Available from: https://www.newstatesman.com/science-tech/coronavirus/2020/04/why-pandemics-create-conspiracy-theories [cited 7 May 2021].
Fletcher R, Kalogeropoulos A, Nielsen RK. Trust in UK government and news media COVID-19 information down, concerns over misinformation from government and politicians up. 2020. Available from: https://reutersinstitute.politics.ox.ac.uk/trust-uk-government-and-news-media-covid-19-information-down-concerns-over-misinformation [cited 10 May 2021].
Davidson M. Vaccination as a cause of autism-myths and controversies. Dialogues Clin Neurosci. 2017;19(4):403–7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789217/ [cited 7 May 2021].
Burki T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit Health. 2020;2(10):504–5 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7508526/ [cited 8 May 2021].
Islam S, Kamal AM, Kabir A, Southern DL, Khan SH, Hasan SMM, et al. COVID-19 vaccine rumors and conspiracy theories: The need for cognitive inoculation against misinformation to improve vaccine adherence. PLoS One. 2021;16(5):e0251605 Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0251605 [cited 8 May 2021].
MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161–4 Available from: https://pubmed.ncbi.nlm.nih.gov/25896383/ [cited 10 May 2021].
Baker JP. The pertussis vaccine controversy in Great Britain, 1974-1986. Vaccine. 2003;21(25-26):4003–10 Available from: https://pubmed.ncbi.nlm.nih.gov/12922137/ [cited 3 May 2021].
Robinson RJ. The whooping-cough immunisation controversy. Arch Dis Child. 1981;56:577–80 Available from: https://adc.bmj.com/content/archdischild/56/8/577.full.pdf [cited 13 May 2021].
Okoli GN, Lam OLT, Racovitan F, Reddy VK, Righolt CH, Neilson C, et al. Seasonal influenza vaccination in older people: A systematic review and meta-analysis of the determining factors. PLoS One. 2020;15(6):e0234702 Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0234702 [cited 14 May 2021].
Public Health England. Disparities in the risk and outcomes of COVID-19. 2020. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908434/Disparities_in_the_risk_and_outcomes_of_COVID_August_2020_update.pdf [cited 20 Apr 2021].
Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J Community Health. 2021;46(2):270–7. https://doi.org/10.1007/s10900-020-00958-x [cited 28 Feb 2022].
Sharma M, Batra K, Batra R. A theory-based analysis of covid-19 vaccine Hesitancy among African Americans in the United States: a recent evidence. Healthcare (Basel). 2021;9(10):1273. https://doi.org/10.3390/healthcare9101273 [cited 28 Feb 2022.]
Lin C, Tu P, Beitsch L. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines. 2021;9(1):16 Available from: https://www.mdpi.com/2076-393X/9/1/16 [cited 25 Apr 2021].
Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39(15):2024–34 Available from: https://www.sciencedirect.com/science/article/pii/S0264410X21001407?via%3Dihub [cited 25 Apr 2021].
Wang Q, Yang L, Jin H, Lin L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prev Med. 2021;150:106694 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8217737/ [cited 19 Aug 2021].
Joshi A, Kaur M, Kaur R, Grover A, Nash D, El-Mohandes A. Predictors of COVID-19 vaccine acceptance, intention, and hesitancy: a scoping review. Front Public Health. 2021;(9):698111. https://doi.org/10.3389/fpubh.2021.698111 [cited 19 Aug 2021.]
Wake AD. The Willingness to Receive COVID-19 Vaccine and Its Associated Factors: “Vaccination Refusal Could Prolong the War of This Pandemic” – A Systematic Review. Risk Manag Healthc Policy. 2021;(14):2609–23. https://doi.org/10.2147/RMHP.S311074 [cited 19 Aug 2021].
Lennon RP, Small ML, Smith RA, Van Scoy LJ, Myrick JG, Martin MA, et al. Unique Predictors of Intended Uptake of a COVID-19 Vaccine in Adults Living in a Rural College Town in the United States. Am J Health Promot. 2022;36(1):180–4. https://doi.org/10.1177/08901171211026132 [cited 28 Feb 2022.]
Li T, Higgins JPT, Deeks JJ. Chapter 5: collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane; 2021. Available from www.training.cochrane.org/handbook.
Waffenschmidt S, Knelangen M, Sieben W, Buhn S, Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol. 2019;19:132 Available from: https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-019-0782-0 [cited 10 May 2021].
Yadete T, Batra K, Netski DM, Antonio S, Patros MJ, Bester JC. Assessing Acceptability of COVID-19 Vaccine Booster Dose among Adult Americans: A Cross-Sectional Study. Vaccines (Basel). 2021;9(12):1424. https://doi.org/10.3390/vaccines9121424 [cited 28 Feb 2022].
O’Connor D, Green S, Higgins JPT. Chapter 5: defining the review question and developing criteria for including studies. In: Higgins JPT, Green S, editors. Cochrane handbook of systematic reviews of intervention. Version 5.1.0 (updated March 2011). Chicester: The Cochrane Collaboration; 2011. Available from www.handbook.cochrane.org.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71 Available from: https://www.bmj.com/content/372/bmj.n71 [cited 20 Apr 2021].
Wolters Kluwer. Ovid MEDLINE® and In-Process & Other Non-indexed citations 1946 to February 12, 2021. Available from: https://ovidsp.dc1.ovid.com/ovid-a/ovidweb.cgi. [cited 12 Feb 2021].
Wolters Kluwer. Ovid Embase 1974 to 2021 February 12. Available from: https://ovidsp.dc1.ovid.com/ovid-a/ovidweb.cgi. [cited 12 Feb 2021].
EBSCO (Firm). CINAHL Plus. Available from: https://www.ebsco.com/products/research-databases/cinahl-database. [cited 12 Feb 2021].
Wolters Kluwer. APA PsycINFO 1967 to February Week 2 2021. Available from: https://ovidsp.dc1.ovid.com/ovid-a/ovidweb.cgi. [cited 12 Feb 2021].
Wolters Kluwer. APA PsycArticles Full Text. Available from: https://ovidsp.dc1.ovid.com/ovid-a/ovidweb.cgi. [cited 12 Feb 2021].
ProQuest (Firm). Sociological Abstracts. Available from: https://www-proquest-com.ezproxye.bham.ac.uk/socabs/index?accountid=8630. [cited 12 Feb 2021].
ProQuest (Firm). Applied Social Sciences Index and Abstracts]. Available from: https://www-proquest-com.ezproxye.bham.ac.uk/assia?accountid=8630. [cited 12 Feb 2021].
MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161–4. https://doi.org/10.1016/j.vaccine.2015.04.036 [cited 02 Aug 2021.]
The EndNote team [computer program]. EndNote X9. Philadelphia: Clarivate; 2013.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan - a web and mobile app for systematic reviews. Syst Revs. 2016;5:210 Available from: https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0384-4 [cited 12 Feb 2021].
Zoom. Video conferencing, web conferencing, webinars, Screen Sharing 2018. Available from: https://zoom.us/ [cited 22 Apr 2021].
Hartling L, Hamm M, Milne A. Validity and inter-rater reliability testing of Quality Assessment Instruments. Rockville: Agency for Healthcare Research and Quality (US); 2012. Table 2, Interpretation of Fleiss’ kappa (K) (from Landis and Koch 1977: Available from: https://www.ncbi.nlm.nih.gov/books/NBK92295/table/methods.t2/
Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12):e011458 Available from: https://pubmed.ncbi.nlm.nih.gov/27932337/ [cited 22 Apr 2021].
StataCorp [computer program]. Stata statistical software: release 16. College Station: StataCorp LLC; 2019.
Abdelhafiz AS, Mohammed Z, Ibrahim ME, Ziady HH, Alorabi M, Ayyad M, et al. Knowledge, Perceptions, and Attitude of Egyptians Towards the Novel Coronavirus Disease (COVID-19). J Community Health. 2020;45:881–90 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7173684/ [cited 12 Feb 2021].
Ali KF, Whitebridge S, Jamal MH, Alsafy M, Atkin SL. Perceptions, Knowledge and Behaviors Related to COVID-19 Among Social Media Users: Cross-Sectional Study. J Med Internet Res. 2020;22(9):e19913 Available from: https://www.jmir.org/2020/9/e19913/ [cited 12 Feb 2021].
Alley SJ, Stanton R, Browne M, To QG, Khalesi S, Williams SL, et al. As the Pandemic Progresses, How Does Willingness to Vaccinate against COVID-19 Evolve? Int J Environ Res Public Health. 2021;18(2):797 Available from: https://www.mdpi.com/1660-4601/18/2/797#. [cited 12 Feb 2021].
Attwell K, Lake J, Sneddon J, Gerrans P, Blyth C, Lee J. Converting the maybes: Crucial for a successful COVID-19 vaccination strategy. PLoS One. 2021;16(1):e0245907 Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0245907 [cited 12 Feb 2021].
Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: A multi-methods study in England. Vaccine. 2020;38(49):7789–98 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7569401/ [cited 12 Feb 2021].
Biasio LR, Boonaccorsi G, Lorini C, Pecorelli S. Assessing COVID-19 vaccine literacy: a preliminary online survey. Hum Vaccin Immunother. 2020;17(5):1304–12 Available from: https://pubmed.ncbi.nlm.nih.gov/33118868/ [cited 12 Feb 2021].
Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38(45):7002–6 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7498238/ [cited 12 Feb 2021].
Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults. Ann Intern Med. 2020;173(12):964–73 Available from: https://pubmed.ncbi.nlm.nih.gov/32886525/ [cited 12 Feb 2021].
Garcia LY, Cerda AA. Contingent assessment of the COVID-19 vaccine. Vaccine. 2020;38(34):5424–9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7315946/ [cited 12 Feb 2021].
Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum Vaccin Immunother. 2020;16(12):3074–80 Available from: https://www.tandfonline.com/doi/full/10.1080/21645515.2020.1819741 [cited 12 Feb 2021].
Lin Y, Hu Z, Zhao Q, Alias H, Danaee M, Wong LP. Understanding COVID-19 vaccine demand and hesitancy: A nationwide online survey in China. PLoS Negl Trop Dis. 2020;12(12):e0008961 Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0008961 [cited 12 Feb 2021].
Mercadante AR, Law AV. Will they, or Won’t they? Examining patients’ vaccine intention for flu and COVID-19 using the Health Belief Model. Res Soc Adm Pharm. 2020;20:1551–7411 Available from: https://www.sciencedirect.com/science/article/pii/S1551741120312407?via%3Dihub [cited 12 Feb 2021].
Mouchtouri VA, Agathagelidou E, Kofonikolas K, Rousou X, Dadouli K, Pinaka O, et al. Nationwide Survey in Greece about Knowledge, Risk Perceptions, and Preventive Behaviors for COVID-19during the General Lockdown in April 2020. Int J Environ Res Public Health. 2020;17(23):8854 Available from: https://pubmed.ncbi.nlm.nih.gov/33260789/ [cited 12 Feb 2021].
Murphy J, Vallieres F, Bentall RP, Shevlin M, McBride O, Hartman TK, et al. Psychological characteristics associated withCOVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat Commun. 2021;12:29 Available from: https://www.nature.com/articles/s41467-020-20226-9 [cited 12 Feb 2021].
Prati G. Intention to receive a vaccine against SARS-CoV-2 in Italy and its association with trust, worry and beliefs about the origin of the virus. Health Educ Res. 2020;35(6):505–11 Available from: https://pubmed.ncbi.nlm.nih.gov/33367772/ [cited 12 Feb 2021].
Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine. 2020;38(42):6500–7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7440153/ [cited 12 Feb 2021].
Romer D, Jamieson KH. Conspiracy theories as barriers to controlling the spread of COVID-19 in the U.S. Soc Sci Med. 2020;263:113356 Available from: https://pubmed.ncbi.nlm.nih.gov/32967786/ [cited 2021 Feb 12].
Sallam M, Dababseh D, Eid H, Al-Mahzoum K, Al-Haidar A, Taim D, et al. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. Vaccines. 2021, 9(1):42 Available from: https://pubmed.ncbi.nlm.nih.gov/33445581/ [cited 12 Feb 2021].
Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccin Immunother. 2020;17(6):1612–21 Available from: https://pubmed.ncbi.nlm.nih.gov/33242386/ [cited 12 Feb 2021].
Ward JK, Alleaume C, Peretti-Watel P. The French public's attitudes to a future COVID-19 vaccine: The politicization of a public health issue. Soc Sci Med. 2020;265:113414 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7537647/ [cited 12 Feb 2021].
Williams L, Gallant AJ, Rasmussen S, Nicholls LAB, Cogan N, Deakin K, et al. Towards intervention development to increase the uptake of COVID-19 vaccination among those at high risk: Outlining evidence-based and theoretically informed future intervention content. Br J Health Psychol. 2020;25(4):1039–54 Available from: https://bpspsychub.onlinelibrary.wiley.com/doi/10.1111/bjhp.12468 [cited 12 Feb 2021.]
Wong LP, Alias H, Wong PF, Lee HY, AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum Vaccin Immunother. 2020;16(9):2204–14 Available from: https://pubmed.ncbi.nlm.nih.gov/32730103/ [cited 12 Feb 2021.]
Zeballos DR, Jaldin MLL, Canaviri BN, Escalante LFP, Fernandez AMCA, Ticona JPA. Social media exposure, risk perception, preventive behaviors and attitudes during the COVID-19 epidemic in La Paz, Bolivia: A cross sectional study. PLoS One. 2021;16(1):e0245859 Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0245859 [cited 12 Feb 2021].
European Centre for Disease Prevention and Control. Season influenza vaccination in Europe Vaccination recommendations and coverage rates in the EU Member States for eight influenza seasons: 2007–2008 to 2014–2015. 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/influenza-vaccination-2007%E2%80%932008-to-2014%E2%80%932015.pdf [cited 8 May 2021].
Perlman S, Wamai RG, Bain PA, Welty T, Ogembo JG. Knowledge and Awareness of HPV Vaccine and Acceptability to Vaccinate in Sub-Saharan Africa: A Systematic Review. PLoS One. 2014;9(3):e90912 Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0090912 [cited 12 May 2021].
Public Health England. Seasonal influenza vaccine uptake in GP patients: winter season 2018 to 2019. 2019. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/804889/Seasonal_influenza_vaccine_uptake_in_GP_patients_1819.pdf [cited 10 May 2021].
NHS. COVID-19 vaccine programme Maximising vaccine uptake in undeserved communities: a framework for systems, sites and local authorities leading vaccination delivery. 2021. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2021/03/C1226-maximising-vaccine-uptake-in-underserved-communities-a-framework-.pdf [cited 9 May 2021].
GOVUK. Factors influencing COVID-19 vaccine uptake among minority ethnic groups. 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/952716/s0979-factors-influencing-vaccine-uptake-minority-ethnic-groups.pdf [cited 10 Apr 2021].
National Institute for Health and Care Excellence. Flu vaccination: increasing uptake. NICE guidelines NG103. London: National Institute for Health and Care Excellence; 2018. Available from: https://www.nice.org.uk/guidance/ng103/chapter/Context [cited 2 May 2021]
Fajar JK, Harapan H. Socioeconomic and Attitudinal Variables Associated with Acceptance and Willingness to Pay Towards Dengue Vaccine: A Systematic Review. Arch Clin Infect Dis. 2017;12(3):e13914 Available from: https://sites.kowsarpub.com/archcid/articles/13914.html [cited 5 May 2021].
Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396(10255):898–908 Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31558-0/fulltext [cited 11 May 2021].
Harapan H, Anwar S, Setiawan AM, Sasmono RT. Dengue vaccine acceptance and associated factors in Indonesia: A community-based cross-sectional survey in Aceh. Vaccine. 2016;34(32):3670–5 Available from: https://www.sciencedirect.com/science/article/pii/S0264410X16303164?via%3Dihub [cited 11 May 2021].
Fossett K. The myth about women and the Covid-19 vaccine that won’t die. 2021. Available from: https://www.politico.com/newsletters/women-rule/2021/03/26/the-myth-about-women-and-the-covid-19-vaccine-that-wont-die-492263 [cited 6 May 2021].
Obregon R, Chitnis K, Morry C, Feek W, Bates J, Galway M, et al. Achieving polio eradication: a review of health communication evidence and lessons learned in India and Pakistan. Bull World Health Organ. 2009;87:624–30 Available from: https://www.who.int/bulletin/volumes/87/8/08-060863.pdf [cited 7 May 2021].
Prichard EC, Christman SD, et al. Front Psychol. 2020;11:3130 Available from: https://www.frontiersin.org/article/10.3389/fpsyg.2020.597671 [cited 5 May 2021].
The Royal Society. COVID-19 vaccine deployment: Behaviour, ethics, misinformation and policy strategies. 2020. Available from: https://royalsociety.org/-/media/policy/projects/set-c/set-c-vaccine-deployment.pdf [cited 4 May 2021].
Graham HM, Wardle H, Law C, Platt L, Philo D. Health behaviour and health behaviour change among adults in England. Available from: https://orcid.org/0000-0001-7949-6819. [cited 8 Feb 2022].
Syan SK, Gohari MR, Levitt EE, Belisario K, Gillarrd J, DeJesus J, et al. COVID-19 Vaccine Perceptions and Differences by Sex, Age, and Education in 1,367 Community Adults in Ontario. Front Public Health. 2021;9:2296–565 Available from: https://www.frontiersin.org/article/10.3389/fpubh.2021.719665 [cited 2 Feb 2022].
Tang C, Wu X, Chen X, et al. Examining income-related inequality in health literacy and health-information seeking among urban population in China. BMC Public Health. 2019;19:221. https://doi.org/10.1186/s12889-019-6538-2 [cited 9 Feb 2022.]
Gendler Y, Ofri L. Investigating the Influence of Vaccine Literacy, Vaccine Perception and Vaccine Hesitancy on Israeli Parents’ Acceptance of the COVID-19 Vaccine for Their Children: A Cross-Sectional Study. Vaccines 2021;9 1391. Available from: https://doi.org/10.3390/vaccines9121391 [cited 8 Feb 2022].
Cascini F, Pantovic A, Al-Ajlouni, Failla G, Ricciardi W. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: A systematic review. EClinicalMedicine. 2021;40:101113. https://doi.org/10.1016/j.eclinm.2021.101113 [cited 8 Feb 2022.]
Laaksonen M, Prättälä R, Helasoja V, Income and health behaviours, et al. Evidence from monitoring surveys among Finnish adults. J Epidemiol Community Health. 2003;57:711–7. https://doi.org/10.1136/jech.57.9.711 [cited 8 Feb 2022].
Mudd AL, van Lenthe FJ, Verra SE, et al. Socioeconomic inequalities in health behaviors: exploring mediation pathways through material conditions and time orientation. Int J Equity Health. 2021;20:184. https://doi.org/10.1186/s12939-021-01522-2 [cited 8 Feb 2022.]
Lennon RP, Block R, Schneider EC, Zephrin L, Shah A. Underserved Population Acceptance of Combination Influenza-COVID-19 Booster Vaccines. Vaccine. 2022;40(4):562–7. https://doi.org/10.1016/j.vaccine.2021.11.097 [cited 28 Feb 2022.]
Hobson-West P. Understanding vaccination resistance: moving beyond risk. Health Risk Soc. 2012;5(3):273–83 Available from: https://www.tandfonline.com/doi/abs/10.1080/13698570310001606978?journalCode=chrs20 [cited 10 May 2021].
Bloom BR, Lambert PH. Chapter 26 – Vaccine Acceptance: Barriers, Perceived Risks, Benefits, and Irrational Beliefs. In: The Vaccine Book. 2nd ed. Massachusetts: Academic Press; 2016.
Lamorte W. The Health Belief Model. 2019. Available from: https://sphweb.bumc.bu.edu/otlt/MPH-Modules/SB/BehavioralChangeTheories/BehavioralChangeTheories2.html [cited 10 May 2021].
Schneider CR, Dryhurst S, Kerr J, Freeman ALJ, Recchia G, Spiegelhalter D, et al. COVID-19 risk perception: a longitudinal analysis of its predictors and associations with health protective behaviours in the United Kingdom. J Risk Res. 2021;24(3):294–313 Available from: https://www.tandfonline.com/doi/full/10.1080/13669877.2021.1890637 [cited 10 May 2021].
World Health Organisation. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020. Available from: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations [cited 10 Apr 2021].
McCleskey J, Gruda D. Risk-taking, resilience, and state anxiety during the COVID-19 pandemic: A coming of (old) age story. Personal Individ Differ. 2021;170:110485 Available from: https://www.sciencedirect.com/science/article/pii/S0191886920306760 [cited 10 May 2021].
Oyetunji TP, Ogunmola OA, Oyelakin TT, Olorunsogbon OF, Ajayi FO. COVID-19-related risk perception, anxiety and protective behaviours among Nigerian adults: a cross-sectional study. Z Gesundh Wiss. 2021;21:1–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7950426/. [cited 11 May 2021].
Public Health England. UK secures extra 60 million Pfizer/BioNTech COVID-19 vaccines [press release]. 2021. Available from: https://www.gov.uk/government/news/uk-secures-extra-60-million-pfizerbiontech-covid-19-vaccines [cited 28 Apr 2021].
CNBC. CDC director says U.S. is planning for Covid vaccine booster shots ‘just in case’. 2021. Available from: https://www.cnbc.com/2021/05/11/covid-booster-shots-cdc-director-says-us-planning-just-in-case.html [cited 26 May 2021].
Israel Ministry of Health. Coronavirus in Israel – general situation. 2021. Available from: https://datadashboard.health.gov.il/COVID-19/general [cited 26 May 2021].
GOV.UK. Coronavirus (COVID-19) in the UK – UK Summary. 2021. Available from: https://coronavirus.data.gov.uk/ [cited 26 Aug 2021].
Evans AM, Brand MJ. Comparing the Effects of Hypothetical Moral Preferences on Real-Life and Hypothetical Behavior: Commentary on Bostyn, Sevenhant, and Roets (2018). Adv Methods Pract Psychol Sci. 2019;30(9):1380–2 Available from: https://journals.sagepub.com/doi/full/10.1177/0956797618815482 [cited 15 May 2021].
Giubilini A, Douglas T, Savulescu J. The moral obligation to be vaccinated: utilitarianism, contractualism, and collective easy rescue. Med Health Care Philos. 2018;21(4):547–60 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6267229/ [cited 6 May 2021].
Public Health England. JCVI advises on COVID-19 vaccine for people aged under 40 [press release]. 2021. Available from: https://www.gov.uk/government/news/jcvi-advises-on-covid-19-vaccine-for-people-aged-under-40 [cited 26 May 2021].
Public Health England. COVID-19 vaccination and blood clotting. 2021. Available from: nhs.uk/CoronavirusVaccination [cited 24 Aug 2021]
Pavord S, Scully M, Hunt BJ, Lester W. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N Engl J Med. 2021; Available from: https://pubmed.ncbi.nlm.nih.gov/34379914/ [cited 24 Aug 2021].
European Medicines Agency. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. 2021. Available from: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots [cited 26 Aug 2021].
Department of Health & Social Care. JCVI statement on COVID-19 vaccination of children and young people aged 12 to 17 years: 4 August 2021. 2021. Available from: https://www.gov.uk/government/publications/jcvi-statement-august-2021-covid-19-vaccination-of-children-and-young-people-aged-12-to-17-years/jcvi-statement-on-covid-19-vaccination-of-children-and-young-people-aged-12-to-17-years-4-august-2021 [cited 26 Aug 2021].
PharmaNewsIntelligence. European Countries Suspend Use of AstraZeneca’s COVID-19 Vaccine. 2021. Available from: https://pharmanewsintel.com/news/european-countries-suspend-use-of-astrazenecas-covid-19-vaccine [cited 24 Aug 2021].
Smith M. Extent of damage to AstraZeneca vaccine’s perceived safety in Europe revealed. 2021. Available from: https://yougov.co.uk/topics/international/articles-reports/2021/03/07/extent-damage-astrazeneca-vaccines-perceived-safet(popup:related_entities/correlated/Richard_Branson) [cited 26 Aug 2021].
BBC News. Covid vaccine: How many people in the UK have been vaccinated so far?. 2021. Available from: https://www.bbc.co.uk/news/health-55274833 [cited 26 Aug 2021].
British Science Association. Vaccine hesitancy and false alarms. 2021. Available from: https://www.britishscienceassociation.org/blog/vaccine-hesitancy [cited 26 Aug 2021].
World Health Organisation. Vaccine Inequity Undermining Global Economic Recovery [press release]. 2021. Available from: https://www.who.int/news/item/22-07-2021-vaccine-inequity-undermining-global-economic-recovery [cited 8 Feb 2022].
OECD Policy Responses to Coronavirus (COVID-19). Coronavirus (COVID-19) vaccines for developing countries: an equal shot at recovery [press release] 2021. Available from: https://www.oecd.org/coronavirus/policy-responses/coronavirus-covid-19-vaccines-for-developing-countries-an-equal-shot-at-recovery-6b0771e6/ [cited 8 Feb 2022].
NHS. Coronavirus (COVID-19) vaccines. 2021. Available from: https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/coronavirus-vaccine/ [cited 26 Aug 2021].
Acknowledgements
I would like to thank the Public Health and Population Sciences team (Dr Laura Jones, Dr. Derek Ward, Dr. Jayne Parry) for their advice throughout this review, and Dr. Benjamin Fletcher at the Institute of Applied Health Research for support with statistical analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ET undertook the literature searches, all stages of screening, data-extraction and quality and risk of bias assessment. ET analysed and interpreted the survey data and was responsible for the writing of the manuscript. SC participated in both independent title and abstract and full-text screening of eligible papers, and independently undertook quality and risk of bias assessment for 10% of studies. SD participated in the resolution of screening, quality and risk of bias assessment disagreements between ET and SC and advised throughout all stages of searching, data collection and extraction, and data analysis. SD participated in decisions regarding the review focus, search strategy and points of discussion. SG participated in decisions regarding the focus of the review, search strategy and points of discussion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Eligibility Criteria. Eligibility criteria for the research question, using the SPIDER search tool.
Additional file 2.
Search Strategy. A detailed description of the search strategies for each database searched in the review.
Additional file 3.
Study Characteristics Table. Summary table detailing the characteristics of each included cross-sectional study.
Additional file 4.
Study Results Table. Summary table detailing the results of each included cross-sectional study.
Additional file 5.
AXIS Summary Table [41]. Summary table of results of the appraisal of cross-sectional studies (AXIS) for each study.
Additional file 6.
Data Extraction Form. A copy of the data extraction form used to extract data from each study included in the review.
Additional file 7.
AXIS Form. A copy of the AXIS form used for the assessment of bias and quality of each study included in the review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Terry, E., Cartledge, S., Damery, S. et al. Factors associated with COVID-19 vaccine intentions during the COVID-19 pandemic; a systematic review and meta-analysis of cross-sectional studies. BMC Public Health 22, 1667 (2022). https://doi.org/10.1186/s12889-022-14029-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14029-4