Abstract
Background
Inflammation has an important role in the pathogenesis of schizophrenia. The aim of this study was to investigate the levels of tumor necrosis factor (TNF) and matrix metalloproteinase-2 (MMP-2) in male patients with treatment-resistant schizophrenia (TRS) and chronic medicated schizophrenia (CMS), and the relationship with psychopathology.
Methods
The study enrolled 31 TRS and 49 cm male patients, and 53 healthy controls. Serum MMP-2 and TNF-α levels were measured by the Luminex liquid suspension chip detection method. Positive and Negative Syndrome Scale (PANSS) scores were used to evaluate symptom severity and Repeatable Battery for the Assessment of Neuropsychological Status was used to assess cognitive function.
Results
Serum TNF-α and MMP-2 levels differed significantly between TRS, CMS and healthy control patients (F = 4.289, P = 0.016; F = 4.682, P = 0.011, respectively). Bonferroni correction demonstrated that serum TNF-α levels were significantly elevated in CMS patients (P = 0.022) and MMP-2 levels were significantly higher in TRS patients (P = 0.014) compared to healthy controls. In TRS patients, TNF-α was negatively correlated with age (r=-0.435, P = 0.015) and age of onset (r=-0.409, P = 0.022). In CMS patients, MMP-2 and TNF-α were negatively correlated with PANSS negative and total scores, and TNF-α was negatively correlated with PANSS general psychopathology scores (all P < 0.05). MMP-2 levels were positively correlated with TNF-α levels (P < 0.05), but not with cognitive function (P > 0.05).
Conclusion
The results indicate the involvement of inflammation in the etiology of TRS and CMS. Further studies are warranted.
Similar content being viewed by others
Introduction
Schizophrenia is a complex, heterogeneous, and chronically severe mental disorder characterized by a range of symptoms, including positive and negative symptoms, and cognitive deficits [1]. Despite advancements in pharmacological treatments, approximately one-third of patients will develop treatment-resistant schizophrenia (TRS), which fails to respond adequately to standard antipsychotic medications [2]. Additionally, a subset of patients with schizophrenia become chronic and require continuous medication, which can lead to protracted symptoms and a persistent burden of disease [3, 4]. However, the reasons behind the suboptimal treatment outcomes in these populations are not fully understood. Further explorations of the underlying pathophysiological mechanisms are needed.
Recent studies have suggested that inflammation may play a critical role in the etiology of schizophrenia [5, 6]. One of the key inflammatory cytokines implicated in this process is tumor necrosis factor-alpha (TNF-α); the level of TNF-α is altered in patients with schizophrenia [7]. TNF-α has an established role in the inflammatory response and has been associated with various psychiatric symptoms, such as affective symptoms and cognitive function, as well as being involved in the etiology of the acute and chronic phases of schizophrenia [8,9,10]. The relationship between TNF-α levels and the clinical manifestations of schizophrenia presents a potential avenue for understanding the inflammatory etiology of schizophrenia as well as variations in symptoms.
Matrix metalloproteinases (MMPs) constitute a group of zinc-dependent proteases involved in the processing of active factors, such as cell-surface receptors, neurotrophic factors, chemokines, and cytokines, as well as the regulation of neuroinflammation [11]. MMPs contribute to synaptogenesis, plasticity, and long-term potentiation, play a role in converting pro-TNF-α into mature secretory proteins, and disrupt the integrity of the blood-brain barrier (BBB) [12, 13]. MMP-2 is one of the cores of the 25 known MMP enzymes. The connection between MMP-2 and inflammatory cytokines suggests a complex interplay that may contribute to the neuropathological changes observed in schizophrenia [14]. For instance, interleukin-6 up-regulates the production of MMP-2, and MMP-2 activates TNF-α [15,16,17]. Nonetheless, there are limited studies of MMP-2 in patients with schizophrenia, particularly in the context of treatment-resistant and chronically medicated patients.
Cognitive deficits have been widely demonstrated in patients with schizophrenia at various stages, including cases without psychopharmacological treatment, first-episode psychosis, and TRS [18, 19]. Neuroimmunity has been implicated as an etiological factor in the cognitive deficits of patients with schizophrenia [20]. In patients with schizophrenia, TNF-α has been associated with various domains of cognitive function [7, 21]. Studies in mouse models have demonstrated that inhibition of MMP-2 expression by the increased integrity of the BBB diminishes the decline in cognitive function [22, 23]. Nevertheless, the exact etiology of cognitive deficits in patients with TRS remains unclear.
Therefore, exploring the interaction of TNF-α and MMP-2 in patients with TRS and chronic medicated schizophrenia (CMS) could provide insight into the mechanisms driving treatment resistance and chronicity. The hypothesis of this study is that TNF-α and MMP-2 levels are altered in patients with TRS and CMS, and correlate with psychopathological symptoms. The aims of this research are to investigate (1) whether the levels of TNF-α and MMP-2 in patients with TRS and CMS differed compared to healthy controls, and (2) the relationship between TNF-α and MMP-2 levels with clinical symptoms and cognitive function.
Methods
Subjects
This observational, cross-sectional study using a case-control design recruited a total of 31 TRS and 49 cm male patients from the Fourth People’s Hospital of Lianyungang. Five patients were treated with clozapine alone, and the rest were treated with typical or atypical antipsychotics, with the drug dose finally converted to a chlorpromazine-equivalent dose. The diagnosis of schizophrenia was determined using the Structured Clinical Interview DSM-IV. The inclusion criteria were male sex, age 18–60 years, Han Chinese ethnicity, and absence of anti-inflammatory or antibiotic medication for at least 4 weeks prior to enrolment.
A semi-structured questionnaire was used to collect general data from the patients, which included age, sex, education, smoking status, height, weight, duration of illness, and age of onset. During the same period, 53 healthy controls from the local community of Lianyungang were matched to the patient group in terms of age, sex, education, smoking, and body mass index (BMI), without a diagnosis in accordance with the Axis I criteria for a major disease. A family history of mental illness was excluded. Exclusion criteria for all participants were comorbidities of major medical morbidity, degenerative neurological disorders, endocrine system disorders, and alcohol or substance dependence. The health status of all participants was determined by physical examination and laboratory tests such as blood count, liver and renal function tests, glucose levels, and thyroid function.
Informed consent was given by all participants or their guardians. The protocol was approved by the Ethics Committee of the Fourth People’s Hospital of Lianyungang City.
Clinical and cognitive assessment
The severity of clinical symptoms in patients with schizophrenia was assessed by two experienced psychiatrists using the Positive and Negative Syndrome Scale (PANSS). The correlation coefficient among the scores of PANSS exceeded 0.8. Cognitive function of the subjects was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which consists of five subtests assessing immediate memory, visuospatial/structural, verbal, attentional, and delayed memory [24]. The raw total scores of the subscales were subsequently processed into standardized scores. The RBANS has demonstrated good reliability and validity in patients with schizophrenia and in the Chinese population [25].
Defining TRS and CMS
Patients with TRS were defined as those who had been poorly effective on two antipsychotics consecutively for more than 6 months, whose equivalent dose of chlorpromazine was > 600 mg/day, and who had scores ≥ 3 for each of the eight PANSS subscales (P1, P2, P3, N1, N4, N6, G5, and G9) [26,27,28]. CMS patients were those who had a stable treatment effect of one antipsychotic medication for more than 6 months, an equivalent dose of chlorpromazine of < 600 mg/day, score < 3 for each of the eight aforementioned PANSS subscales, and a total PANSS score < 60 [26, 28].
Measurement of serum MMP-2 and TNF-α levels
Fasting peripheral blood samples were drawn from each participant between 7:00 and 9:00 a.m. Each sample was centrifuged at 3500 rpm for 15 min and stored at -80 °C until analyzed. Clinical symptoms and cognitive function were performed after obtaining blood samples. Serum MMP-2 and TNF-α levels were measured using the Luminex liquid suspension chip detection method according to product instructions (R&D Systems, Minneapolis, MN, USA). All blood samples measurements were made in duplicate by a technician blinded to the clinical implications of the samples. The intra- and inter-assay variability of MMP-2 and TNF-α measurements was 2.05% and 2.93%, respectively.
Statistical analyses
SPSS version 19.0 (IBM, Armonk, NY, USA) was used for statistical analyses, The Kolmogorov-Smirnov test was performed to assess the normal distribution of the variables. Continuous variables were analyzed using analysis of variance (ANOVA). Results are expressed as mean ± standard deviation. Variables that were not normally distributed were analyzed using the Mann–Whitney U-test and expressed as median with 25th and 75th quartiles. Categorical variables were tested using chi-square test. Differences were considered statistically significant at P < 0.05. Analysis of covariance (ANCOVA) was performed with TNF-α and MMP-2 as the dependent variables, with diagnosis as fix factor, age, BMI, smoking, and education as covariates. Bonferroni correction was performed for multiple testing, the Bonferroni-corrected P-value between the TRS and CMS groups was the original P-value × 4 for each of the PANSS scores. Cohen’s d value determined effect size. Pearson’s or Spearman’s correlation analysis was performed to explore the relationship between variables. Stepwise multiple regression was performed to determine the relationship between variables after controlling for confounding factors of age, education, smoking, chlorpromazine equivalent dose, duration of illness, and age of onset.
Results
Sample characteristics
Comparison of demographic data, clinical symptoms, and cognitive function between TRS, CMS, and healthy control groups are shown in Table 1. The range of years of education in the patient and healthy groups was 6–16 years. No significant differences were evident in age, education, BMI, and smoking status among the three groups (all P > 0.05). Differences in age of onset were not significantly different between the TRS and CMS groups (P = 0.236). Significant differences included the duration of illness, PANSS total scores and scores of the subscales, and the RBANS total scores and differences of the subscales (all P < 0.05). The Bonferroni post-hoc test showed that both the RBANS total and index scores TRS and CMS groups in the TRS and CMS groups were significantly lower than those of the healthy control group (all P < 0.05), with no significant difference found between TRS and CMS groups (all P > 0.05). Post-hoc tests revealed statistically significant differences between the TRS and CMS groups in the overall and subscale PANSS scores (all P < 0.05).
Serum TNF-α and MMP-2 levels in TRS and CMS patients, and healthy controls
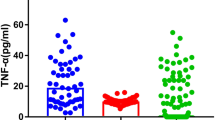
Serum TNF- α and MMP-2 levels varied significantly across patients with TRS and CMS, and the healthy controls (F = 4.289, P = 0.016; F = 4.682, P = 0.011, respectively). After controlling for age, BMI, smoking and education, ANCOVA revealed significant differences in the levels of TNF-α (F = 4.505, P = 0.013) and MMP-2 (F = 5.490, P = 0.005) between the TRS and CMS patients, and healthy controls. Bonferroni correction showed that TNF-α levels were increased with significant variances between CMS and healthy controls (P = 0.022). There was no significant difference between TRS and healthy controls (P = 0.104). The post-hoc results showed that elevated serum MMP-2 levels were significantly different between TRS and healthy controls (P = 0.014), but not between CMS and TRS patients (P = 0.986), and healthy controls (P = 0.10) (Fig. 1A and B).
Association of MMP-2 and TNF-α levels with psychopathological and cognitive function in patients with TRS
In patients with TRS, correlation analysis revealed that serum MMP-2 levels were positively associated with TNF-α (r = 0.379, P = 0.036) (Fig. 2A) and duration of illness (r = 0.438, P = 0.014). TNF-α was negatively related to age (r = − 0.435, P = 0.015) and age of onset (r = − 0.409, P = 0.022), after controlling for age and age of onset. There was no correction between MMP-2, TNF-α, and the PANSS total score and subscales, or the RBANS total score and subscales (all P > 0.05).
Subsequently, after controlling for confounders of age, education, smoking, and chlorpromazine equivalent dose, BMI, age of onset, and duration of illness, stepwise multiple regression demonstrated that MMP-2 was correlated with TNF-α (B = 0.628, t = 2.487, P = 0.019).
Association of MMP-2 and TNF-α levels with psychopathological and cognitive function in patients with CMS
In patients with CMS, correlation analyses showed that serum MMP-2 levels were positively correlated with TNF-α (r = 0.730, P < 0.001) (Fig. 2B) and negatively correlated with the PANSS negative subscores (r = − 0.332, P = 0.020) (Fig. 3A) and total scores (r = − 0.339, P = 0.017) (Fig. 3B). TNF-α levels were negatively correlated with the PANSS general psychopathology scores (r = − 0.288, P = 0.045), negative subscores (r = − 0.424, P = 0.002), and total scores (r = − 0.385, P = 0.006). No association was observed between MMP-2 and TNF-α with the RBANS total score and subscales (all P > 0.05).
After controlling for confounding factors with stepwise multiple regression analyses, MMP-2 levels were associated with TNF-α (B = 1.137, t = 6.640, P < 0.001), PANSS negative subscores (B = − 0.573, t = − 2.570, P = 0.013), and total scores (B = -1.018, t = -2.756, P = 0.008). TNF-α levels were associated with PANSS negative subscores (B = -0.344, t = − 2.515, P = 0.015), total scores (B = − 0,748, t = − 3.429, P = 0.001), and smoking (B = − 3.259, t = − 2.953, P = 0.005) as influencing factors between TNF-α (B = − 0.298, t = − 2.597, P = 0.013) and PANSS general psychopathology subscores.
Discussion
There are four main findings in our study. First, serum MMP-2 levels were significantly elevated in patients with TRS compared to healthy controls, whereas serum TNF-α was significantly increased in patients with CMS. Second, MMP-2 and TNF-α levels were positively correlated in both patients with TRS and CMS. Third, in patients with CMS, TNF-α and MMP-2 levels were negatively correlated with the PANSS negative subscores and total scores, and TNF-α was negatively correlated with the PANSS general psychopathology subscores. Fourth, no correlation was evident between TNF-α and MMP-2 and cognitive function. To the best of our knowledge, this is the first study to reveal the relationship between elevated MMP-2 and TNF-α, clinical psychopathological symptoms, and cognitive function in TRS and CMS patients.
The finding that serum MMP-2 levels were elevated in patients with TRS is not entirely consistent with previous results. For example, Shibasaki et al. reported that MMP-2 levels were not different from those of healthy controls in a study involving 13 patients with schizophrenia and did not vary before or after electroconvulsive therapy [29]. Omori et al. demonstrated significantly elevated levels of MMP-2 in the cerebrospinal fluid of patients with schizophrenia [30]. The different findings may be related to sample size, ethnicity, source of biological samples, and diagnosis. Previous studies have shown that MMP-2 can increase the permeability of the BBB in mouse models [31, 32]. The significant elevation of serum MMP-2 levels in TRS patients could indicate a particular pathological process in this subgroup. MMP-2 play an important role in degrading the extracellular matrix and modulation of the BBB permeability may contribute to a neuroinflammatory state that underpins resistance to treatment.
In addition, our findings reveal the negative correlation of serum MMP-2 levels with PANSS negative scores and total scores in patients with CMS. A previous study found that cerebrospinal fluid MMP-2 levels were positively associated with core symptoms, psychogenic anxiety, and somatic anxiety in patients with major depressive disorder, but no correlation between cerebrospinal fluid MMP-2 and psychopathological symptoms was observed in patients with schizophrenia [30]. MMP-2 has been implicated in neuroinflammatory processes and neurodegeneration. In particular, MMP-2 modulated the integrity of the BBB and synaptic plasticity, which were posited to underlie certain aspects of schizophrenia pathophysiology [33, 34]. The negative symptoms, characterized by social withdrawal, apathy, and anhedonia, are often resistant to current pharmacotherapies and are linked to poorer functional outcomes [35, 36]. Our findings align with the hypothesis that aberrant neuroimmune activity, reflected in altered MMP-2 serum levels. The negative correlation could indicate that higher MMP-2 levels are associated with increased BBB permeability or impaired synaptic remodeling, potentially leading to exacerbated symptoms.
The major role of TNF-α in the pathophysiology of schizophrenia at various stages has been reported several times in previous studies, including in animal models [37,38,39]. Our study is consistent with previous findings that serum TNF-α levels were elevated in patients with CMS, suggesting a relationship between neuroinflammation and schizophrenia. Furthermore, the increase in TNF-α levels and their association with severity of symptoms as measured by PANSS suggest that ongoing inflammatory processes continue to play a role, even in the context of chronic medication. These findings are consistent with previous observations that persistent inflammation can contribute to the symptom burden in schizophrenia, despite ongoing treatment efforts [40, 41].
Previous studies have shown that patients with TRS not only manifest resistance to conventional dopamine D2 receptor blockers, but may also have abnormalities in glutamate regulation and altered cytokine levels [42, 43], which may combine to contribute to the complexity of the disease and the difficulty of treatment. In addition, prolonged use of dopamine D2 receptor blockers may lead to hypersensitivity of dopamine receptors, which may increase the relapse rate after discontinuation of the drug [44]. TNF-α also mediates glutamatergic activity by inducing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor [45], and the TNF-NFκB-p53 axis restrict dopamine neuron survival in vivo [46], whereas the dopamine and glutamatergic systems have extensive and complex interactions [47]. Although our results indicate that the difference between TNF-α levels in TRS patients and healthy controls was not significant, based on the complex pathophysiology of TRS, we still could not exclude that TNF-α might be involved in the generation of TRS, nor could we rule out the influence of medications on this outcome, and further studies are needed.
Nonetheless, our findings revealed an unexpected negative correlation of MMP-2 and TNF-α with PANSS scores in CMS patients. This is inconsistent with the correlation of TNF-α with PANSS scores reported in previous studies. For example, TNF-α was found to be positively correlated with PANSS negative scores in 47 antipsychotic-responders and 47 antipsychotic non-responders with schizophrenia [48]. In contrast, in patients with schizophrenia who were treated for the first time [49], acutely exacerbated [50], displayed acutely relapsed states [51], and who were medicated in the acute phase [52], TNF-α did not correlate with PANSS scores. In paranoid patients with schizophrenia [40], TNF-α was negatively correlated with PANSS positive scores. In patients with chronic schizophrenia, TNF-α was negatively correlated with PANSS general pathology symptoms and total scores [41]. The relationship between MMP-2 and the severity of clinical symptoms in schizophrenia has not been reported. We speculate that long-term use of antipsychotics may have induced pharmacologic chronic immunomodulatory changes in the present study population. This may be reflected in altered levels of cytokines, which alter the routinely observed relationship between the cytokines and psychiatric measures. This finding emphasizes the complexity of the effects of long-term drug therapy on immune function and may provide new insights into biomarkers and assessment of treatment efficacy in patients with schizophrenia. Future studies should take these factors into account to provide a more nuanced exploration of the immune response in patients on long-term medication and its link to psychiatric severity. Interestingly, we found that serum TNF-α levels were associated with age of onset in patients with TRS. Xiu et al. reported that TNF-α gene − 1031T > C polymorphism was related to age of onset in patients with schizophrenia, suggesting that the TNF-α gene may serve as a modifier of age of onset in schizophrenia [53].
Previous studies have uncovered that MMP-2 and TNF-α have important roles in the progression of other diseases, such as Alzheimer’s disease and Parkinson’s disease [54, 55]. Mouse models have revealed that MMP-2 serves as a sheddase for TNF-α [56], suggesting that synergistic effects of MMP-2 and TNF-α may result in increased neuroinflammation and neurol destruction. The other major finding of the present study was the positive correlation between MMP-2 and TNF-α levels observed in patients with TRS and CMS. This finding may provide new insights into the shared inflammatory pathways that possibly contribute to modifying the pathological process. This interrelation warrants further investigation.
In previous studies, high expression of the MMP-2 gene on mRNA and protein levels in recurrent depressive disorders reported positive effects on cognitive efficiency, such as working memory and executive and attentional functions [57], and inhibition of MMP-2 attenuated cognitive impairment in aged mice [22]. A recent systematic review and meta-analysis indicated the correlation between TNF-α and cognitive deficits in schizophrenia [7]. However, the lack of correlation between MMP-2 and TNF-α and cognitive function in our study was intriguing and suggests that the cognitive deficits in TRS and CMS may not be mediated by a single mechanism. Rather, these deficits may also be related to the interaction or moderating effect between MMP-2 and TNF-α levels, and may also be related to the education of the patients, culture, and assessment instruments. Based on the positive effects of MMP-2 on cognitive efficacy in recurrent depression, how elevated MMP-2 levels in patients with schizophrenia influence cognitive deficits needs further investigation.
Limitations of this study should be acknowledged. Firstly, the cross-sectional design precludes causal inferences. Longitudinal studies are necessary to determine the temporal relationship between MMP-2 and TNF-α levels and clinical symptoms. Secondly, our sample size was relatively small, which may limit the generalizability of our findings. Thirdly, while we controlled for medication use, the heterogeneity in treatment regimens could have influenced the biomarker levels. Fourthly, selectivity errors may result based on defined TRS or CMS inclusion criteria and will need to be avoided in the future by increasing the sample size, introducing samples from multiple sources, and performing sensitivity analyses. Fifthly, participants were not assessed for psychotherapeutic or counseling interventions, which is one of the possible protective factors for non-pharmacological means of treatment that may have an important impact on the course of the illness and response to treatment for patients with schizophrenia [58, 59]. In addition, participants were not assessed for neuroimaging. Neuroimaging is becoming increasingly important in the complex diagnosis of psychiatric disorders and treatment follow-up [60,61,62]. The lack of neuroimaging limits our ability to gain a deeper understanding of the pathomechanisms of schizophrenia from a neurobiological perspective. Therefore, future studies should consider including these important aspects in order to understand more fully the treatment process of psychiatric disorders and to assess the treatment needs and responses of people with schizophrenia. Finally, the lack of cognitive correlation may be due to the cognitive measures used; future studies should include a broader assessment of cognitive function.
In conclusion, our study provides evidence for the involvement of MMP-2 and TNF-α in the pathology of TRS and CMS, with potential implications for the development of new treatment strategies. Future research should aim to elucidate the mechanisms by which these biomarkers influence the disease process and to explore their potential as targets for novel therapeutic interventions.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Faden J, Citrome L. Schizophrenia: one name, many different manifestations. Med Clin North Am. 2023;107(1):61–72.
Elkis H, Buckley PF. Treatment-resistant Schizophrenia. Psychiatr Clin North Am. 2016;39(2):239–65.
Global regional. and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Psychiatry 2022, 9(2):137–150.
Tan W, Chen L, Zhang Y, Xi J, Hao Y, Jia F, Hall BJ, Gu J, Wang S, Lin H, et al. Regional years of life lost, years lived with disability, and disability-adjusted life-years for severe mental disorders in Guangdong Province, China: a real-world longitudinal study. Glob Health Res Policy. 2022;7(1):17.
Ermakov EA, Mednova IA, Boiko AS, Buneva VN, Ivanova SA. Chemokine dysregulation and Neuroinflammation in Schizophrenia: a systematic review. Int J Mol Sci 2023, 24(3).
Fišar Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2023, 120.
Patlola SR, Donohoe G, McKernan DP. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2023;121:110668.
Solmi M, Suresh Sharma M, Osimo EF, Fornaro M, Bortolato B, Croatto G, Miola A, Vieta E, Pariante CM, Smith L, et al. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: a meta-analysis of mean differences and variability. Brain Behav Immun. 2021;97:193–203.
Misiak B, Stanczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res. 2018;192:16–29.
Halstead S, Siskind D, Amft M, Wagner E, Yakimov V, Shih-Jung Liu Z, Walder K, Warren N. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2023;10(4):260–71.
Kim YS, Joh TH. Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol Ther (Seoul). 2012;20(2):133–43.
Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85(13):2813–23.
Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood flow Metabolism: Official J Int Soc Cereb Blood Flow Metabolism. 2001;21(12):1393–400.
Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors - novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt A):1927–39.
Mattos BR, Bonacio GF, Vitorino TR, Garcia VT, Amaral JH, Dellalibera-Joviliano R, Franca SC, Tanus-Santos JE, Rizzi E. TNF-alpha inhibition decreases MMP-2 activity, reactive oxygen species formation and improves hypertensive vascular hypertrophy independent of its effects on blood pressure. Biochem Pharmacol. 2020;180:114121.
Cai YL, Wang ZW. The expression and significance of IL-6, IFN-γ, SM22α, and MMP-2 in rat model of aortic dissection. Eur Rev Med Pharmacol Sci. 2017;21(3):560–8.
Zergoun AA, Zebboudj A, Sellam SL, Kariche N, Djennaoui D, Ouraghi S, Kerboua E, Amir-Tidadini ZC, Chilla D, Asselah F, et al. IL-6/NOS2 inflammatory signals regulate MMP-9 and MMP-2 activity and disease outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2016;37(3):3505–14.
Gebreegziabhere Y, Habatmu K, Mihretu A, Cella M, Alem A. Cognitive impairment in people with schizophrenia: an umbrella review. Eur Arch Psychiatry Clin Neurosci. 2022;272(7):1139–55.
Wada M, Noda Y, Iwata Y, Tsugawa S, Yoshida K, Tani H, Hirano Y, Koike S, Sasabayashi D, Katayama H, et al. Dopaminergic dysfunction and excitatory/inhibitory imbalance in treatment-resistant schizophrenia and novel neuromodulatory treatment. Mol Psychiatry. 2022;27(7):2950–67.
Zhang Q, He H, Cao B, Gao R, Jiang L, Zhang X, Dai J. Analysis of cognitive impairment in schizophrenia based on machine learning: Interaction between psychological stress and immune system. Neurosci Lett. 2021;760:136084.
Chukaew P, Bunmak N, Auampradit N, Siripaiboonkij A, Saengsawang W, Ratta-Apha W. Correlation of BDNF, VEGF, TNF-alpha, and S100B with cognitive impairments in chronic, medicated schizophrenia patients. Neuropsychopharmacol Rep. 2022;42(3):281–7.
Ji Y, Huang W, Chen Y, Zhang X, Wu F, Tang W, Lu Z, Huang C. Inhibition of MMP-2 and MMP-9 attenuates surgery-induced cognitive impairment in aged mice. Brain Res Bull. 2023;204:110810.
Li Q, Michaud M, Shankar R, Canosa S, Schwartz M, Madri JA. MMP-2: a modulator of neuronal precursor activity and cognitive and motor behaviors. Behav Brain Res. 2017;333:74–82.
Goette W. Reconsidering the RBANS factor structure: a Systematic Literature Review and Meta-Analytic factor analysis. Neuropsychol Rev. 2020;30(3):425–42.
Zheng W, Jiang W-L, Zhang X, Cai D-B, Sun J-W, Yin F, Ren P-C, Zhao M, Wu H-W, Xiang Y-Q, et al. Use of the RBANS to evaluate cognition in patients with Schizophrenia and metabolic syndrome: a Meta-Analysis of Case-Control studies. Psychiatr Q. 2021;93(1):137–49.
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, Bloomfield MAP, Bressan RA, Buchanan RW, Carpenter WT, et al. Treatment-resistant Schizophrenia: treatment response and resistance in psychosis (TRRIP) Working Group Consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–29.
Nucifora FC, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol Dis 2019, 131.
Andreasen NC, Carpenter WT Jr., Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–9.
Shibasaki C, Takebayashi M, Itagaki K, Abe H, Kajitani N, Okada-Tsuchioka M, Yamawaki S. Altered serum levels of Matrix Metalloproteinase-2, -9 in response to Electroconvulsive Therapy for Mood disorders. Int J Neuropsychopharmacol 2016, 19(9).
Omori W, Hattori K, Kajitani N, Okada-Tsuchioka M, Boku S, Kunugi H, Okamoto Y, Takebayashi M. Increased Matrix metalloproteinases in Cerebrospinal fluids of patients with Major Depressive Disorder and Schizophrenia. Int J Neuropsychopharmacol. 2020;23(11):713–20.
Younis NS, Mohamed ME. Anethole pretreatment modulates cerebral Ischemia/Reperfusion: the role of JNK, p38, MMP-2 and MMP-9 pathways. Pharmaceuticals 2023, 16(3).
Ruan Z, Zhang D, Huang R, Sun W, Hou L, Zhao J, Wang Q. Microglial Activation Damages Dopaminergic Neurons through MMP-2/-9-Mediated increase of blood-brain barrier permeability in a Parkinson’s Disease Mouse Model. Int J Mol Sci 2022, 23(5).
Hannocks MJ, Zhang X, Gerwien H, Chashchina A, Burmeister M, Korpos E, Song J, Sorokin L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019;75–76:102–13.
Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, Martinez-Avila N, Martinez-Fierro ML. The roles of Matrix metalloproteinases and their inhibitors in Human diseases. Int J Mol Sci 2020, 21(24).
Marder SR, Umbricht D. Negative symptoms in schizophrenia: newly emerging measurements, pathways, and treatments. Schizophr Res. 2023;258:71–7.
Sabe M, Chen C, Perez N, Solmi M, Mucci A, Galderisi S, Strauss GP, Kaiser S. Thirty years of research on negative symptoms of schizophrenia: a scientometric analysis of hotspots, bursts, and research trends. Neurosci Biobehavioral Reviews 2023, 144.
Vallée A. Neuroinflammation in Schizophrenia: the key role of the WNT/β-Catenin pathway. Int J Mol Sci 2022, 23(5).
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M. The role of aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in different phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol. 2019;57(2):778–97.
Bae HJ, Bae HJ, Kim JY, Park K, Yang X, Jung SY, Park SJ, Kim DH, Shin CY, Ryu JH. The effect of lansoprazole on MK-801-induced schizophrenia-like behaviors in mice. Prog Neuropsychopharmacol Biol Psychiatry 2023, 120.
Mednova IA, Boiko AS, Kornetova EG, Semke AV, Bokhan NA, Ivanova SA. Cytokines as potential biomarkers of clinical characteristics of Schizophrenia. Life 2022, 12(12).
Lin C, Chen K, Yu J, Feng W, Fu W, Yang F, Zhang X, Chen D. Relationship between TNF-α levels and psychiatric symptoms in first-episode drug-naïve patients with schizophrenia before and after risperidone treatment and in chronic patients. BMC Psychiatry 2021, 21(1).
de Bartolomeis A, Manchia M, Marmo F, Vellucci L, Iasevoli F, Barone A. Glycine signaling in the Framework of dopamine-glutamate Interaction and postsynaptic density. Implications for treatment-resistant Schizophrenia. Front Psychiatry 2020, 11.
Chen W, Tian Y, Gou M, Wang L, Tong J, Zhou Y, Feng W, Li Y, Chen S, Liu Y, et al. Role of the immune-kynurenine pathway in treatment-resistant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2024;130:110926.
Chouinard G, Samaha A-N, Chouinard V-A, Peretti C-S, Kanahara N, Takase M, Iyo M. Antipsychotic-Induced dopamine supersensitivity psychosis: Pharmacology, Criteria, and Therapy. Psychother Psychosom. 2017;86(4):189–219.
Na K-S, Jung H-Y, Kim Y-K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86.
Kim TW, Koo SY, Riessland M, Cho H, Chaudhry F, Kolisnyk B, Russo MV, Saurat N, Mehta S, Garippa R et al. TNF-NFkB-p53 axis restricts in vivo survival of hPSC-derived dopamine neuron. bioRxiv: the preprint server for biology 2023.
Buck SA, Quincy Erickson-Oberg M, Logan RW, Freyberg Z. Relevance of interactions between dopamine and glutamate neurotransmission in schizophrenia. Mol Psychiatry. 2022;27(9):3583–91.
Enache D, Nikkheslat N, Fathalla D, Morgan BP, Lewis S, Drake R, Deakin B, Walters J, Lawrie SM, Egerton A, et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr Res. 2021;230:1–8.
Azizi E, Zavaran Hosseini A, Soudi S, Noorbala AA. Alteration of serum levels of cytokines in schizophrenic patients before and after treatment with Risperidone. Iran J Allergy Asthma Immunol. 2019;18(3):262–8.
Gül Çakıl A, Kaya H, Sakallı Nural A, Çakmak IB, Okay İT, Göka E. Neutrophil gelatinase-associated lipocalin (NGAL) and tumor necrosis factor-α (TNF-α) levels in patients with schizophrenia. Psychopharmacology. 2023;240(5):1091–101.
Luo Y, He H, Zhang J, Ou Y, Fan N. Changes in serum TNF-alpha, IL-18, and IL-6 concentrations in patients with chronic schizophrenia at admission and at discharge. Compr Psychiatry. 2019;90:82–7.
Pavlovic M, Babic D, Rastovic P, Arapovic J, Martinac M, Jakovac S, Barbaric R. Association of Tumor Necrosis Factor-Alpha with psychopathology in patients with Schizophrenia. Acta Med Okayama. 2023;77(4):395–405.
Xiu M, Zhang G, Chen N, Chen S, Tan Y, Yin G, Man L, Ning Y, Huang X, Teixeira AL, et al. The TNF-alpha gene– 1031T > C polymorphism is associated with onset age but not with risk of schizophrenia in a Chinese population. Neuropsychology. 2019;33(4):482–9.
Tang X, Di X, Liu Y. Protective effects of Donepezil against endothelial permeability. Eur J Pharmacol. 2017;811:60–5.
Marques CR, Fuzeta MA, dos Santos Cunha RM, Pereira-Sousa J, Silva D, Campos J, Teixeira-Castro A, Sousa RA, Fernandes-Platzgummer A, da Silva CL et al. Neurodifferentiation and Neuroprotection Potential of Mesenchymal Stromal Cell-Derived Secretome Produced in Different Dynamic Systems. Biomedicines 2023, 11(5).
De Groef L, Salinas-Navarro M, Van Imschoot G, Libert C, Vandenbroucke RE, Moons L. Decreased TNF Levels and Improved Retinal Ganglion Cell Survival in MMP-2 Null Mice Suggest a Role for MMP-2 as TNF Sheddase. Mediators of Inflammation 2015, 2015:1–13.
Bobińska K, Szemraj J, Gałecki P, Talarowska M. The role of MMP genes in recurrent depressive disorders and cognitive functions. Acta Neuropsychiatrica. 2016;28(4):221–31.
Correll CU. Using patient-centered Assessment in Schizophrenia Care: defining recovery and discussing concerns and preferences. J Clin Psychiatry 2020, 81(3).
Chien WT, Mui J, Gray R, Cheung E. Adherence therapy versus routine psychiatric care for people with schizophrenia spectrum disorders: a randomised controlled trial. BMC Psychiatry. 2016;16:42.
Preller KH, Scholpp J, Wunder A, Rosenbrock H. Neuroimaging biomarkers for drug discovery and development in Schizophrenia. Biol Psychiatry 2024.
Pang TSW, Chun JSW, Wong TY, Chu ST, Ma CF, Honer WG, Chan SKW. A systematic review of neuroimaging studies of clozapine-resistant schizophrenia. Schizophrenia (Heidelb). 2023;9(1):65.
Rasser PE, Ehlkes T, Schall U. Fronto-temporal cortical grey matter thickness and surface area in the at-risk mental state and recent-onset schizophrenia: a magnetic resonance imaging study. BMC Psychiatry 2024, 24(1).
Acknowledgements
We would like to thank the participants in the study.
Funding
The study was supported by the Suzhou Key Technologies Program (SKY2021063), Suzhou clinical Medical Center for mood disorders (No. Szlcyxzx202109), Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116), Lianyungang Science and Technology Bureau of Social Development Key R&D Projects (SF2208) and General Program of Lianyungang Health Committee (NO.202130). The funding sources of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Author information
Authors and Affiliations
Contributions
Haidong Yang and Ruijie Peng wrote the manuscript; Xiaobin Zhang was responsible for study design; Haidong Yang performed the statistical analysis; Man Yang, Jing Zhang and Zhihui Shi were responsible for performing the clinical rating, recruiting the patients, and collecting the samples. All authors have contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
We declare that all experiments on human subjects were conducted in accordance with the Declaration of Helsinki and that all procedures were carried out with the adequate understanding and written consent of the subjects. All experimental protocols were approved by the Ethics Committee of Lian Yun Gang Fourth People’s Hospital. Informed consent was obtained from all the participants and/or their legal guardians. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, H., Peng, R., Yang, M. et al. Association between elevated serum matrix metalloproteinase-2 and tumor necrosis factor-α, and clinical symptoms in male patients with treatment-resistant and chronic medicated schizophrenia. BMC Psychiatry 24, 173 (2024). https://doi.org/10.1186/s12888-024-05621-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05621-6