Abstract
Background
Previous studies have reported associations between obstructive sleep apnea (OSA) and several mental disorders. However, further research is required to determine whether these associations are causal. Therefore, we evaluated the bidirectional causality between the genetic liability for OSA and nine mental disorders by using Mendelian randomization (MR).
Method
We performed two-sample bidirectional MR of genetic variants for OSA and nine mental disorders. Summary statistics on OSA and the nine mental disorders were extracted from the FinnGen study and the Psychiatric Genomics Consortium. The primary analytical approach for estimating causal effects was the inverse-variance weighted (IVW), with the weighted median and MR Egger as complementary methods. The MR Egger intercept test, Cochran’s Q test, Rucker’s Q test, and the MR pleiotropy residual sum and outlier (MR-PRESSO) test were used for sensitivity analyses.
Result
MR analyses showed that genetic liability for major depressive disorder (MDD) was associated with an increased risk of OSA (odds ratio [OR] per unit increase in the risk of MDD, 1.29; 95% CI, 1.11–1.49; P < 0.001). In addition, genetic liability for OSA may be associated with an increased risk of attention-deficit/hyperactivity disorder (ADHD) (OR = 1.26; 95% CI, 1.02–1.56; p = 0.032). There was no evidence that OSA is associated with other mental disorders.
Conclusion
Our study indicated that genetic liability for MDD is associated with an increased risk of OSA without a bidirectional relationship. Additionally, there was suggestive evidence that genetic liability for OSA may have a causal effect on ADHD. These findings have implications for prevention and intervention strategies targeting OSA and ADHD. Further research is needed to investigate the biological mechanisms underlying our findings and the relationship between OSA and other mental disorders.
Similar content being viewed by others
Background
Mental disorders are a significant global health concern and rank among the top 10 causes of burden worldwide. According to estimates, the number of mental disorder cases increased by 48.1% in 2019 compared to 1990, and the proportion of global disability-adjusted life years (DALYs) attributable to mental disorders increased from 2.3–9.4% [1]. However, the etiology of mental disorders remains unclear, and there is evidence to suggest that Obstructive sleep apnea (OSA) may be associated with a variety of mental disorders [2]. OSA is a condition caused by repeated episodes of upper airway collapse and obstruction during sleep associated with arousal from sleep with or without oxygen desaturation [3]. In the general population, the prevalence of OSA is approximately 3–7% in males and 2–5% in females [4]. Both mental disorders and OSA can significantly impact patients’ quality of life. It is crucial to establish a clear causal relationship between these conditions to inform effective prevention and treatment strategies.
In recent years, an increasing number of researches have been devoted to exploring the relationship between OSA and various mental disorders. Two cohort studies suggested that bipolar disorder (BD) and schizophrenia (SCZ) were associated with increased risks of OSA [5, 6]. A Japanese study has also found that preschoolers with autism spectrum disorder (ASD) are more likely to have obstructive sleep apnea than the general population [7]. Moreover, there is evidence of a potential bidirectional relationship between OSA and attention deficit hyperactivity disorder (ADHD), anxiety disorder (ANX), major depressive disorder (MDD), and Post-Traumatic Stress Disorder (PTSD) [8,9,10,11]. However, the ability of these studies to establish causality is insufficient, and even prospective observational studies may be subject to inherent confounding or selection bias. Currently, there are limited studies on this topic, and it remains unclear whether OSA is the cause or a downstream effect of mental disorders. Therefore, any potential causal relationship still requires further research.
In this case, the genetic epidemiological method of Mendelian randomization (MR) is a powerful tool to evaluate the causal relationship between OSA and mental disorders. MR uses single nucleotide polymorphisms (SNPs) as instrumental variables to estimate their effect on the outcome of interest, minimizing bias affecting observational epidemiological studies [12,13,14] and thus enhancing causal inference of exposure and outcome. Due to the random allocation of genetic variation during meiosis and the natural causal effect of genetic variation on phenotype, SNPs are independent of potential confounders, and therefore confounders and reverse causality bias can be minimized [15]. Two-sample MR refers to MR analysis using two independent samples from different studies or databases, which are typically collected from publicly available large-scale genome-wide association studies (GWAS), and has the advantage of increased statistical power [16]. Therefore, we conducted a two-sample bidirectional MR analysis using the latest GWAS to investigate the potential association between OSA and mental disorders.
Method
Study design
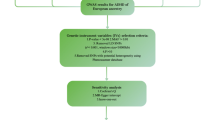
A two-sample bidirectional MR was used to evaluate the potential causal association between obstructive sleep apnoea and nine mental disorders (Fig. 1). The MR design is based on three fundamental assumptions three basic assumptions: (1) genetic variants must be highly correlated with exposure; (2) genetic variants cannot be associated with any potential confounders; (3) genetic variants influence outcome solely through the exposure [15]. This study used summary-level data from publicly available GWAS. Ethical approval was obtained for all original studies.
Data source
Obstructive sleep apnea
Summary statistics for OSA were obtained from the FinnGen database with 375,657 individuals of European ancestry. FinnGen is a large public-private partnership aimed at collecting and analyzing genomic and health data from 500,000 Finnish biobank participants [17]. The GWAS included 38,998 OSA cases and 336,659 controls. Diagnostic criteria for OSA cases were based on ICD codes (ICD-10: G47.3; ICD-9: 3472), derived from the Finnish National Hospital Discharge Registry and the Causes of Death Registry. More details on the OSA GWAS can be found at https://r9.risteys.finngen.fi/endpoints/G6_SLEEPAPNO/ and https://finngen.gitbook.io/documentation/methods/phewas/.
Mental disorders
We used summary statistics for 9 mental disorders from different studies [18,19,20,21,22,23,24,25,26], which were obtained from the Psychiatric Genomics Consortium (PGC). The mental disorders included in this study are ADHD, AN, ANX, autism spectrum disorder (ASD), BD, MDD, obsessive-compulsive disorder (OCD), PTSD, and SCZ. For consistency with the OSA data, genetic data of European ancestry were used for all nine mental disorders to avoid heterogeneity. Sample sizes for each GWAS study are listed below: ADHD(38,691 cases and 186,843 controls), AN(16,992 cases and 55,525 controls), ANX(7,016 cases and 14,745 controls), ASD(18,381 cases and 27,969 controls), BD(41,917 cases and 371,549 controls), MDD(246,363 cases and 561,190 controls), OCD(2,688 cases and 7,037 controls), PTSD(23,212 cases and 151,447 controls), SCZ(53,386 cases and 77,258 controls). A detailed description of the data sources can be found in Additional file 1.
Selection of instrument variables
The number of instrumental variables (IVs) determines statistical power and the presence of confounding factors, so the appropriate number of IVs is needed. We used four different p-value thresholds, p < 5 × 10− 6 (ANX, ASD, OCD, PTSD), p < 5 × 10− 7 (AN), p < 5 × 10− 8 (ADHD, BD, MDD, OSA), and p < 5 × 10− 12 (SCZ), and without linkage disequilibrium (10,000 kilobase pairs apart and r2 < 0.001) to select SNPs as instrumental variables. The linkage disequilibrium (LD) reference was obtained from http://fileserve.mrcieu.ac.uk/ld/1kg.v3.tgz. After clumped SNPs for independence, PhenoScanner [27, 28] was used to assess previous associations with potential confounding traits. PhenoScanner is a curated database holding publicly available results from large-scale genome-wide association studies. To meet the assumptions of the MR design, we excluded SNPs that were strongly associated with other traits or diseases (p < 5 × 10− 8) to rule out possible pleiotropic effects. To evaluate the weak instrument bias, we calculated the instrument strength (F-statistic) for each IV according to the following formula: \( F= \frac{{beta}^{2}}{{se}^{2}}\) (beta: the effect size of SNP on exposure; se: its corresponding standard error) [29]. We removed SNPs associated with exposure if they could not be matched to SNPs in the outcome. Details of the SNPs that were selected as IVs are shown in Additional files 2 and 3.
Statistical analysis
In MR analysis, the inverse-variance weighted (IVW) model is the main method for assessing the bidirectional relationship between exposure and outcome. However, if there is horizontal pleiotropy and invalid instrument bias, IVW cannot provide unbiased estimates of causal effects [30, 31]. Therefore, we use two different MR methods that are relatively robust to horizontal pleiotropy, although at the cost of reduced statistical power [32]. First, causal effects are estimated using the weighted median method, in which only 50% of the SNPs need to be valid instruments [33]. Second, MR Egger estimates causal effects by setting an intercept that allows horizontal pleiotropy to be unbalanced or directed [31, 34]. In addition, we used several sensitivity analyses including the MR Egger intercept test, Cochran’s Q test, Rucker’s Q test and the MR pleiotropy residual sum and outlier (MR-PRESSO) test to determine the validity and robustness of the results. The MR Egger regression provides a test for directional pleiotropy through its intercept [35]. The Cochran’s Q test and Rucker’s Q test were performed to assess the heterogeneity, and if heterogeneity was present, outlier SNPs were excluded by observing the funnel plot or sorting RSSobs in MR-PRESSO and the MR analysis was repeated [36]. MR-PRESSO was used to detect pleiotropic bias, identify outliers, and obtain the corrected results by removing outliers [37].
Associations between genetic liability for OSA and risk of mental disorders were expressed as odds ratios (ORs) and their 95% confidence intervals (CIs). We indexed the strength of evidence against the no association by the exact p-value. A p-value less than 0.0056 (0.05/9) was considered to be statistically significant evidence for a causal association. A p-value below 0.05, but above the Bonferroni-corrected threshold, was considered suggestive evidence for a potential causal association. We used an online tool called mRnd (https://shiny.cnsgenomics.com/mRnd/) to calculate the statistical power of the MR analysis [38]. All statistical analyses were performed using R (version 4.3.1) with the packages “TwoSampleMr”, “MR-PRESSO” and “ieugwasr”.
Results
Genetically predicted obstructive sleep apnea on mental disorders
The associations of OSA with 9 mental disorders are demonstrated in Fig. 2. We observed only a weak association between genetic liability for OSA and increased risk of ADHD (IVW, OR = 1.26; 95% CI, 1.02–1.56; p = 0.032), and there was no evidence that other mental disorders were associated with OSA. The results of the estimation using the weighted median and MR Egger methods, as well as information on statistical power, Q P-value and Pintercept-value, are presented in Additional file 4. The F-statistic of each IV we selected for OSA was greater than 10, indicating a low probability of weak instrument bias [39]. The MR-Egger intercept analysis did not indicate horizontal pleiotropy. The Rucker’s Q test revealed possible heterogeneity of individual SNPs between the effect estimates of OSA and SCZ (Rucker’s Q = 13.964, Q p-value = 0.030). Therefore, we excluded one outlier SNP (rs59333125) and performed a second MR analysis, which showed no heterogeneity (Rucker’s Q = 6.670, Q p-value = 0.246). For the SNPs we used, MR-PRESSO did not detect any potential outliers.
Genetically predicted mental disorders on obstructive sleep apnea
The associations of 9 mental disorders with OSA are demonstrated in Fig. 3. The genetic liability for MDD had an effect estimate consistent with an increased risk of OSA. However, Cochran’s Q test indicated the presence of heterogeneity (Cochran’s Q = 43.195, Q p-value = 0.009), we excluded two outlier SNPs (rs4141983, rs9529218) and performed a second MR analysis (IVW, OR = 1.37; 95%CI, 1.20–1.57; p < 0.001), which did not show heterogeneity (Cochran’s Q = 29.474, Q p-value = 0.132). The results of the estimation using the weighted median and MR Egger methods, as well as information on statistical power, Q P-value and Pintercept-value, are presented in Additional file 5. The scatterplot, leave-one-out-sensitivity forest plot, and funnel plot of MR estimation results for MDD associated with OSA are provided in Additional file 6. F-statistic greater than 10 for each IV for the 9 mental disorders, indicating a small magnitude of weak instrument bias. MR-Egger intercept analysis did not identify any pleiotropic SNPs. And Cochran’s Q test suggested potential heterogeneity in ADHD (Cochran’s Q = 28.816, Q p-value = 0.001, BD (Cochran’s Q = 38.784, Q p-value = 0.039), PTSD (Cochran’s Q = 22.605, Q p-value = 0.047) and SCZ (Cochran’s Q = 38.169, Q p-value = 0.012), so we excluded outlier SNPs for ADHD (rs7844069, rs2025286), BD (rs10994415), PTSD (rs1268149), and SCZ (rs145071536, rs16851048) and performed a second MR analysis, which showed no heterogeneity (Additional file 5). One outlier SNP in ASD (rs28729902) was excluded using the MR-PRESSO test. After correction for possible outliers, the causal effect estimates for ADHD, ASD, BD, PTSD and SCZ were still not statistically significant.
Associations between genetic liability for mental disorders and risk of obstructive sleep apnea. For exposure phenotype ASD, the result is shown after removing outliers with the MR-PRESSO test. For exposure phenotype ADHD, BD, MDD, PTSD and SCZ, the figure shows the results of the second MR analysis with outlier SNPs removed due to heterogeneity.
Discussion
In our study, we performed a bidirectional MR analysis based on several large genetic populations to assess the association between genetic liability for OSA and the risk of mental disorders. Our results found possible genetic evidence that OSA was associated with an increased risk of ADHD. In the opposite direction, genetic liability for MDD was associated with an increased risk of OSA.
Our study provides suggestive evidence of a possible association between OSA and ADHD based on larger populations. Although few observational studies have conclusively confirmed the potential causal relationship between OSA and ADHD, there are still evidences that support our findings. A meta-analysis showed that children with Sleep-disordered breathing (SDB) are at increased risk of presenting with ADHD symptoms such as inattention and hyperactivity [40]. OSA is usually accompanied by decreased oxygen saturation and sleep disruption. These symptoms may affect brain development, which in turn affects cognitive function and leads to poor concentration [41, 42]. From a pathophysiological perspective, inflammatory cytokines (C-reactive protein and interleukin-6) are elevated in children with SDB, which may lead to cognitive dysfunction [43,44,45]. In addition, several studies have shown that adenotonsillectomy (a treatment for OSA) can improve ADHD symptoms and cognitive problems [8, 40, 46,47,48]. However, the relationship between OSA and ADHD may be reciprocal rather than in the traditional one-way relationship, although a reverse relationship was not observed in our study. Symptoms of ADHD overlap with a diagnosis of OSA, and attentional deficits have been reported in up to 95% of OSA patients [49]. A case-control study found that 28 out of 30 ADHD patients had comorbid sleep disorders, 15 of whom had OSA [50]. In summary, the relationship between OSA and ADHD is complex and needs to be further explored in future studies.
Our study found that MDD was associated with an increased risk of OSA incidence, which is consistent with a recent MR study [51]. However, observational studies similar to ours are limited. Only one population-based longitudinal study suggested that a bidirectional link between MDD and OSA exists. This study, which included 27,073 depressed patients and 135,365 controls, demonstrated that having depression was associated with an increased risk of future OSA (HR = 2.30; 95%CI, 2.11–2.50) [10]. More observational studies consider OSA as a risk factor for MDD [52,53,54,55], so the relationship between MDD and OSA seems to be reciprocal as well. Two systematic reviews showed that both the prevalence of MDD among patients with OSA and the prevalence of OSA among patients with MDD were higher than in the general population [56, 57]. This may be due to a partial overlap of symptoms and diagnostic criteria or common underlying mechanisms between MDD and OSA [58,59,60]. However, the following pathophysiological mechanisms support our derived unidirectional association between MDD and OSA. First, increased inflammatory cytokines in depressed patients may lead to neurological damage and altered circadian rhythms, which may increase the risk of OSA and exacerbate the symptoms of OSA [61, 62]. Second, patients with depressive disorders are usually associated with central nervous system 5-HT dysfunction, with decreased plasma tryptophan (a 5-HT precursor), decreased 5-HT metabolites in cerebrospinal fluid, and decreased 5-HT1 receptor binding [63]. The 5-HT reduction affects dilator muscles of the upper airway, narrowing the size of the upper airway, which may contribute to the incidence of OSA [64]. Third, some sleep medications and benzodiazepines are used to treat depression, and their tranquillizing effects may decrease the muscle tone of upper airway dilator muscles, thereby increasing the risk of OSA [65,66,67]. In conclusion, the relationship between MDD and OSA has not been clarified, and further research into pathogenesis is needed to provide effective and feasible treatments.
The main strength of this study is the use of two-sample bidirectional MR to assess the relationship between OSA and mental disorders, minimizing confounders and reverse causality present in observational studies. Moreover, we limited the population of the GWAS study to Europeans to minimize heterogeneity arising from ethnic differences. In addition, the IVs we used were extracted from the most recent GWAS with a large sample size, and the likelihood of weak instrument bias was minimal and only related to the exposures we were focused on.
However, there are several limitations to this study. First, both OSA and the nine mental disorders are binary exposures, and we could not know whether there would be selective bias due to underdiagnosis [29], as well as exclusive restriction bias due to the possibility that genetic variants would influence outcomes via continuous risk factors [68]. In addition, due to the lack of individual-level data from the GWAS study, we were unable to know whether the severity of the disease and medication would cause potential bias. Second, we used different thresholds for screening IVs, but this may have led to inconsistencies in the reliability of the results. Fewer IVs, while greatly reducing the potential for pleiotropy, reduced statistical power. Third, the biological roles and mechanisms of SNPs are not currently fully understood [69], and the presence of horizontal pleiotropy cannot be completely ruled out, although we performed sensitivity analyses using various methods to rule out horizontal pleiotropy. Fourth, MR estimates reflect the cumulative effects of exposure over individuals’ lifetimes, which are likely to be stronger than in observational studies and clinical trials. However, it is difficult to determine the relationship between OSA and mental disorders using randomized clinical trials due to the limitations of ethical rules and the uncertainty of etiology, and more observational studies are needed in the future to validate our findings. Finally, limiting the ancestry of the population to Europeans reduced the bias due to population stratification, but also limited the extrapolation of the results to other races.
Conclusion
Our study provided genetic evidence that MDD is associated with an increased risk of OSA without a bidirectional relationship. In addition, we also found genetic evidence that OSA is a potential causal risk factor for ADHD. We did not find a causal relationship between OSA and AN, ANX, ASD, BD, OCD, PTSD, and SCZ. Clinically, these findings contribute to the identification, treatment, and prevention of OSA and ADHD in patients. Further investigation is required to better understand the biological mechanisms underlying the relationship between OSA and other mental disorders. This will aid clinicians in providing more effective treatment for these conditions.
Data availability
All data used in this study are obtained from open access databases or published manuscripts. FinnGen: https://r9.risteys.finngen.fi/endpoints/G6_SLEEPAPNO. https://storage.googleapis.com/finngen-public-data-r9/summary_stats/finngen_R9_G6_SLEEPAPNO.gz. PGC: https://pgc.unc.edu/for-researchers/download-results.
Abbreviations
- ADHD:
-
Attention-deficit/hyperactivity disorder
- AN:
-
Anorexia nervosa
- ANX:
-
Anxiety disorder
- ASD:
-
Autism spectrum disorder
- BD:
-
Bipolar disorder
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association studies
- IVs:
-
Instrumental variables
- IVW:
-
Inverse variance weighted
- LD:
-
Linkage disequilibrium
- MDD:
-
Major depressive disorder
- MR:
-
Mendelian randomization
- MR-PRESSO:
-
Mendelian randomization pleiotropy residual sum and outlier test
- OCD:
-
Obsessive-compulsive disorder
- OR:
-
Odds ratio
- OSA:
-
Obstructive sleep apnea
- PGC:
-
Psychiatric Genomics Consortium
- PTSD:
-
Post-traumatic stress disorder
- SCZ:
-
Schizophrenia
- SDB:
-
Sleep-disordered breathing
- SNP:
-
Single nucleotide polymorphism
References
Ferrari AJ, Santomauro DF, Herrera AMM, Shadid J, Ashbaugh C, Erskine HE, et al. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50. https://doi.org/10.1016/S2215-0366(21)00395-3.
Lin W-C, Winkelman JW. Obstructive sleep apnea and severe mental illness: evolution and consequences. Curr Psychiat Rep. 2012;14:503–10. https://doi.org/10.1007/s11920-012-0307-6.
Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med. 2019;86:2–9. https://doi.org/10.3949/ccjm.86.s1.02.
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. https://doi.org/10.1513/pats.200709-155MG.
Chang E-T, Chen S-F, Chiang J-H, Wang L-Y, Hsu C-Y, Shen Y-C. Risk of obstructive sleep apnea in patients with bipolar disorder: a nationwide population-based cohort study in Taiwan. Psychiatry Clin Neurosci. 2019;73:163–8. https://doi.org/10.1111/pcn.12802.
Wu Y-Y, Chang E-T, Yang Y-C, Chen S-F, Hsu C-Y, Shen Y-C. Risk of obstructive sleep apnea in patients with schizophrenia: a nationwide population-based cohort study. Soc Psych Psych Epid. 2020;55:1671–7. https://doi.org/10.1007/s00127-020-01870-4.
Hirata I, Mohri I, Kato-Nishimura K, Tachibana M, Kuwada A, Kagitani-Shimono K, et al. Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Res Dev Disabil. 2016;49–50:86–99. https://doi.org/10.1016/j.ridd.2015.11.002.
Luis Urbano G, Janine Tablizo B, Moufarrej Y, Tablizo MA, Chen ML, Witmans M. The link between Pediatric Obstructive Sleep Apnea (OSA) and attention deficit hyperactivity disorder (ADHD). Children-Basel. 2021;8:824. https://doi.org/10.3390/children8090824.
Diaz SV, Brown LK. Relationships between obstructive sleep apnea and anxiety. Curr Opin Pulm Med. 2016;22:563–9. https://doi.org/10.1097/MCP.0000000000000326.
Pan M-L, Tsao H-M, Hsu C-C, Wu K-M, Hsu T-S, Wu Y-T, et al. Bidirectional association between obstructive sleep apnea and depression: a population-based longitudinal study. Med (Baltim). 2016;95:e4833. https://doi.org/10.1097/MD.0000000000004833.
McCall CA, Watson NF. A narrative review of the association between post-traumatic stress disorder and obstructive sleep apnea. J Clin Med. 2022;11:415. https://doi.org/10.3390/jcm11020415.
Smith GD, Ebrahim S. Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. https://doi.org/10.1093/ije/dyg070.
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. https://doi.org/10.1002/sim.3034.
Davey Smith G, Holmes MV, Davies NM, Ebrahim S. Mendel’s laws, mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur J Epidemiol. 2020;35:99–111. https://doi.org/10.1007/s10654-020-00622-7.
Emdin CA, Khera AV, Kathiresan S, Mendelian Randomization. JAMA. 2017;318:1925–6. https://doi.org/10.1001/jama.2017.17219.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, EPIC- InterAct Consortium. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543–52. https://doi.org/10.1007/s10654-015-0011-z.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18. https://doi.org/10.1038/s41586-022-05473-8.
Demontis D, Walters GB, Athanasiadis G, Walters R, Therrien K, Nielsen TT, et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet. 2023;55:198–208. https://doi.org/10.1038/s41588-022-01285-8.
Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatr. 2016;21:1391–9. https://doi.org/10.1038/mp.2015.197.
Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51:1207–14. https://doi.org/10.1038/s41588-019-0439-2.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44. https://doi.org/10.1038/s41588-019-0344-8.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29. https://doi.org/10.1038/s41588-021-00857-4.
Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52. https://doi.org/10.1038/s41593-018-0326-7.
International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatr. 2018;23:1181–8. https://doi.org/10.1038/mp.2017.154.
Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558. https://doi.org/10.1038/s41467-019-12576-w.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8. https://doi.org/10.1038/s41586-022-04434-5.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–3. https://doi.org/10.1093/bioinformatics/btz469.
Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–9. https://doi.org/10.1093/bioinformatics/btw373.
Li J, Zhao L, Ding X, Cui X, Qi L, Chen Y. Obstructive sleep apnea and the risk of Alzheimer’s disease and Parkinson disease: a mendelian randomization study OSA, Alzheimer’s disease and Parkinson disease. Sleep Med. 2022;97:55–63. https://doi.org/10.1016/j.sleep.2022.06.004.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. https://doi.org/10.7554/eLife.34408.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. https://doi.org/10.1093/ije/dyv080.
Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum Mol Genet. 2018;27:R195–208. https://doi.org/10.1093/hmg/ddy163.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. https://doi.org/10.1002/gepi.21965.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74. https://doi.org/10.1093/ije/dyw220.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med. 2017;36:1783–802. https://doi.org/10.1002/sim.7221.
Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48:728–42. https://doi.org/10.1093/ije/dyy258.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. https://doi.org/10.1038/s41588-018-0099-7.
Brion M J A, Shakhbazov K, Visscher PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501. https://doi.org/10.1093/ije/dyt179.
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–42. https://doi.org/10.1177/0962280210394459.
Sedky K, Bennett DS, Carvalho KS. Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: a meta-analysis. Sleep Med Rev. 2014;18:349–56. https://doi.org/10.1016/j.smrv.2013.12.003.
Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–16. https://doi.org/10.1542/peds.2004-0227.
Paavonen EJ, Porkka-Heiskanen T, Lahikainen AR. Sleep quality, duration and behavioral symptoms among 5-6-year-old children. Eur Child Adoles Psy. 2009;18:747–54. https://doi.org/10.1007/s00787-009-0033-8.
Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–93. https://doi.org/10.1164/rccm.200610-1519OC.
Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–9. https://doi.org/10.1016/j.sleep.2007.04.013.
Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulm. 2009;44:417–22. https://doi.org/10.1002/ppul.20981.
Türkoğlu S, Tahsin Somuk B, Sapmaz E, Bilgiç A. Effect of adenotonsillectomy on sleep problems, attention deficit hyperactivity disorder symptoms, and quality of life of children with adenotonsillar hypertrophy and sleep-disordered breathing. Int J Psychiat Med. 2019;54:231–41. https://doi.org/10.1177/0091217419829988.
Ahmadi MS, Poorolajal J, Masoomi FS, Haghighi M. Effect of adenotonsillectomy on attention deficit-hyperactivity disorder in children with adenotonsillar hypertrophy: a prospective cohort study. Int J Pediatr Otorhi. 2016;86:193–5. https://doi.org/10.1016/j.ijporl.2016.05.012.
Amiri S, AbdollahiFakhim S, Lotfi A, Bayazian G, Sohrabpour M, Hemmatjoo T. Effect of adenotonsillectomy on ADHD symptoms of children with adenotonsillar hypertrophy and sleep disordered breathing. Int J Pediatr Otorhinolaryngol. 2015;79:1213–7. https://doi.org/10.1016/j.ijporl.2015.05.015.
Youssef NA, Ege M, Angly SS, Strauss JL, Marx CE. Is obstructive sleep apnea associated with ADHD? Ann Clin Psychiatry. 2011;23:213–24.
Miano S, Amato N, Foderaro G, Pezzoli V, Ramelli GP, Toffolet L, et al. Sleep phenotypes in attention deficit hyperactivity disorder. Sleep Med. 2019;60:123–31. https://doi.org/10.1016/j.sleep.2018.08.026.
Mi C, Hou A, Liu Y, Qi X, Teng J. Assessing the causal relationship between psychiatric disorders and obstructive sleep apnea: a bidirectional Mendelian randomization. Frontiers in Psychiatry, 2024, 15[2024-03-19]. https://doi.org/10.3389/fpsyt.2024.1351216.
Lu M-K, Tan H-P, Tsai I-N, Huang L-C, Liao X-M, Lin S-H. Sleep apnea is associated with an increased risk of mood disorders: a population-based cohort study. Sleep Breath. 2017;21:243–53. https://doi.org/10.1007/s11325-016-1389-x.
Lang CJ, Appleton SL, Vakulin A, McEvoy RD, Vincent AD, Wittert GA, et al. Associations of Undiagnosed Obstructive Sleep Apnea and Excessive Daytime Sleepiness with Depression: an Australian Population Study. J Clin Sleep Med. 2017;13:575–82. https://doi.org/10.5664/jcsm.6546.
Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med. 2013;9:417–23. https://doi.org/10.5664/jcsm.2652.
Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. https://doi.org/10.1001/archinte.166.16.1709.
Garbarino S, Bardwell WA, Guglielmi O, Chiorri C, Bonanni E, Magnavita N. Association of anxiety and depression in Obstructive Sleep Apnea patients: a systematic review and Meta-analysis. Behav Sleep Med. 2020;18:35–57. https://doi.org/10.1080/15402002.2018.1545649.
Stubbs B, Vancampfort D, Veronese N, Solmi M, Gaughran F, Manu P, et al. The prevalence and predictors of obstructive sleep apnea in major depressive disorder, bipolar disorder and schizophrenia: a systematic review and meta-analysis. J Affect Disord. 2016;197:259–67. https://doi.org/10.1016/j.jad.2016.02.060.
Luik AI, Noteboom J, Zuurbier LA, Whitmore H, Hofman A, Tiemeier H. Sleep apnea severity and depressive symptoms in a population-based study. Sleep Health. 2015;1:128–32. https://doi.org/10.1016/j.sleh.2015.03.002.
Kendzerska T, Gershon AS, Hawker GA, Tomlinson GA, Leung RS. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: a historical cohort study. Eur Respir J. 2017;49:1601361. https://doi.org/10.1183/13993003.01361-2016.
Vanek J, Prasko J, Genzor S, Ociskova M, Kantor K, Holubova M, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–8. https://doi.org/10.1016/j.sleep.2020.03.017.
Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. https://doi.org/10.1016/j.bbi.2007.01.010.
Vgontzas AN, Zoumakis E, Lin H-M, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. https://doi.org/10.1210/jc.2003-031929.
Jans La, Riedel W, Markus WJ, Blokland CR. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatr. 2007;12:522–43. https://doi.org/10.1038/sj.mp.4001920.
Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Resp Crit Care. 2003;167:563–9. https://doi.org/10.1164/rccm.200202-107OC.
Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E. Zolpidem-polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav. 1988;29:807–9. https://doi.org/10.1016/0091-3057(88)90212-2.
Guilleminault C. Benzodiazepines, breathing, and sleep. Am J Med. 1990;88:S25–8. https://doi.org/10.1016/0002-9343(90)90282-i.
Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. 2015;11:165–75. https://doi.org/10.5664/jcsm.4466.
Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33:947–52. https://doi.org/10.1007/s10654-018-0424-6.
Choi KW, Chen C-Y, Stein MB, Klimentidis YC, Wang M-J, Koenen KC, et al. Assessment of Bidirectional relationships between physical activity and depression among adults a 2-Sample mendelian randomization study. JAMA Psychiatry. 2019;76:399–408. https://doi.org/10.1001/jamapsychiatry.2018.4175.
Acknowledgements
We sincerely appreciate FinnGen and the PGC for providing publicly available GWAS data. We would also like to thank other faculty members in the Department of Epidemiology and Health Statistics for their help.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HM L wrote the main manuscript as well as analysed the data. XM W and H F used PhenoScanner to exclude instrumental variables associated with other traits or diseases. CP OY and JH P made data curation. SZ Z and XB H reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All data used in this work are publicly available from studies with relevant participant consent and ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, H., Wang, X., Feng, H. et al. Obstructive sleep apnea and mental disorders: a bidirectional mendelian randomization study. BMC Psychiatry 24, 304 (2024). https://doi.org/10.1186/s12888-024-05754-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05754-8