Abstract
Background
Eating disorders (EDs) are serious, often chronic, conditions associated with pronounced morbidity, mortality, and dysfunction increasingly affecting young people worldwide. Illness progression, stages and recovery trajectories of EDs are still poorly characterised. The STORY study dynamically and longitudinally assesses young people with different EDs (restricting; bingeing/bulimic presentations) and illness durations (earlier; later stages) compared to healthy controls. Remote measurement technology (RMT) with active and passive sensing is used to advance understanding of the heterogeneity of earlier and more progressed clinical presentations and predictors of recovery or relapse.
Methods
STORY follows 720 young people aged 16–25 with EDs and 120 healthy controls for 12 months. Online self-report questionnaires regularly assess ED symptoms, psychiatric comorbidities, quality of life, and socioeconomic environment. Additional ongoing monitoring using multi-parametric RMT via smartphones and wearable smart rings (‘Ōura ring’) unobtrusively measures individuals’ daily behaviour and physiology (e.g., Bluetooth connections, sleep, autonomic arousal). A subgroup of participants completes additional in-person cognitive and neuroimaging assessments at study-baseline and after 12 months.
Discussion
By leveraging these large-scale longitudinal data from participants across ED diagnoses and illness durations, the STORY study seeks to elucidate potential biopsychosocial predictors of outcome, their interplay with developmental and socioemotional changes, and barriers and facilitators of recovery. STORY holds the promise of providing actionable findings that can be translated into clinical practice by informing the development of both early intervention and personalised treatment that is tailored to illness stage and individual circumstances, ultimately disrupting the long-term burden of EDs on individuals and their families.
Similar content being viewed by others
Background
Eating disorders (EDs) are serious mental health conditions characterised by disturbances in eating behaviours, thoughts, and emotions, with significant physical and psychological consequences [1, 2]. Affecting about one in every six young females and one in 20 males, they pose a growing global public health concern, comparable to anxiety and depression [3, 4]. However, EDs have historically received little attention in research, leaving significant gaps in understanding their progression, variations in illness durations, and optimal treatment selection.
The peak onset of EDs occurs during the transitional period from adolescence to young adulthood, impacting socio-emotional, cognitive, and educational development [5]. This vulnerability is compounded by evidence suggesting that EDs are progressive disorders, where longer untreated illness duration is associated with poorer treatment outcomes [6, 7], greater symptom interconnectivity [8, 9], and neurobiological and behavioural changes that drive progression [10], altogether underscoring the critical importance of early intervention in ED management [11, 12].
Clinical staging models, that define the illness phenotypes along developmental lines with escalating symptom severity, offer a promising framework for understanding and intervening in the progressive nature of EDs [13, 14]. This contrasts with traditional approaches that view conditions as static and typically derive diagnostic criteria from advanced presentations, impeding early detection of the conditions in their nascent form. Establishing the underlying biopsychosocial processes at each stage that maintain illness, enhance progression or support recovery may inform stage-specific treatment to prevent further progression. These models, successfully adopted in psychiatry, including psychosis [15], are of current interest in EDs [16]. A proposed 4-stage model for Anorexia Nervosa (AN) ranges from an at-risk phase with attenuated symptoms to a chronic phase with severe, enduring symptoms [17]. However, variability remains in defining ED stages in terms of duration, symptom profiles, and treatment with much research solely focusing on AN.
The implication of staging models for prevention and early intervention proposes the possibility of symptom recovery at each stage, yet EDs exhibit low sustained recovery rates with only half achieving full remission with best available treatments [18]. This complexity is exacerbated by inconsistent conceptualisations of ED recovery that are predominantly biomedical (e.g., weight restoration, absence of ED behaviours), neglecting psychosocial dimensions and ED cognitions (e.g., subjective well-being, freedom from weight concerns) [19, 20]. Relapse risks persist until these underlying factors improve [21, 22]. Patients often describe their recovery as a protracted process with multiple ‘ups and downs’ that may take years to stabilise [23]. This intermediate state of partial improvement without regaining pre-illness health and functioning highlights the need for a more nuanced definition of ED recovery, using physical, behavioural, and psychological indices, and delineating partially and fully recovered groups.
Remote measurement technology (RMT) provides an unobtrusive, cost-efficient means to capture individuals’ daily behaviours and physiology using digital devices, gaining wider application in research across conditions [24, 25]. Active RMT enables delivery of smartphone-based assessments for detecting momentary changes. For instance, speech characteristics (incl. pitch, pauses, speaking rate) collected through smartphone microphones in app-based tasks can serve as scalable digital biomarkers of health outcomes, including depression severity, by providing information on cognitive, neuromuscular, and physiological aspects [26]. Passive RMT continuously gathers background data via smartphone and wearable sensors (e.g., location, heart rate, activity, screentime). The sensor data indicate behavioural markers relevant to clinical states (e.g., circadian rhythm, autonomic arousal, sociability). This range of domains measured by RMT reflects the proposed multidimensional nature of ED recovery, promising to elucidate the recovery process and outcome predictors. Research applying RMT to EDs is significantly lacking [27].
Through combining a traditional prospective cohort design with continuous remote monitoring, the STORY study (Illness Stages, Progression, and Recovery Trajectories of Eating Disorders in Young People) gathers comprehensive data from a large, deeply and dynamically phenotyped cohort of young people with a range of ED presentations. It will inform conceptual models of illness stages, progression, and recovery across illness durations, diagnoses, and age groups. STORY is part of the UKRIFootnote 1-funded ‘EDIFY’ consortium which unites a UK-wide, multi-disciplinary team of investigators with the shared aim of improving prevention and early intervention for young people with EDs [28].
Study objectives
The primary aim of the STORY study is twofold. The first (objectives 1–3) is to identify how biopsychosocial and neurocognitive symptom profiles differ between earlier and more progressed stages of EDs and which variables maintain illness, enhance progression or support recovery. The second (objectives 4–6) is to explore recovery processes and the factors that influence them by obtaining real-world data from participants’ daily lives.
-
Objective 1: To use a multi-modal assessment protocol to cross-sectionally and longitudinally compare young people with earlier and later illness stages in terms of their biopsychosocial profiles and how these change over time within and across ED diagnostic groups.
-
Objective 2: To identify baseline biopsychosocial predictors of outcome at 6 and 12 months within and across ED diagnostic and illness duration groups.
-
Objective 3: To use cognitive tasks with illness-relevant stimuli to compare young people with earlier and later-stage illnesses in terms of their cognitive profiles over time within and across ED diagnostic and illness duration groups.
-
Objective 4: To use biological and psychological RMT measures to compare young people presenting with an earlier-stage ED with healthy young people.
-
Objective 5: To assess differences in recovery trajectories within and across ED groups.
-
Objective 6: To identify early RMT predictors of ED recovery or lack of recovery at 12 months.
Methods
Study design
STORY is a multi-centre prospective cohort study, using ongoing remote monitoring for one year. Data will be collected via self-report online assessments at baseline, 6 and 12 months, via smartphones and wearable devices throughout the study period, and via neurocognitive measures completed in person by a subset of participants at baseline and 12 months. A further follow-up at 24 months is planned, recognising that ED recovery can continue over several years. These assessments are distinct from the main STORY study and not detailed in this protocol.
Study sample
The total sample size target is 840 young people aged 16–25 years, capturing the critical period where EDs commonly manifest and progress while ensuring cognitive maturity to provide consent and complete study measures. Participants are divided into three groups based on symptom profiles and illness duration at baseline:
-
▶ 480 young people with an earlier-stage ED (illness duration ≤ 3 years);
-
▶ 240 young people with a later-stage ED (illness duration > 3 years);
-
▶ 120 healthy controls (HCs).
The 3-year cut-off reflects more responsive treatment patterns in first-episode EDs of fewer than three years [29]. Symptom profiles distinguish between restricting-type presentations that involve severe limitations in food intake (e.g., Anorexia Nervosa [AN], Avoidant restrictive food intake disorder [ARFID]), and bingeing/bulimic-type presentations that involve episodes of binge eating, sometimes followed by compensatory actions, like purging or excessive exercise (e.g., Bulimia Nervosa [BN], Binge Eating Disorder [BED]). Individuals with atypical and subthreshold ED presentations (i.e., those exhibiting clinically significant symptoms without meeting full diagnostic criteria) are included to capture a comprehensive spectrum of ED symptomatology [30]. HCs have no current or past ED or other major mental disorders.
For the earlier-stage ED group, an estimated 100 recoveries within each of the two diagnostic groups are needed to test the predictive validity of RMTs, if 10 variables are to be entered into the predictive model [31]. Assuming a recovery rate of 50% at 12 months [18] and accounting for a 20% dropout rate, 480 participants are required to detect a medium-sized effect with 80% power (f = 0.15, α = 0.05; G*Power 3.1), aiming for an equal distribution of the two diagnostic groups.
The sample size for the later-stage ED group considers the total group for the comparisons between longer and shorter illness durations and varies between outcomes due to selective participation in some measures (e.g., neuroimaging). A sample of 480 provides 90% power to detect a small within-between group interaction effect (f = 0.08, α = 0.05), with two groups assessed twice (baseline, 12-months). Therefore, 240 participants with later-stage EDs will be recruited. A subsample of 100 for the additional in-person assessments, provides 95% power for small-medium interaction effects (f = 0.18, α = 0.05) with the two illness duration groups assessed at two time points.
A sample of 120 HCs represents 25% of the condition group for comparison analyses between control, ED and illness progression subgroups. The eligibility criteria are summarised in Table 1.
Study procedures
Recruitment
ED participants are recruited from an established network of 50 + FREED early intervention servicesFootnote 2 and specialist child, adolescent and adult ED services across the UK. Clinicians conduct a preliminary assessment of the inclusion criteria and provide study materials to potential participants to review. Participants are also identified via primary care services, waiting lists of ED services, third-sector organisations (e.g., ED charities), schools and universities, relevant websites, social media, posters in public places, and existing research cohorts (e.g., ESTRA, GLAD and EDGI cohorts [32,33,34]). This wide recruitment strategy is hoped to allow for greater diversity in our sample than typically found in the research base, to ensure representation of various demographic groups, including those who do not commonly present to ED services (e.g., males, minoritised ethnic groups, those from the LGBTQ + community, those with higher body weight, those from rural locations) [35].
Screening
Interested individuals scan a QR code on recruitment materials linking to the online screening questionnaire to assess eligibility and inform group allocation (incl. sociodemographics, medical and ED history). Symptoms consistent with a current full or subthreshold diagnosis of an ED, as well as lack thereof for HCs, are confirmed via the Eating Disorder Diagnostic Scale (EDDS) [36]. ED illness duration is determined using adapted questions from the comprehensive onset interview used in FREED early intervention services [29]. Eligible participants are contacted by the research team and directed to an electronic consent form, where they can opt into optional study components. Researchers will follow up with participants where necessary, for instance, to confirm diagnoses, comorbidities or willingness to use the study devices.
Study assessments
Following consent, participants self-complete the online baseline assessments via Research Electronic Data Capture software (REDCap), a web application for managing online surveys [37]. These assessments are repeated at 6 and 12 months (see 2.4.1 for measures). REDCap sends automatic survey invitations and reminders to participants for the duration of the study.
At baseline, participants are sent an Android study smartphone (where not already owned) and Ōura smart ring (where consented) and attend an enrolment session online or in-person (subject to preference) with a researcher for assisted setup of the devices. The remote monitoring starts following the setup of the devices and lasts for 12 months (see 2.4.2 for active and passive measures).
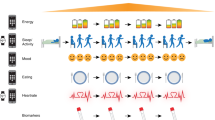
Additional optional in-person cognitive testing and neuroimaging assessments are completed at baseline and 12 months. Optional qualitative interviews are conducted at 6 and 12 months. See Table 2 for the complete schedule of observations and Fig. 1 for participant flow through the study.
Remuneration
Participants receive a total of £50 for completing the online assessments (£20 at baseline, £15 for each follow-up), and £25 for completing the app-based assessments at the end of the data collection period. While the Ōura rings are to be returned by participants after the data collection periods, the study smartphone can be kept. In-person cognitive testing and neuroimaging assessments are reimbursed with £25 per assessment visit (adding up to an additional £100), plus travel costs. All monetary reimbursements are made via bank transfer.
Ethical approval and consent to participate
STORY is conducted according to the Declaration of Helsinki and Good Clinical Practice, adhering to principles outlined in the NHS Research Governance Framework for Health and Social Care. Ethical approval was obtained in October 2023 from the London-Bloomsbury Research Ethics Committee (REC reference: 23/PR/0927). All staff working on the study have received training in study conduct, informed consent and risk assessment. All data is pseudonymised and stored securely in a research database per the General Data Protection Regulation.
Emphasis is placed on informed decision-making regarding participation and signed informed consent is obtained from all participants. Participants’ relationships with care teams are not impacted by participation or withdrawal from the study. If necessary, participants are signposted to third-sector organisations for additional support or encouraged to seek help in the NHS for clinical management.
Outcome measures
Core outcome measures are grouped into online assessments measuring psychological, social, and functional outcomes at baseline, 6 and 12 months (see 2.4.1), and continuous active and passive RMT measures over the study period (see 2.4.2). Additional outcome measures include in-person cognitive and neuroimaging assessments at baseline and 12 months (see 2.4.3), and qualitative interviews at 6 and 12 months (see 2.4.4).
Online questionnaires
The primary outcome is the Eating Disorder Examination Questionnaire (EDE-Q) [38] global score at 12 months which provides data informative to the dual study aims of STORY investigating illness progression (higher scores indicating greater severity) and recovery (global score < 2.8; additional criterion of BMI > 18.5 kg/m2 for AN [39]).
Secondary outcomes are:
-
▶ ED-related attitudes and behaviours (Eating Disorder Scale, ED-15 [40]; six questions from the Avon Longitudinal Study of Parents and Children [41]).
-
▶ Motivation and readiness to change eating difficulties (two visual analogue scales; VAS).
-
▶ Muscularity-related attitudes (muscularity-oriented body image subscale of the Drive for Muscularity Scale, DMS [42]).
-
▶ Mood states and emotions (Profile of Mood States, POMS [43]; Positive and Negative Affect Scale, PANAS [44]).
-
▶ Depression symptoms (Patient Health Questionnaire; PHQ-8 [45]).
-
▶ Anxiety symptoms (Generalized Anxiety Disorder Questionnaire, GAD-7 [46]).
-
▶ Obsessive–compulsive symptoms (Obsessive Compulsive Inventory-Child Version, OCI-CV [47].
-
▶ Autistic traits (Autism Spectrum Quotient, AQ-10 [48]) at baseline only.
-
▶ Symptomatic and functional impairment (Psychological Outcome Profiles, PSYCHLOPS [49]; Work and Social Adjustment Scale–Youth-Version, WSAS-Y [50]).
-
▶ Emotion regulation difficulties (Difficulties in Emotion Regulation Scale, DERS-16 [51]).
-
▶ Loneliness (UCLA Loneliness Scale – Short form, UCLA-4 [52]).
-
▶ Addiction-reinforcing risk personality traits, e.g., impulsivity (Substance Use Risk Profile Scale, SURPS [53]) at baseline only.
-
▶ Mobile phone and social media use (13 questions from the Study of Cognition, Adolescents and Mobile Phones study [54], Motivations for Social Media Use Scale, MSMU [55]).
-
▶ Alcohol use (Alcohol Use Disorders Identification Test; AUDIT [56]) and smoking (two questions from Perman-Howe and colleagues [57]).
Remote data collection
Remote monitoring consists of active and passive components, following procedures established in previous research programmes [58, 59]. The open-source RADAR-base platform used to support the RMT data collection is described elsewhere [60].
Active RMT (aRMT) app
Participants install a purpose-built app that is part of the RADAR-base and was successfully applied in multiple projects. The app notifies participants to complete assessments according to the study schedule:
-
▶ ED symptoms and motivation to change eating difficulties every two weeks (ED-15 [40]; two VAS). Participants are invited to enter their weight monthly.
-
▶ Anxiety and depressive symptoms every two weeks (GAD-7 [46]; PHQ-8 [45]).
-
▶ Short speech tasks once a month, as used in previous studies [26]. A first scripted speech task asks participants to record themselves reading aloud excerpts from Aesop’s fable “The North Wind and The Sun” [61], which is reasonably phonetically balanced while relatively short, taking less than a minute to read aloud [62]. A second, free-response task asks participants to briefly speak about something they have coming up in the next week and how they feel about it (Appendix A). Participants can rerecord their response up to five times, if they are interrupted, or skip the task. The data is recorded, encrypted and uploaded to a secure server, then processed to extract linguistic and paralinguistic features (acoustic, prosodic, e.g., pitch, speaking rate, intensity) for analysis using similar pipelines to Cummins et al. and Zhang et al. [26, 63].
-
▶ Every 12 weeks, participants are prompted to complete brief in-the-moment assessments known as experience sampling method (ESM). ESM assesses mood changes, social interactions, and physical states in daily life. The schedule is initiated at six semi-random times per day within 90-min blocks between 08.30 and 22.00 for six consecutive days. Each ESM assessment consists of approximately 28 items and takes less than two minutes to complete (Appendix B). This intensity of assessment has demonstrated good acceptability in other clinical populations [64].
Passive RMT (pRMT) app
Participants install a second purpose-built pRMT app that is part of the RADAR base. This runs in the background and collects ongoing data via smartphone sensors, to test potential digital markers of change in ED symptoms and impairments. These include relative location data,Footnote 3 ambient light and noise, weather conditions, sociability (e.g., via Bluetooth proximity data, length and duration of calls, keystrokes, number of text messages and emails), app use, and battery life. The pRMT app requires the Android operating system; participants who own non-compatible phones will be provided with Android smartphones.
Wearables sensors
Participants are invited to wear an ‘Ōura’ ring for the duration of the study (12 months), which collects ongoing data on sleep, autonomic arousal and physical activity, including heart rate, heart rate variability, step count, electrodermal activity, sleep efficiency, latency and fragmentation, skin temperature and oxygen saturation (SpO2). To access the Ōura app, participants enter deidentified login credentials generated by the research team. The Ōura app interface will not display any measured health data apart from the ring’s battery life and synch status. The pseudonymised data collected by the ring is synchronised with a smartphone app via Bluetooth, transmitted to Ōura Servers via WiFi, and then pulled to secure sFTP storage located in King’s College London.
The Ōura ring was selected due to the range of measurements available, improved accuracy in sleep tracking, competitive pricing, and ability to be safely implemented in an ED population (see 2.7). The Ōura ring has been shown to provide valid physical measurements comparable to gold-standard methods (e.g., polysomnography) in adult and adolescent populations [65, 66]. The minimal, aesthetically appealing design is aimed to minimise stigma and burden for the user.
Cognitive tasks and neuroimaging
Reward behaviour, inhibitory control and food-related decision-making are assessed via three cognitive tasks completed in person with a researcher present. These are a face-affective go/no-go task [67], a Pavlovian to Instrumental Transfer task [68], and a food choice task, where participants rate 42 food images for perceived healthiness and tastiness compared to a self-chosen ‘neutral’ reference item [69]. Additionally, participants complete the following five tasks utilising eye-tracking technology (Tobii TX300 eye tracker):
-
▶ Visual probe task [70]: Participants view high or low-calorie food items alongside resembling non-food objects, followed by a probe presented randomly over one stimulus which participants must respond to with a keypress. Response latency, time to first fixation and fixation duration are collected to assess attentional biases toward food cues.
-
▶ Two naturalistic scenes: Participants view a 124-s clip from the 1995-film ‘Welcome to the Dollhouse’ depicting a social situation of a young female attempting to find a table in a school cafeteria [71], followed by a 40-s clip of people being interviewed in the street [72]. During both videos, eye-tracking data will be collected to measure social attention and comprehension.
-
▶ Films Expressions Task [73]: Participants match a descriptive emotional verb (e.g., “shocked”) to a corresponding face image out of three, each being displayed for 500ms. Reaction times, accuracy and eye-movement data provide insight into participants’ emotion recognition abilities.
-
▶ Gap-Overlap task [74]: Participants view a centrally presented stimulus and then shift their attention to a peripheral stimulus presented randomly to either side. This task assesses the speed and accuracy of shifts of low-level overt attention. Attentional disengagement is manipulated via the timing and ordering of stimulus presentation, relative to a baseline condition.
Neuroimaging assessments include task-negative functional Magnetic Resonance Imaging (fMRI) and arterial spin labelling (ASL) to measure regional interactions in a resting state. The Amsterdam Resting-State Questionnaire (ARSQ) [75] is administered prior to the scan to measure cognitive state and thought wandering. Resting state scans also provide control images for the following tasks:
-
▶ Monetary Incentive Delay task [76]: Participants respond to visual stimuli to either win or avoid losing money, capturing neural substrates of different processing stages of reward-based learning and motivation control in the context of temporal discounting.
-
▶ Stop signal task [77]: Participants have to respond or withhold their response to a visual stimulus. The task yields an estimate of the participant’s reactive response inhibition serving as a proxy for impulse control.
-
▶ Movie-watching [78]: Participants watch a short clip from the movie ‘Despicable Me’ while in the scanner. This allows to measure natural and real functional brain states in response to continuous and immersive sensory stimulation that may not otherwise be detectable in traditional task-based designs.
Qualitative interviews
Participants are invited to online interviews at 6 and 12 months to investigate personal accounts of ED recovery. This information will complement quantitative data by offering a contextual understanding of individuals’ lived experiences and psychosocial dimensions of recovery (e.g., coping strategies). The interviews additionally serve as a process evaluation, exploring participants’ experiences within the study and RMT specifically. Understanding potential challenges and comfort levels with the study apps and devices will help refine and optimise their integration into future studies.
Adverse events and study withdrawal
Due to STORY’s observational nature, it is not anticipated that participation increases significant risks of harm to participants. There may be several reasons for withdrawal from the study:
-
1.
Participant chooses to no longer participate. Participants are informed of the voluntary nature of participation and their right to withdraw without providing a reason, with no impact on their care.
-
2.
The research team withdraws the participant in the event of inter-current illness, adverse event, protocol violation, administrative or other reasons.
-
3.
Participant loses capacity for continued participation.
Should a participant decide to withdraw from the study, efforts will be made to follow up to establish the reason for withdrawal to gather data on the acceptability of the study. Data from withdrawn participants will be included in the final analysis unless otherwise requested. In case of lack of engagement or missing data for more than three days, follow-up efforts will be made with participants via email and text message (if consented) up to three times before they are withdrawn from the study. Similarly, researchers will conduct random checks on the completion of aRMT measures, prompting participants as needed to ensure continued engagement and data quality.
Statistical and analysis plan
The STORY study is exploratory and not using directional hypotheses. Analyses will be pre-registered (e.g., https://osf.io/) and any reports will clearly distinguish between a-priori and additional post-hoc/exploratory analyses. Datasets will be prepared, stored and shared in line with open science best practices and FAIR principles (www.go-fair.org/fair-principles) to allow replication.
To meet our first aim, various modelling approaches are used to characterise ED symptoms during illness progression and stages and identify outcome predictors. For example, network analysis methods are used that conceptualise factors (e.g., ED symptoms, comorbidities, other traits) as nodes and their associations as edges connecting the nodes to represent the psychopathology of EDs in a network of interconnected symptoms. To gain mechanistic insights and reveal differences that characterise ED subgroups and illness progression, our analyses further include comparisons between (i) controls, anorexic-type and bulimic-type subgroups, (ii) patient groups with different illness duration, and (iii) the initial and follow-up assessments.
To meet our second aim, features obtained from biosensors, questionnaires, tasks, and ESM assessments are used for analyses within and between groups. Initial raw data from smartphones and wearables is aggregated to generate feature sets. Time-independent and dependent probabilistic models are applied to investigate biological and psychological markers of recovery or illness progression, trajectory, and stage classification in EDs and identify predictors of outcome, including Mixture latent Markov (MLM) models. MLM models allow to identify unobserved subgroups (clusters) within the data that share similar symptom trajectories over time. This allows to explore how ED symptom patterns evolve differently across participant groups. Anomaly/novelty detection methods are used to investigate deviations from baseline data and the relationship between these changes and their symptoms.
Qualitative data is analysed using thematic analysis [79]. The thematic framework initially draws upon qualitative patient and public involvement (PPI) work conducted prior to STORY (see 2.7) and remains subject to development throughout analysis, as codes and themes are identified in the data.
The results of the study will be disseminated as widely as possible into the scientific and broader community, including via publications in peer-reviewed journals, scholarly book chapters, presentations at conferences, and publications in proceedings.
Patient and public involvement
The original proposal of EDIFY was co-developed with eight young people with lived ED experience. The EDIFY project has a youth advisory board of 15 young experts-by-experience, six of whom are directly involved with the STORY study, having provided advice on STORY’s design (e.g., feasibility; attractiveness; questionnaire protocol; recovery definitions), and development of the study materials (e.g., designing documents; helping to avoid jargon; developing the recruitment video [https://www.youtube.com/watch?v=gRyVHnKYw4Y]). Youth advisors will continue to provide advice and feedback throughout the study.
Extensive pilot work has informed the acceptability of RMTs within STORY’s target population and its safe integration into the study. The perceived impact of RMT on weight- and food-related behaviours and attitudes was assessed as part of a qualitative interview study with former participants from the RADAR-MDD study who reported an ED diagnosis during their participation [58]. In an iterative process, the youth advisory board of the wider EDIFY consortium provided further in-depth feedback around the choice and integration of the wearable device. Overall, having access to measured health metrics was perceived to increase preoccupation with activity, weight and diet, thereby adversely impact ED symptomatology. In response to the feedback received, a smart ring was chosen as the wearable in STORY in contrast to other fitness-focused activity trackers used in similar studies (e.g., Fitbit, Garmin [31, 59]), and its use has been made optional. Additionally, access to data measured by the ring in the accompanying app can be restricted remotely by the research team allowing complete blinding.
Discussion
While public and scientific awareness of EDs has grown over the past decades, the factors that perpetuate illness or are associated with sustained recovery remain poorly understood. STORY’s multidimensional data, capturing participants’ experiences in naturalistic everyday settings, will explore both neurobiological and psychosocial correlates of illness progression and recovery. This holds the potential for actionable results, paving the way for a more bespoke approach to treatment, aiming for earlier recovery and reduced chronicity. Integrating a qualitative component to complement the comprehensive quantitative assessments will foster a holistic understanding of recovery to shape interventions that resonate with individuals’ diverse needs.
The use of RMT in ED research is nascent and typically only over short periods [80,81,82]; its application to the STORY population and study duration is novel. The continuous monitoring of biopsychosocial factors promises to improve understanding of complex recovery processes and to explore under-researched factors potentially influencing ED progression, such as circadian rhythm and heart-rate variability [83, 84]. In the long run, these technologies could revolutionise clinical care. In contrast to existing ED treatment models, typically based on population effects or clinical expertise, personalised devices can monitor multidimensional outcomes and individual treatment responses in real time to inform clinical decisions (e.g., adjusting treatment type or intensity) [85]. Measurement-based care has proven effective in managing both physical and mental health conditions [86]. However, implementing RMT in an ED population presents unique challenges, most notably the use of wearable devices that are commonly associated with fitness and diet tracking. Such technology has been shown to trigger, maintain and worsen ED symptomatology in clinical and non-clinical populations [87, 88], mirrored in reluctance amongst individuals with an ED history to participate in RMT studies [89]. To understand how RMT can be safely integrated into ED research and clinical practice, we encourage future research to follow processes similar to those in STORY (e.g., PPI; close consultation with experts-by-experience; process evaluations).
The STORY study prioritises diverse representation by using liberal inclusion criteria and including groups commonly underrepresented in research, such as people of the global majority, individuals with under-researched EDs (e.g., ARFID) and those with persistent symptoms [35]. Individuals are eligible if they show significant ED symptoms at screening but have not been formally diagnosed yet which will help capture the full spectrum of ED experiences and severities. Recognising frequent psychological or neurodevelopmental comorbidities of EDs (e.g., mood or anxiety disorders, obsessive–compulsive disorder, autism), participants are not necessarily excluded for these unless significantly impaired or at safety risk. STORY further proactively explores diversity-related aspects (e.g., ethnicity, sexuality, gender, socio-economic background), to identify potential disparities in care and improve support for minority and marginalised groups. Finally, by encompassing an age range that straddles common divisions in research, policy, and service provision (i.e., < 18s vs. ≥ 18s), data from STORY allows for a more integrated and inclusive understanding of EDs in youth.
The STORY study is an ambitious project not without its challenges, primarily in participant recruitment and retention due to its longitudinal design, large number of variables measured, and transient study population. Recruitment challenges are likely to be eased by the wide reach of the study and broad inclusion criteria. To reduce attrition, participants are remunerated for individual assessments and allegiance to the study is fostered using purpose-designed study merchandise (e.g., tote bags, travel mugs), newsletters and events as successfully used in previous studies. Retention will be further aided by contact with dedicated research team members who provide technical support as needed, remind participants of the importance of data collection, and motivate them to contribute study data, as evidenced in previous longitudinal RMT studies [89]. The STORY study prioritises capturing young people’s experiences with EDs, enabling an in-depth exploration of individual factors related to illness progression and recovery. This focus excludes family or caregiver perspectives, known to influence ED development and recovery, and future research including both parties could provide valuable insights. However, focusing on individual experiences allows for a controlled design, avoiding potential biases introduced by family interactions during data collection.
Ultimately, the comprehensive data gathered from the STORY study, together with other initiatives within the EDIFY research programme, aspires to redefine the approach towards understanding and treating EDs. By spreading awareness and learning more about these disorders, we hope to identify them earlier and encourage people to seek help sooner, thereby fostering swifter recovery and diminishing long-term complications. Understanding the data-driven stories of young people with EDs is a crucial first step in rewriting those of young people in the future.
Availability of data and materials
No datasets were generated or analysed during the current study.
Notes
UK Research and Innovation (UKRI) is a national funding agency investing in science and research in the UK.
First Episode Rapid Early Intervention for Eating Disorders (FREED).
GPS location data is obfuscated; that is, providing relative location data, not absolute coordinates, preventing identification of an individual’s home address or precise geographical location.
Abbreviations
- AE:
-
Adverse event
- AN:
-
Anorexia Nervosa
- AQ-10:
-
10-item autism spectrum quotient questionnaire
- ARFID:
-
Avoidant Restrictive Food Intake Disorder
- aRMT:
-
Active remote measurement technology
- ARSQ:
-
Amsterdam Resting State Questionnaire
- ASD:
-
Autism spectrum disorder
- ASL:
-
Arterial spin labelling
- AUDIT:
-
Alcohol use disorders identification test
- BED:
-
Binge Eating Disorder
- BMI:
-
Body Mass Index
- BN:
-
Bulimia Nervosa
- DERS-16:
-
16-item difficulties in emotion regulation scale
- DMS:
-
Drive for muscularity scale
- DSM:
-
Diagnostic and statistical manual
- ED:
-
Eating disorder
- ED-15:
-
Eating disorder scale
- EDDS:
-
Eating disorder diagnostic scale
- EDE-Q:
-
Eating disorder examination questionnaire
- EDGI UK:
-
Eating Disorders Genetics Initiative UK
- EDIFY consortium:
-
Eating Disorders: Delineating Illness and Recovery Trajectories to Inform Personalised Prevention and Early Intervention in Young People
- ESM:
-
Experience sampling method
- ESTRA Study:
-
Earlier detection and stratification of eating disorders and comorbid mental illnesses
- fMRI:
-
Functional magnetic resonance imaging
- FREED:
-
First episode and Rapid Early intervention for Eating Disorders
- GAD-7:
-
7-item generalised anxiety disorder questionnaire
- G/NG-T:
-
Go/No-Go Task
- GLAD Study:
-
Genetic Links to Anxiety and Depression
- GPS:
-
Global positioning system
- MID:
-
Monetary incentive delay task
- MLM:
-
Mixture latent Markov model
- MRI:
-
Magnetic resonance imaging
- MSMU:
-
Motivations for social media use questionnaire
- OCD:
-
Obsessive compulsive disorder
- OCI-CV:
-
Obsessive Compulsive Inventory
- PANAS:
-
Positive and negative affect scale
- PHQ-8:
-
8-item patient health questionnaire
- PIT:
-
Pavlovian to instrumental transfer task
- POMS:
-
Profile of mood states questionnaire
- PPI:
-
Patient and Public Involvement
- pRMT:
-
passive remote measurement technology
- PSYCHLOPS:
-
Psychological outcome profiles questionnaire
- RADAR-CNS:
-
Remote assessment of disease and relapse-Central nervous system
- RADAR-MDD:
-
Remote assessment of disease and relapse-Major depressive disorder
- REC:
-
Research Ethics Committee
- REDCap:
-
Research electronic data capture
- RMT:
-
Remote measurement technology
- sFTP:
-
Secure File Transfer Protocol
- SST:
-
Stop-signal task
- SURPS:
-
Substance use risk profile scale
- UCLA-4:
-
4-item UCLA-Loneliness scale
- VAS:
-
Visual analogue scale
- WSAS-Y:
-
Work and social adjustment scale-Youth version
References
Schmidt U, Adan R, Böhm I, Campbell IC, Dingemans A, Ehrlich S, et al. Eating disorders: The big issue. The Lancet Psychiatry. 2016;3(4):313–5.
Treasure J, Duarte TA, Schmidt U. Eating Disorders. The Lancet. 2020;396(10227):800–911.
Qian J, Wu Y, Liu F, Zhu Y, Jin H, Zhang H, et al. An update on the prevalence of eating disorders in the general population: a systematic review and meta-analysis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2022;27(2):415–28.
van Hoeken D, Hoek HW. Review of the burden of eating disorders: mortality, disability, costs, quality of life, and family burden. Curr Opin Psychiatry. 2020;33(6):521–7.
Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27(1):281–95.
Ambwani S, Cardi V, Albano G, Cao L, Crosby RD, Macdonald P, et al. A multicenter audit of outpatient care for adult anorexia nervosa: Symptom trajectory, service use, and evidence in support of “early stage” versus “severe and enduring” classification. Int J Eat Disord. 2020;53(8):1337–48.
Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr Opin Psychiatry. 2016;29(6):340–5.
Christian C, Williams BM, Hunt RA, Wong VZ, Ernst SE, Spoor SP, et al. A network investigation of core symptoms and pathways across duration of illness using a comprehensive cognitive–behavioral model of eating-disorder symptoms. Psychol Med. 2021;51(5):815–24.
Slof-Op ’t Landt MCT, Dingemans AE, Giltay EJ. Eating disorder psychopathology dimensions based on individual co-occurrence patterns of symptoms over time: a dynamic time warp analysis in a large naturalistic patient cohor. Eat Weight Disord. 2022;27(8):3649–63.
Steinglass JE, Walsh BT. Neurobiological model of the persistence of anorexia nervosa. J Eat Disord. 2016;4(1):19.
National Institute for Health and Care Excellence. Eating disorders: recognition and treatment NICE guideline [NG69]. [Internet]. 2017. [cited 2024 April 19]. Available from: https://www.nice.org.uk/guidance/ng69.
Royal College of Psychiatrists Position statement on early intervention for eating disorders 2019 [Available from: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/position-statements/ps03_19.pdf?sfvrsn=b1283556_2.
McGorry PD, Purcell R, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging: a heuristic model for psychiatry and youth mental health. Med J Aust. 2007;187(S7):S40–2.
Cosci F, Fava GA. Staging of Mental Disorders: Systematic Review. Psychother Psychosom. 2013;82(1):20–34.
Shah JL, Jones N, van Os J, McGorry PD, Gülöksüz S. Early intervention service systems for youth mental health: integrating pluripotentiality, clinical staging, and transdiagnostic lessons from early psychosis. The Lancet Psychiatry. 2022;9(5):413–22.
Hyam LE, Phillips M, Gracie L, Allen K, Schmidt U. Clinical staging across eating disorders: a scoping review protocol. BMJ Open. 2023;13(11):e077377.
Treasure J, Willmott D, Ambwani S, Cardi V, Clark Bryan D, Rowlands K, Schmidt U. Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness. J Clin Med. 2020;9(3):630.
Solmi M, Monaco F, Højlund M, Monteleone AM, Trott M, Firth J, et al. Outcomes in people with eating disorders: a transdiagnostic and disorder-specific systematic review, meta-analysis and multivariable meta-regression analysis. World Psychiatry. 2024;23(1):124–38.
Bardone-Cone AM, Hunt RA, Watson HJ. An Overview of Conceptualizations of Eating Disorder Recovery, Recent Findings, and Future Directions. Curr Psychiatry Rep. 2018;20(9):79.
Hower H, LaMarre A, Bachner-Melman R, Harrop EN, McGilley B, Kenny TE. Conceptualizing eating disorder recovery research: Current perspectives and future research directions. J Eat Disord. 2022;10(1):165.
de Vos JA, LaMarre A, Radstaak M, Bijkerk CA, Bohlmeijer ET, Westerhof GJ. Identifying fundamental criteria for eating disorder recovery: a systematic review and qualitative meta-analysis. J Eat Disord. 2017;5(1):34.
Miles S, Nedeljkovic M, Phillipou A. Investigating differences in cognitive flexibility, clinical perfectionism, and eating disorder-specific rumination across anorexia nervosa illness states. Eat Disord. 2023;31(6):610–31.
Wetzler S, Hackmann C, Peryer G, Clayman K, Friedman D, Saffran K, et al. A framework to conceptualize personal recovery from eating disorders: A systematic review and qualitative meta-synthesis of perspectives from individuals with lived experience. Int J Eat Disord. 2020;53(8):1188–203.
Melbye S, Kessing LV, Bardram JE, Faurholt-Jepsen M. Smartphone-Based Self-Monitoring, Treatment, and Automatically Generated Data in Children, Adolescents, and Young Adults With Psychiatric Disorders: Systematic Review. JMIR Ment Health. 2020;7(10):e17453.
Hickey BA, Chalmers T, Newton P, Lin C-T, Sibbritt D, McLachlan CS, et al. Smart Devices and Wearable Technologies to Detect and Monitor Mental Health Conditions and Stress: A Systematic Review. Sensors. 2021;21(10):3461.
Cummins N, Dineley J, Conde P, Matcham F, Siddi S, Lamers F, et al. Multilingual markers of depression in remotely collected speech samples: A preliminary analysis. J Affect Disord. 2023;341:128–36.
Presseller EK, Patarinski AGG, Fan SC, Lampe EW, Juarascio AS. Sensor technology in eating disorders research: A systematic review. Int J Eat Disord. 2022;55(5):573–624.
Hemmings A, Sharpe H, Allen K, Bartel H, Campbell IC, Desrivières S, et al. EDIFY (Eating Disorders: Delineating Illness and Recovery Trajectories to Inform Personalised Prevention and Early Intervention in Young People): project outline. BJPsych Bulletin. 2023;47(6):328–36. https://doi.org/10.1192/bjb.2022.83.
Brown A, McClelland J, Boysen E, Mountford V, Glennon D, Schmidt U. The FREED Project (first episode and rapid early intervention in eating disorders): service model, feasibility and acceptability. Early Interv Psychiatry. 2018;12(2):250–7.
Crow SJ, Agras WS, Halmi K, Mitchell JE, Kraemer HC. Full syndromal versus subthreshold anorexia nervosa, bulimia nervosa, and binge eating disorder: A multicenter study. Int J Eat Disord. 2002;32(3):309–18.
Matcham F, Barattieri di San Pietro C, Bulgari V, de Girolamo G, Dobson R, Eriksson H, et al. Remote assessment of disease and relapse in major depressive disorder (RADAR-MDD): a multi-centre prospective cohort study protocol. BMC Psychiatry. 2019;19(1):72.
King's College London ESTRA: Earlier detection and stratification of eating disorders and comorbid mental illnesses 2022 [Available from: https://www.kcl.ac.uk/research/estra.
Bulik CM, Thornton LM, Parker R, Kennedy H, Baker JH, MacDermod C, et al. The Eating Disorders Genetics Initiative (EDGI): study protocol. BMC Psychiatry. 2021;21(1):234.
Bright SJ, Hübel C, Young KS, Bristow S, Peel AJ, Rayner C, et al. Sociodemographic, mental health, and physical health factors associated with participation within re-contactable mental health cohorts: an investigation of the GLAD Study. BMC Psychiatry. 2023;23(1):542.
Halbeisen G, Brandt G, Paslakis G. A Plea for Diversity in Eating Disorders Research. Front Psychiatry. 2022;13:820043
Stice E, Telch CF, Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2000;12(2):123–31.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Fairburn CG, Beglin S. Eating Disorder Examination Questionnaire (EDE-Q). In: Fairburn CG, editor. Cognitive Behaviour Therapy and Eating Disorders. New York, NY: Guilford Press; 2008. p. 317–60.
Schmidt U, Ryan EG, Bartholdy S, Renwick B, Keyes A, O’Hara C, et al. Two-year follow-up of the MOSAIC trial: A multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49(8):793–800.
Tatham M, Turner H, Mountford VA, Tritt A, Dyas R, Waller G. Development, psychometric properties and preliminary clinical validation of a brief, session-by-session measure of eating disorder cognitions and behaviors: The ED-15. Int J Eat Disord. 2015;48(7):1005–15.
Chua YW, Lewis G, Easter A, Lewis G, Solmi F. Eighteen-year trajectories of depressive symptoms in mothers with a lifetime eating disorder: findings from the ALSPAC cohort. Br J Psychiatry. 2020;216(2):90–6.
McCreary DR. The Drive for Muscularity Scale: Description, psychometrics, and research findings. In: Thompson JK, Cafri G, editors. The muscular ideal: Psychological, social, and medical perspectives. Washington, DC: American Psychological Association; 2007. p. 87–106.
McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971.
Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063.
Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1):163–73.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Foa EB, Coles M, Huppert JD, Pasupuleti RV, Franklin ME, March J. Development and Validation of a Child Version of the Obsessive Compulsive Inventory. Behav Ther. 2010;41(1):121–32.
Allison C, Auyeung B, Baron-Cohen S. Toward Brief “Red Flags” for Autism Screening: The Short Autism Spectrum Quotient and the Short Quantitative Checklist in 1,000 Cases and 3,000 Controls. J Am Acad Child Adolesc Psychiatry. 2012;51(2):202-12.e7.
Ashworth M, Shepherd M, Christey J, Matthews V, Wright K, Parmentier H, et al. A client-generated psychometric instrument: The development of ‘PSYCHLOPS.’ Couns Psychother Res. 2004;4(2):27–31.
Jassi A, Lenhard F, Krebs G, Gumpert M, Jolstedt M, Andrén P, et al. The Work and Social Adjustment Scale, Youth and Parent Versions: Psychometric Evaluation of a Brief Measure of Functional Impairment in Young People. Child Psychiatry Hum Dev. 2020;51(3):453–60.
Bjureberg J, Ljótsson B, Tull MT, Hedman E, Sahlin H, Lundh L-G, et al. Development and Validation of a Brief Version of the Difficulties in Emotion Regulation Scale: The DERS-16. J Psychopathol Behav Assess. 2016;38(2):284–96.
Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39(3):472–80.
Woicik PA, Stewart SH, Pihl RO, Conrod PJ. The substance use risk profile scale: A scale measuring traits linked to reinforcement-specific substance use profiles. Addict Behav. 2009;34(12):1042–55.
Toledano MB, Mutz J, Röösli M, Thomas MSC, Dumontheil I, Elliott P. Cohort Profile: The Study of Cognition, Adolescents and Mobile Phones (SCAMP). Int J Epidemiol. 2019;48(1):25–6.
Rodgers RF, McLean SA, Gordon CS, Slater A, Marques MD, Jarman HK, Paxton SJ. Development and Validation of the Motivations for Social Media Use Scale (MSMU) Among Adolescents. Adolescent Res Rev. 2021;6(4):425–35.
Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791–804.
Perman-Howe PR, Horton M, Robson D, McDermott MS, McNeill A, Brose LS. Harm perceptions of nicotine-containing products and associated sources of information in UK adults with and without mental ill health: A cross-sectional survey. Addiction. 2022;117(3):715–29.
Matcham F, Leightley D, Siddi S, Lamers F, White KM, Annas P, et al. Remote Assessment of Disease and Relapse in Major Depressive Disorder (RADAR-MDD): recruitment, retention, and data availability in a longitudinal remote measurement study. BMC Psychiatry. 2022;22(1):136.
Bruno E, Biondi A, Böttcher S, Vértes G, Dobson R, Folarin A, et al. Remote Assessment of Disease and Relapse in Epilepsy: Protocol for a Multicenter Prospective Cohort Study. JMIR Res Protoc. 2020;9(12):e21840.
Ranjan Y, Rashid Z, Stewart C, Conde P, Begale M, Verbeeck D, et al. RADAR-Base: Open Source Mobile Health Platform for Collecting, Monitoring, and Analyzing Data Using Sensors, Wearables, and Mobile Devices. JMIR Mhealth Uhealth. 2019;7(8):e11734.
International Phonetic Association. Handbook of the International Phonetic Association: A guide to the use of the International Phonetic Alphabet. Cambridge: Cambridge University Press; 1999.
Lammert Adam C, Melot J, Sturim Douglas E, Hannon Daniel J, DeLaura R, Williamson James R, et al. Analysis of Phonetic Balance in Standard English Passages. J Speech Lang Hear Res. 2020;63(4):917–30.
Zhang Y, Folarin AA, Dineley J, Conde P, de Angel V, Sun S, et al. Identifying depression-related topics in smartphone-collected free-response speech recordings using an automatic speech recognition system and a deep learning topic model. J Affect Disord. 2024;355:40–9.
Vachon H, Viechtbauer W, Rintala A, Myin-Germeys I. Compliance and Retention With the Experience Sampling Method Over the Continuum of Severe Mental Disorders: Meta-Analysis and Recommendations. J Med Internet Res. 2019;21(12): e14475.
Kristiansson E, Fridolfsson J, Arvidsson D, Holmäng A, Börjesson M, Andersson-Hall U. Validation of Oura ring energy expenditure and steps in laboratory and free-living. BMC Med Res Methodol. 2023;23(1):50.
Miller DJ, Sargent C, Roach GD. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors. 2022;22(16):6317.
Chami R, Cardi V, Lawrence N, MacDonald P, Rowlands K, Hodsoll J, Treasure J. Targeting binge eating in bulimia nervosa and binge eating disorder using inhibitory control training and implementation intentions: a feasibility trial. Psychol Med. 2022;52(5):874–83.
Vogel V, Dittrich M, Horndasch S, Kratz O, Moll GH, Erim Y, et al. Pavlovian-to-instrumental transfer in Anorexia Nervosa: A pilot study on conditioned learning and instrumental responding to low- and high-calorie food stimuli. Eur J Neurosci. 2020;51(8):1794–805.
Steinglass J, Foerde K, Kostro K, Shohamy D, Walsh BT. Restrictive food intake as a choice—A paradigm for study. Int J Eat Disord. 2015;48(1):59–66.
Werthmann J, Simic M, Konstantellou A, Mansfield P, Mercado D, van Ens W, Schmidt U. Same, same but different: Attention bias for food cues in adults and adolescents with anorexia nervosa. Int J Eat Disord. 2019;52(6):681–90.
Kerr-Gaffney J, Jones E, Mason L, Hayward H, Murphy D, Loth E, Tchanturia K. Social attention in anorexia nervosa and autism spectrum disorder: Role of social motivation. Autism. 2022;26(7):1641–55.
Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, et al. Visual orienting in the early broader autism phenotype: disengagement and facilitation. J Child Psychol Psychiatry. 2009;50(5):637–42.
Garrido L, Furl N, Draganski B, Weiskopf N, Stevens J, Tan GC-Y, et al. Voxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosics. Brain. 2009;132(12):3443–55.
Glennon JM, D’Souza H, Mason L, Karmiloff-Smith A, Thomas MSC. Visuo-attentional correlates of Autism Spectrum Disorder (ASD) in children with Down syndrome: A comparative study with children with idiopathic ASD. Res Dev Disabil. 2020;104:103678.
Diaz BA, Van Der Sluis S, Moens S, Benjamins J, Migliorati F, Stoffers D, et al. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition. Front Human Neuroscience. 2013;7:446.
Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. Neuroimage. 2000;12(1):20–7.
Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev. 2016;64:35–62.
Eickhoff SB, Milham M, Vanderwal T. Towards clinical applications of movie fMRI. Neuroimage. 2020;217: 116860.
Braun V, Clarke V. Conceptual and design thinking for thematic analysis. Qualitative Psychology. 2022;9(1):3–26.
Flatt RE, Thornton LM, Smith T, Mitchell H, Argue S, Baucom BRW, et al. Retention, engagement, and binge-eating outcomes: Evaluating feasibility of the Binge-Eating Genetics Initiative study. Int J Eat Disord. 2022;55(8):1031–41.
Bulik CM, Butner JE, Tregarthen J, Thornton LM, Flatt RE, Smith T, et al. The Binge Eating Genetics Initiative (BEGIN): study protocol. BMC Psychiatry. 2020;20(1):307.
Presseller EK, Lampe EW, Zhang F, Gable PA, Guetterman TC, Forman EM, Juarascio AS. Using Wearable Passive Sensing to Predict Binge Eating in Response to Negative Affect Among Individuals With Transdiagnostic Binge Eating: Protocol for an Observational Study. JMIR Res Protoc. 2023;12: e47098.
Cooper AR, Loeb KL, McGlinchey EL. Sleep and eating disorders: current research and future directions. Curr Opin Psychol. 2020;34:89–94.
Peyser D, Scolnick B, Hildebrandt T, Taylor JA. Heart rate variability as a biomarker for anorexia nervosa: A review. Eur Eat Disord Rev. 2021;29(1):20–31.
Tam HE, Ronan K. The application of a feedback-informed approach in psychological service with youth: Systematic review and meta-analysis. Clin Psychol Rev. 2017;55:41–55.
Parikh A, Fristad MA, Axelson D, Krishna R. Evidence Base for Measurement-Based Care in Child and Adolescent Psychiatry. Child Adolesc Psychiatr Clin N Am. 2020;29(4):587–99.
Simpson CC, Mazzeo SE. Calorie counting and fitness tracking technology: Associations with eating disorder symptomatology. Eat Behav. 2017;26:89–92.
Boldi A, Silacci A, Boldi M-O, Cherubini M, Caon M, Zufferey N, et al. Exploring the impact of commercial wearable activity trackers on body awareness and body representations: A mixed-methods study on self-tracking. Comput Hum Behav. 2024;151:108036.
Oetzmann C, White KM, Ivan A, Julie J, Leightley D, Lavelle G, et al. Lessons learned from recruiting into a longitudinal remote measurement study in major depressive disorder. npj Digital Med. 2022;5(1):133.
Acknowledgements
We would like to thank our colleagues within the EDIFY consortium across all involved institutions for their contribution to the development of this protocol. We thank all the members of the EDIFY youth advisory board for their contribution to the device selection procedures, and their invaluable advice throughout the study protocol design. On behalf of the EDIFY consortium: Heike Bartel, Tara French, Jonathan Kelly, Nadia Micali, Sneha Raman, Janet Treasure, Umairah Malik, Diego Rabelo-da-Ponte, Fiona Stephens, Tine Opitz, Nora Trompeter, Jessica Wilkins, Tamsin Parnell, Ruby Abbas, Alice Bromell, Grace Davis, Cameron Eadie, Lara Gracie, Beck Heslop, Katie McKenzie, Eniola Odubanjo, Chris Sims, Tallulah Street, Andreia Tavares-Semedo, Eleanor Wilkinson, Lucy Zocek.
Funding
This work is supported by the Medical Research Council/Arts and Humanities Research Council/Economic and Social Research Council Adolescence, Mental Health and the Developing Mind initiative as part of the EDIFY programme (grant number MR/W002418/1). This paper also represents independent research funded by the National Institute of Health Research (NIHR) Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust (SlaM) and King’s College London (KCL). US receives salary support from the NIHR BRC at SLaM and KCL. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. CK and AH are supported by NIHR Maudsley BRC PhD scholarships. KA is supported by the Medical Research Council as part of grant MR/X030539/1 (Eating Disorders Clinical Research Network).
Author information
Authors and Affiliations
Consortia
Contributions
U.S. and H.S. developed the main conceptual ideas for the EDIFY programme. U.S., H.S., C.K., M.P., S.M., B.İ., A.H., L.G., and I.C. were responsible for the conception and design of the STORY study. U.S., H.S., C.K., M.P., and S.M. developed and wrote the protocol for ethical approval. C.K. and M.P. were responsible for the initial drafting of this manuscript. C.B., I.C., D.D., A.H., B.İ., S.M., H.S., and U.S. have contributed to reviewing and revising the manuscript critically for important intellectual content. U.S., H.S., B.İ., C.K., S.M., and C.B. are responsible for the coordination of the study across sites. All local research site PIs are responsible for the coordination of the study in their respective Trust area. P.C., R.D., A.F., and Z.R. developed the RADAR-base system and the apps used for data collection and management, and data protection, security, and storage systems. P.C., R.D., A.F., and Z.R. also developed an analytic method for handling data collected via the RADAR-base system. N.C. and J.D. have contributed to the implementation of the speech tasks. L.M. has contributed to the implementation of the eye-tracking tasks. All authors have been involved in reviewing the manuscript and given approval for it to be published. All authors have agreed to be accountable for all aspects of the work, ensuring that questions relating to the accuracy or the integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kuehne, C., Phillips, M.D., Moody, S. et al. Characterising illness stages and recovery trajectories of eating disorders in young people via remote measurement technology (STORY): a multi-centre prospective cohort study protocol. BMC Psychiatry 24, 409 (2024). https://doi.org/10.1186/s12888-024-05841-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05841-w