Abstract
Background
Electroconvulsive therapy (ECT) is a highly effective treatment for depressive disorder. However, the use of ECT is limited by its cognitive side effects (CSEs), and no specific intervention has been developed to address this problem. As transcranial direct current stimulation (tDCS) is a safe and useful tool for improving cognitive function, the main objective of this study was to explore the ability to use tDCS after ECT to ameliorate the cognitive side effects.
Methods
60 eligible participants will be recruited within two days after completing ECT course and randomly assigned to receive either active or sham stimulation in a blinded, parallel-design trial and continue their usual pharmacotherapy. The tDCS protocol consists of 30-min sessions at 2 mA, 5 times per week for 2 consecutive weeks, applied through 15-cm2 electrodes. An anode will be placed over the left dorsolateral prefrontal cortex (DLPFC), and a cathode will be placed over the right supraorbital cortex. Cognitive function and depressive symptoms will be assessed before the first stimulation (T0), after the final stimulation (T1), 2 weeks after the final stimulation (T2), and 4 weeks after the final stimulation (T3) using the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Discussion
We describe a novel clinical trial to explore whether the administration of tDCS after completing ECT course can accelerates recovery from the CSEs. We hypothesized that the active group would recover faster from the CSEs and be superior to the sham group. If our hypothesis is supported, the use of tDCS could benefit eligible patients who are reluctant to receive ECT and reduce the risk of self-inflicted or suicide due to delays in treatment.
Trial registration details
The trial protocol is registered with https://www.chictr.org.cn/ under protocol registration number ChiCTR2300071147 (date of registration: 05.06.2023). Recruitment will start in November 2023.
Similar content being viewed by others
Introduction
Background
Electroconvulsive therapy (ECT) stands out as an effective and rapid treatment for major depressive disorder (MDD) patients [1]. However, concerns about potential cognitive side effects (CSEs) deter patients from consenting to ECT [2]. While most deficits, including memory, executive function, attention, and processing speed, are transient [3, 4], sensitive assessment tools suggest that spatial recognition memory impairment can persist for more than a month following ECT [5, 6]. Furthermore, patients reported a deleterious effect on memory persisting 24 weeks post-ECT using Global Self-Evaluation—Memory [7, 8].
The underlying cause of cognitive impairment following ECT remains unclear. Hippocampal enlargement after ECT is posited to have a significant correlation with cognitive dysfunction [9,10,11,12], potentially attributable to the interference of neuroplastic alterations with existing synaptic connections following ECT [9, 10]. Besides, a consistently replicated finding points to reduced prefrontal cerebral blood flow (CBF) and metabolism post-ECT [13,14,15,16]. Moreover, challenging conventional perspectives, the question arises: are the antidepressant effects and the CSEs of ECT a consequence of the seizure, the electrical stimulation, or a combination of both [17,18,19]?
To mitigate CSEs, researchers have pursued various approaches. Significantly, advancements in electrode placement and electrical parameters, such as high dosage right unilateral ECT and ultra-brief pulse width ECT, have effectively reduced CSEs [20,21,22]. However, some studies indicate that compared to right unilateral ECT, bilateral ECT demonstrates higher efficacy and may offer a quicker onset of action [23,24,25]. Similarly, brief pulse width right unilateral ECT showed slightly higher effectiveness in treating depression with fewer sessions than ultra-brief pulse width right unilateral ECT [20]. In addition, extensive research has been dedicated to pharmacological interventions [26]. Notably, although promising medications like liothyronine [27], memantine [28, 29], and galantamine [30] have been reported to potentially alleviate CSEs, further rigorous trials are indispensable to verify their appropriateness for routine clinical application. In sum, exploring alternative strategies becomes imperative.
Transcranial direct current stimulation (tDCS) is a non-invasive, effective, and affordable brain stimulation tool with mild side effects [31, 32]. Consequently, tDCS has been widely applied in the field of neuropsychiatry to enhancing cognitive function. Studies have demonstrated that tDCS applied to frontal and temporal regions, with the most commonly stimulated site being the dorsolateral prefrontal cortex (DLPFC), can modulate specific neural circuits, thereby elicit a transdiagnostic enhancement of working memory and attention [33]. This effect has been observed in various conditions, including Depressive Disorder [34], Schizophrenia [35], Bipolar Disorder [36], Attention-Deficit/Hyperactivity Disorder [37], Alzheimer's disease [38] and Parkinson's disease [39]. Furthermore, some articles have revealed that anodal tDCS over the DLPFC can decrease response time [40, 41], improves executive function [42], boosts episodic memories [43, 44] and promotes other cognitive performance [45] in healthy participants. Additionally, the increased CBF [46,47,48] and cortical activity [49,50,51] induced by anodal stimulation are likely to improve the ECT-induced reduction in frontal CBF and metabolism [13, 14, 16]. However, such research remains largely unexplored to date. Therefore, utilization tDCS to ameliorate CSEs in ECT appears promising and merits further investigation.
Method
Objective
The primary objective of this study is to determine whether the implementation of active tDCS after completing the ECT course accelerates recovery from the CSEs, especially the spatial recognition memory.
Secondary, the study aims at examining the long-term (after 4 weeks) effects in improving cognitive function and the whether the implementation of tDCS after the ECT course decrease the relapse of depressive symptoms.
Study design and setting
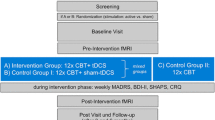
This is a randomized, double-blind, sham-controlled, interventional trial. 60 eligible participants will be recruited within two days after the last ECT session and randomly allocated into two groups in a 1:1 ratio: active tDCS (intervention group) and sham tDCS (control group). Assessments will be conducted at the following time points: before the first tDCS session (T0), immediately after the last tDCS session (T1), 2 weeks post-tDCS (T2), and 4 weeks post-tDCS (T3), using the Cambridge Neuropsychological Test Automated Battery (CANTAB). Table 2 shows schedule of enrolment, intervention, and assessments. Throughout the study, there was no interference or restriction for the patient ‘s physician in charge to adjust psychiatric medications according to the patient’ s condition. Figure 1 represents the research procedure schematically.
This study, approved by the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval Number: 2023–203) and registered with the China Clinical Trials Center (Registration Number: ChiCTR2300071147), will be conducted at one of Southwest China's largest hospitals, the First Affiliated Hospital of Chongqing Medical University, where approximately 40 to 60 patients receive ECT daily. The study design is in accordance with the 2013 Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement [52].
Recruitment
Participants will be recruited from the Psychiatry Department by the researchers. The study will be advertised on the hospital's website, and we will distribute leaflets to the patients two days prior to their discharge. Prior to screening, all potential participants will receive verbal and written explanations about the study's procedures and potential benefits or risks. Every participant will voluntarily sign the informed consent form (Supplementary Material: informed consent) and can withdraw from the trial at any time. Written informed consent will be obtained from all participants and their legal guardians before their formal inclusion in the study. Compliance will be improved by using WeChat (a popular social communication app in China) for appointment reminders and offering transportation cost reimbursements to reduce dropout among financially constrained participants.
Participants
Participants will be assessed based on inclusion and exclusion criteria highlighted in Table 1. Throughout the study, researchers may opt to discontinue a participant's involvement due to urgent medical circumstances or if the participant's condition changes to the extent that they no longer meet the eligibility criteria for participation.
Randomization
Random sequences will be generated by a statistician with no other connection to the trial using Microsoft Office Excel 2017. The randomization sequence list will remain concealed from the investigators. Allocation concealment will be achieved through consecutively numbered, opaque, and sealed envelopes. A research nurse, uninvolved in any other study procedures, will assign the participants to their group to ensure objectivity and minimize bias.
Blinding and unblinding
The tDCS treatment device consists of two parts: a computer and a small stimulator. To prevent participants from being directly aware of their group assignment, the treatment is carried out in a dedicated room, where the patient will sit on a couch positioned away from the computer and the stimulator will be placed in an opaque bag behind the patient. The psychiatrist administering tDCS knows the group assignments but cannot share this with participants or engage in the study’s evaluation or analysis. Additionally, the assessors and data analysts will be kept unaware of the intervention groups. To evaluate the adequacy of blinding procedures, participants will be invited to make an educated guess regarding their treatment assignment after the tDCS sessions.
If there is a suspicion of a severe adverse reaction or if clinical psychiatrists determine it is essential for participant safety, disclosure of group membership may be required.
Intervention
tDCS will be administered using a battery-driven direct current stimulator (EM8010S, Yimai Medical Technology Co., Ltd. Wuhan, China) via a pair of identical squares (3 × 5 cm) saline-soaked sponge electrodes secured in place with elastic bands. The anodal electrodes will be positioned over the F3 site, corresponding to the DLPFC following the EEG10– 20 international system. Conversely, the cathodal electrode will be placed above the Fp2 site, corresponding to the right supraorbital region. Within two days after completing the ECT course, all study participants will receive tDCS five times per week, once daily, for two consecutive weeks. In the active tDCS condition, each session will deliver a direct current of 2 mA for 30 min. In the sham tDCS condition, stimulation will be administered using the same active tDCS montage. The stimulation intensity will be set at 2 mA, but the current will be applied for 1 min with a 30-s ramp-up and ramp-down. In prior studies [53], this approach ensured participant blinding regarding the stimulation type (active vs. sham).
During the intervention, participants will continue their usual pharmacotherapy, but transcranial magnetic stimulation or other neuromodulation treatments are forbidden. The intervention will be discontinued if there are any severe adverse effects or withdrawal. This trial is deemed of minimal risk to study participants. Therefore, there are no provisions for ancillary or posttrial care.
ECT regimen
The ECT regimen, initially up to four times in the first week and then adjusted to three times weekly, is determined by the treating psychiatrist. It will be conducted using a Thymatron DGx apparatus, with bitemporal electrode placement 5 cm above the outer angle of the orbit. The initial electric charge is calculated using the half-age method, with subsequent dosages adjusted based on EEG seizure activity, increasing by 5% each session. The ECT uses square-wave stimulation at 900 mA, with 125 bidirectional pulses per second and a brief-pulse width of 1.5 ms. Anesthesia involves intravenous atropine (0.5 mg), propofol (2 mg/kg), and succinylcholine (1 mg/kg). Subsequent dosages will be adjusted according to the patient's response to achieve appropriate seizure morphology. We will assess seizure adequacy based on morphology and duration, including polyspike wave, 3-Hz spike and wave activity, and postictal suppression [54]. ECT effectiveness will be defined by induced electroencephalograph seizure durations exceeding 25 s [55].
Outcome
To assess cognitive changes, we will employ the CANTAB, a tool previously demonstrated to be highly sensitive in evaluating the cognitive side effects of ECT [5, 6]. Additionally, we have chosen a set of five tests designed to assess frontal lobe function. To mitigate potential learning effects, different parallel versions of the same test will be employed during various assessment phases. Assessments of cognitive function and depressive symptoms will be conducted before the first tDCS session (T0), immediately after the last tDCS session (T1), 2 weeks post-tDCS (T2), and 4 weeks post-tDCS (T3). The detailed visit procedure is shown in Table 2.
Primary outcome
The primary outcome measured is the change in Spatial Recognition Memory (SRM), which assesses spatial recognition memory using a forced-choice paradigm. In the first phase, participants are presented with a series of 5 white boxes, each at a different spatial location on the screen. During this phase, participants are instructed to remember the locations of these white boxes. In the second phase, two boxes are simultaneously presented. The target box occupies a location from the first phase, while the distractor box is placed in a previously unused location. Participants must recognize and click on the target box. This process is repeated four times, with new target and distractor locations in each test. The percentage of correct responses will be recorded as part of this assessment.
Second outcome
-
1.
Spatial Working Memory (SWM) assesses the subject's ability to retain and manipulate spatial information in working memory. The outcome measures include between errors, within errors, and strategy employed. A high strategy score represents poor use of strategy, and a low score equates to effective use.
-
2.
Verbal Recognition Memory (VRM) evaluates immediate and delayed verbal information memory. Outcome measures include the total number of correct words in the free recall phase, the total of all correctly identified words in the immediate recognition phase, and the delayed recognition phase.
-
3.
Rapid Visual Information Processing (RVP) assesses visual sustained attention. Outcome measures include mean latency and total correct responses.
-
4.
Stockings of Cambridge (SOC) evaluates spatial planning and spatial working memory. Outcome measures include the number of n-move problems solved in the minimum moves, mean moves for n-move problems, mean initial thinking time for n-move problems, and mean subsequent thinking time for n-move problems.
-
5.
Assessing the degree of depressive symptom with the Hamilton Depression Rating Scale (HAMD-24) [56] and the Self-rated Depression Scale (SDS) [57, 58].
Harms
All participants will complete the tDCS adverse effects questionnaire (tDCS-AEQ) after each tDCS session to evaluate potential adverse effects of the intervention. Common adverse reactions, as indicated by a systematic review [59], include tingling sensations, itching, mild skin redness, and discomfort in the stimulation area. Participants will rate their experienced adverse events on a 0 to 5 scale. Serious adverse events (SAEs) or reactions are considered unlikely based on the review. Only unexpected serious adverse events or reactions unrelated to these clinical procedures will be reported as SAEs.
Date collection methods and management
CANTAB is a computer-administered battery of tasks, and the primary outcome variables from the CANTAB tasks will be automatically calculated by the software. A clinical senior psychiatrist blinded to the group allocation will assess the severity of depressive symptoms using HAMD-24.
All participant demographic and scale related results will be recorded in the case report form (CRF). For patient privacy, irrelevant personal information like names, contact numbers, and addresses will be omitted during data entry, using patient numbers for identification. The original CRFs are securely stored and accessible only to the project leader and principal investigator.
Our trial does not have a data monitoring committee, but the subject group has a dedicated person to oversee. We will notify patients to come for tDCS via WeChat (a popular social communication app in China) and reimburse their transportation costs to improve patient compliance.
Statistical analysis
Sample size
Based on primary cognitive function outcome—the SRM correct rate [5], we calculated the sample size. Sample size was determined via PASS software (PASS 15, NCSS, LLC. Kaysville, UT, USA) for a power of 0.80 and a two-tailed α level of 0.05. Parameters were derived from a prior study [5] (mean SRM correct rate at 1-month follow-up: 64.67%, SE: 3.88, SD: 15.03, dropout rate: 37.5%). Calculations yielded permissible error (δ) = 16.20 (20% of mean), and σ = 15.03. Recruitment won't exceed original participants; thus, 24 patients per group are needed. Thus, 24 patients per group are needed. Based on a 20% dropout rate, each group ended up with 30 participants.
Statistical methods
To assess the normality or approximate normality of the data, we will employ a combination of histograms and the Shapiro–Wilk test. For normally or approximately normally distributed data, we will report the results as mean ± standard deviation (SD). If the data exhibit skewness, we will express the results as medians and quartiles. Categorical data will be presented as absolute numbers and percentages. Appropriate statistical tests, such as t-tests, nonparametric tests, or chi-square tests, were used in assessing differences in baseline characteristics between groups.
Consideration of repeated measurement data, we employed three strategies for presentation. Firstly, a line graph was utilized to depict values (whether rates or averages) at distinct time points. Secondly, we applied mixed regression models to assess both between-group and time effects for both primary and secondary outcomes. We will employ the stepwise selection approach to construct parsimonious regression models. These adjustments will account for additional factors that may potentially influence these associations, including age, ECT sessions, HAMD-24 score, SDS score, gender, current medication usage, disease duration, etc. Age and gender were entered in all models regardless of their statistical significance. Thirdly, we examined simple effects at different time points and adjusted p-values to reduce the risk of false positives.
No interim analysis and subgroup analysis will be performed. Transforming the data into a longitudinal format to meet the fitting requirements of mixed regression models. The statistical analysis of our data will be conducted using SPSS version 25.0 and R version 4.1.3. We define statistical significance as a two-tailed p-value of less than 0.05.
Protocol amendments
Significant protocol changes will be informed to the Ethics Committee. If approved, updates will be notified in writing to involved parties, and the Chinese Clinical Trial Registry record will be adjusted.
Dissemination policy
Before the first patient enrolls, trial details will be available on the Chinese Clinical Trial Registry. All kinds of results will be shared. They will be published and discussed in international conferences and peer-reviewed journals.
Discussion
This is the first RCT aimed at using tDCS to assist in the recovery of CSEs caused by ECT in depression patients. Given that CSEs might hinder patients from opting for ECT [60], identifying a rehabilitation strategy could assist patients in alleviating these concerns.
Depressive patients exhibit an imbalance in cortical metabolism between the two hemispheres, with lower metabolism observed in the left frontal lobe compared to the right frontal lobe [61, 62]. ECT can induce a short-term reduction of CBF and metabolism in frontal lobe, but this alteration subsequently normalizes within around one month [13].
By utilizing the anode at left DLPFC and the cathode at right DLPFC, tDCS aims to rectify the cortical imbalance in depression, ultimately ameliorating depressive symptoms [61]. Although the mechanisms underlying the impact of tDCS on cognitive function remain not entirely clear, early research suggests that tDCS may elicit long-term effects extending beyond the stimulation period [49, 63, 64]. Otherwise, these enduring effects are likely mediated through the modulation of N-methyl-D-aspartate and gamma-aminobutyric acid receptor activity [65, 66].
Cognitively, depression in the elderly is intricately linked with dementia; past studies have suggested that depressive symptoms might be a precursor to dementia [67, 68]. Despite improvements in depressive symptoms, elderly patients often continue to exhibit deficits in visuospatial ability, information-processing speed, and delayed memory [69]. This complexity adds to the challenge of distinguishing whether cognitive deficits in elderly patients post-ECT are due to ECT itself or pre-existing conditions. Further research indicates that while ECT treatment may lead to short-term cognitive decline in elderly patients [70], there is potential for long-term cognitive improvement [71]. Therefore, enhancing cognitive function during symptom remission in elderly patients with depression is of critical clinical importance, a goal that requires further dedicated efforts [72].
There are also limitations to this RCT. Firstly, our study did not include patients over the age of 65, a demographic with a significant need for cognitive improvement. Secondly, The lack of pre-ECT cognitive assessments in our study restricted our thorough evaluation of cognitive functions in patients with depression. Thirdly, due to financial constraints as an investigator-initiated study, there is no independent Trial Steering or Monitoring Committee. However, a research management group composed of study researchers will oversee daily operations and data authenticity. Fourthly, administering tDCS in a hospital poses challenges for discharged patients in terms of transportation, potentially leading to higher drop-out rates. lastly, the research is conducted at a single site, potentially limiting the external validity of the findings.
Availability of data and materials
Not applicable.
Abbreviations
- CANTAB:
-
Cambridge Neuropsychological Test Automated Battery
- CBF:
-
Cerebral blood flow
- DLPFC:
-
Dorsolateral prefrontal cortex
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- ECT:
-
Electroconvulsive therapy
- HAMD-24:
-
24-Item Hamilton Rating Scale for Depression
- MDD:
-
Major depressive disorder
- RCT:
-
Randomized controlled trial
- RVP:
-
Rapid visual information processing
- SDS:
-
Self-rated depression scale
- SRM:
-
Spatial recognition memory
- SWM:
-
Spatial working memory
- SD:
-
Standard deviation
- tDCS:
-
Transcranial direct current stimulation
- tDCS-AEQ:
-
Transcranial direct current stimulation adverse effects questionnaire
References
Espinoza RT, Kellner CH. Electroconvulsive Therapy. N Engl J Med. 2022;386(7):667–72.
Brown SK, Nowlin RB, Sartorelli R, Smith J, Johnson K. Patient experience of Electroconvulsive Therapy: a retrospective review of clinical outcomes and satisfaction. J ECT. 2018;34(4):240–6.
Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568–77.
Andrade C, Arumugham SS, Thirthalli J. Adverse effects of Electroconvulsive Therapy. Psychiatr Clin North Am. 2016;39(3):513–30.
Falconer DW, Cleland J, Fielding S, Reid IC. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB) to assess the cognitive impact of electroconvulsive therapy on visual and visuospatial memory. Psychol Med. 2010;40(6):1017–25.
Fernie G, Bennett DM, Currie J, Perrin JS, Reid IC. Detecting objective and subjective cognitive effects of electroconvulsive therapy: intensity, duration and test utility in a large clinical sample. Psychol Med. 2014;44(14):2985–94.
Berman RM, Prudic J, Brakemeier EL, Olfson M, Sackeim HA. Subjective evaluation of the therapeutic and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(1):16–26.
Brakemeier EL, Berman R, Prudic J, Zwillenberg K, Sackeim HA. Self-evaluation of the cognitive effects of electroconvulsive therapy. J ect. 2011;27(1):59–66.
Van der Jager AJ, van Dellen JE, Mandl E, Somers RCW, Boks M, Sommer MPM, Nuninga IEC. Changes in perfusion, and structure of hippocampal subfields related to cognitive impairment after ECT: a pilot study using ultra high field MRI. J Affect Disord. 2023;325:321–8.
van Oostrom I, van Eijndhoven P, Butterbrod E, van Beek MH, Janzing J, Donders R, Schene A, Tendolkar I. Decreased cognitive functioning after Electroconvulsive Therapy is related to increased hippocampal volume: exploring the role of Brain plasticity. J ect. 2018;34(2):117–23.
Gryglewski G, Lanzenberger R, Silberbauer LR, Pacher D, Kasper S, Rupprecht R, Frey R, Baldinger-Melich P. Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul. 2021;14(4):927–37.
Argyelan M, Lencz T, Kang S, Ali S, Masi PJ, Moyett E, Joanlanne A, Watson P, Sanghani S, Petrides G, et al. ECT-induced cognitive side effects are associated with hippocampal enlargement. Transl Psychiatry. 2021;11(1):516.
Abbott CC, Gallegos P, Rediske N, Lemke NT, Quinn DK. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33–46.
Schmidt EZ, Reininghaus B, Enzinger C, Ebner C, Hofmann P, Kapfhammer HP. Changes in brain metabolism after ECT-positron emission tomography in the assessment of changes in glucose metabolism subsequent to electroconvulsive therapy–lessons, limitations and future applications. J Affect Disord. 2008;106(1–2):203–8.
Singh A, Kar SK. How electroconvulsive therapy works? Understanding the neurobiological mechanisms. Clin Psychopharmacol Neuroscience: Official Sci J Korean Coll Neuropsychopharmacol. 2017;15(3):210–21.
Bolwig TG. Neuroimaging and electroconvulsive therapy: a review. J ECT. 2014;30(2):138–42.
Deng ZD, Robins PL, Regenold W, Rohde P, Dannhauer M, Lisanby SH. How electroconvulsive therapy works in the treatment of depression: is it the seizure, the electricity, or both? Neuropsychopharmacology. 2024;49(1):150–62.
Regenold WT, Noorani RJ, Piez D, Patel P. Nonconvulsive Electrotherapy for Treatment Resistant Unipolar and bipolar major depressive disorder: a proof-of-concept Trial. Brain Stimul. 2015;8(5):855–61.
Sackeim HA. Is the Seizure an unnecessary component of Electroconvulsive Therapy? A startling possibility. Brain Stimul. 2015;8(5):851–4.
Tor PC, Bautovich A, Wang MJ, Martin D, Harvey SB, Loo C. A systematic review and Meta-analysis of brief Versus Ultrabrief Right Unilateral Electroconvulsive Therapy for Depression. J Clin Psychiatry. 2015;76(9):e1092-1098.
Verwijk E, Spaans HP, Comijs HC, Kho KH, Sienaert P, Bouckaert F, Obbels J, Scherder EJ, Stek ML, Kok RM. Relapse and long-term cognitive performance after brief pulse or ultrabrief pulse right unilateral electroconvulsive therapy: a multicenter naturalistic follow up. J Affect Disord. 2015;184:137–44.
Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, Berman RM, Brakemeier EL, Perera T, Devanand DP. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71–83.
Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, McClintock SM, Tobias KG, Martino C, Mueller M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry: J Mental Sci. 2010;196(3):226–34.
Fink M. What was learned: studies by the consortium for research in ECT (CORE) 1997–2011. Acta Psychiatr Scand. 2014;129(6):417–26.
Kellner CH, Cicek M, Ables JL. Letter to the editor: electrode placement in electroconvulsive therapy - bilateral is still the ‘gold standard’ for some patients. Psychol Med. 2017;47(8):1510–1.
Verdijk J, van Kessel MA, Oud M, Kellner CH, Hofmeijer J, Verwijk E, van Waarde JA. Pharmacological interventions to diminish cognitive side effects of electroconvulsive therapy: a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;145(4):343–56.
Mohagheghi A, Arfaie A, Amiri S, Nouri M, Abdi S, Safikhanlou S. Preventive effect of liothyronine on electroconvulsive therapy-induced memory deficit in patients with major depressive disorder: a double-blind controlled clinical trial. Biomed Res Int. 2015;2015: 503918.
Abbasinazari M, Adib-Eshgh L, Rostami A, Beyraghi N, Dabir S, Jafari R. Memantine in the prevention or alleviation of electroconvulsive therapy induces cognitive disorders: a placebo controlled trial. Asian J Psychiatr. 2015;15:5–9.
Alizadeh NS, Maroufi A, Jamshidi M, Hassanzadeh K, Gharibi F, Ghaderi E. Effect of Memantine on Cognitive performance in patients under Electroconvulsive Therapy: a double-blind Randomized Clinical Trial. Clin Neuropharmacol. 2015;38(6):236–40.
Matthews JD, Siefert CJ, Blais MA, Park LT, Siefert CJ, Welch CA, Dubois CM, van Nieuwenhuizen AO, Rooney KO, Seabrook RC, et al. A double-blind, placebo-controlled study of the impact of galantamine on anterograde memory impairment during electroconvulsive therapy. J ECT. 2013;29(3):170–8.
Moffa AH, Brunoni AR, Fregni F, Palm U, Padberg F, Blumberger DM, Daskalakis ZJ, Bennabi D, Haffen E, Alonzo A, et al. Safety and acceptability of transcranial direct current stimulation for the acute treatment of major depressive episodes: analysis of individual patient data. J Affect Disord. 2017;221:1–5.
Aparício LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR. A systematic review on the acceptability and tolerability of Transcranial Direct current stimulation treatment in neuropsychiatry trials. Brain Stimul. 2016;9(5):671–81.
Begemann MJ, Brand BA, Ćurčić-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. 2020;50(15):2465–86.
Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry: J Mental Sci. 2012;200(1):52–9.
Mondino M, Brunelin J, Palm U, Brunoni AR, Poulet E, Fecteau S. Transcranial Direct Current Stimulation for the Treatment of Refractory Symptoms of Schizophrenia. Current evidence and future directions. Curr Pharm Design. 2015;21(23):3373–83.
Bersani FS, Minichino A, Bernabei L, Spagnoli F, Corrado A, Vergnani L, Mannarelli D, Pauletti C, Fattapposta F, Biondi M, et al. Prefronto-cerebellar tDCS enhances neurocognition in euthymic bipolar patients. Findings from a placebo-controlled neuropsychological and psychophysiological investigation. J Affect Disord. 2017;209:262–9.
Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neuroscience. 2021;46(1):E14-e33.
Cai M, Guo Z, Xing G, Peng H, Zhou L, Chen H, McClure MA, He L, Xiong L, He B, et al. Transcranial Direct Current Stimulation improves cognitive function in mild to moderate Alzheimer Disease: a Meta-analysis. Alzheimer Dis Assoc Disord. 2019;33(2):170–8.
Cammisuli DM, Cignoni F, Ceravolo R, Bonuccelli U, Castelnuovo G. Transcranial Direct Current Stimulation (tDCS) as a useful Rehabilitation Strategy to improve cognition in patients with Alzheimer’s Disease and Parkinson’s Disease: an updated systematic review of Randomized controlled trials. Front Neurol. 2021;12:798191.
Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA. A systematic review and Meta-analysis of the effects of Transcranial Direct Current Stimulation (tDCS) over the Dorsolateral Prefrontal cortex in healthy and neuropsychiatric samples: influence of Stimulation parameters. Brain Stimul. 2016;9(4):501–17.
Dubreuil-Vall L, Chau P, Ruffini G, Widge AS, Camprodon JA. tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 2019;12(6):1456–63.
Miler JA, Meron D, Baldwin DS, Garner M. The Effect of Prefrontal Transcranial Direct current stimulation on attention network function in healthy volunteers. Neuromodulation: J Int Neuromodulation Soc. 2018;21(4):355–61.
Sandrini M, Manenti R, Gobbi E, Rusich D, Bartl G, Cotelli M. Transcranial direct current stimulation applied after encoding facilitates episodic memory consolidation in older adults. Neurobiol Learn Mem. 2019;163: 107037.
Sandrini M, Manenti R, Brambilla M, Cobelli C, Cohen LG, Cotelli M. Older adults get episodic memory boosting from noninvasive stimulation of prefrontal cortex during learning. Neurobiol Aging. 2016;39:210–6.
Bashir S, Al-Hussain F, Hamza A, Asim Niaz T, Albaradie R, Habib SS. Cognitive function assessment during 2 mA transcranial direct current stimulation in DLPFC in healthy volunteers. Physiological Rep. 2019;7(20):e14264.
Sherwood MS, Madaris AT, Mullenger CR, McKinley RA. Repetitive Transcranial Electrical Stimulation Induces Quantified Changes in Resting Cerebral Perfusion Measured from Arterial Spin Labeling. Neural Plast. 2018;2018: 5769861.
Baeken C, Remue J, Vanderhasselt MA, Brunoni AR, De Witte S, Duprat R, Koster EHW, De Raedt R, Wu GR. Increased left prefrontal brain perfusion after MRI compatible tDCS attenuates momentary ruminative self-referential thoughts. Brain Stimul. 2017;10(6):1088–95.
Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neuroscience. 2013;33(28):11425–31.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of physiology. 2000;527(Pt 3):633–9.
Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22(2):495–504.
Muccio M, Walton Masters L, Pilloni G, He P, Krupp L, Datta A, Bikson M, Charvet L, Ge Y. Cerebral metabolic rate of oxygen (CMRO(2)) changes measured with simultaneous tDCS-MRI in healthy adults. Brain Res. 2022;1796: 148097.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, Cohen LG, Dowthwaite G, Ellrich J, Flöel A, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiology. 2017;128(9):1774–809.
Porter RJ, Douglas K, Knight RG. Monitoring of cognitive effects during a course of electroconvulsive therapy: recommendations for clinical practice. J ect. 2008;24(1):25–34.
Kranaster L, Hoyer C, Janke C, Sartorius A. Bispectral index monitoring and seizure quality optimization in electroconvulsive therapy. Pharmacopsychiatry. 2013;46(4):147–50.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62.
Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13(6):508–15.
Zung WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry. 1965;12:63–70.
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133–45.
Sackeim HA. Modern electroconvulsive therapy: vastly improved yet greatly underused. JAMA Psychiat. 2017;74(8):779–80.
Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, Brunelin J, Nakamura-Palacios EM, Marangolo P, Venkatasubramanian G, et al. Evidence-based guidelines and secondary Meta-analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric disorders. Int J Neuropsychopharmacol. 2021;24(4):256–313.
Brunoni AR, Ferrucci R, Fregni F, Boggio PS, Priori A. Transcranial direct current stimulation for the treatment of major depressive disorder: a summary of preclinical, clinical and translational findings. Prog Neuro-psychopharmacol Biol Psychiatry. 2012;39(1):9–16.
Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15(4):619–26.
Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901.
Leow LA, Marcos A, Nielsen E, Sewell D, Ballard T, Dux PE, Filmer HL. Dopamine alters the Effect of Brain Stimulation on decision-making. J Neuroscience: Official J Soc Neurosci. 2023;43(41):6909–19.
Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex (New York NY: 1991). 2008;18(3):648–51.
Wang S, Blazer DG. Depression and cognition in the elderly. Ann Rev Clin Psychol. 2015;11:331–60.
Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimäki M, Sabia S. Trajectories of depressive symptoms before diagnosis of dementia: a 28-Year follow-up study. JAMA Psychiat. 2017;74(7):712–8.
Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF 3rd, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatric Psychiatr. 2006;14(5):419–27.
Lisanby SH, McClintock SM, Alexopoulos G, Bailine SH, Bernhardt E, Briggs MC, Cullum CM, Deng ZD, Dooley M, Geduldig ET, et al. Neurocognitive effects of Combined Electroconvulsive Therapy (ECT) and Venlafaxine in Geriatric Depression: phase 1 of the PRIDE study. Am J Geriatric Psychiatry. 2020;28(3):304–16.
Lisanby SH, McClintock SM, McCall WV, Knapp RG, Cullum CM, Mueller M, Deng ZD, Teklehaimanot AA, Rudorfer MV, Bernhardt E, et al. Longitudinal Neurocognitive effects of Combined Electroconvulsive Therapy (ECT) and pharmacotherapy in major depressive disorder in older adults: phase 2 of the PRIDE study. Am J Geriatric Psychiatry: Official J Am Association Geriatric Psychiatry. 2022;30(1):15–28.
Kumar S, Mulsant BH, Liu AY, Blumberger DM, Daskalakis ZJ, Rajji TK. Systematic review of Cognitive effects of Electroconvulsive Therapy in Late-Life Depression. Am J Geriatric Psychiatry: Official J Am Association Geriatric Psychiatry. 2016;24(7):547–65.
Acknowledgements
The authors wish to express their gratitude to both the study participants and research assistants for their valuable dedication and substantial contributions.
Trial status
Recruitment is scheduled to commence in November 2023.
Funding
This study was supported by the Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0107). The funders played no part in shaping the study's design, data gathering, data analysis, data interpretation, or manuscript composition. This protocol has not undergone independent peer review from the funding body.
Author information
Authors and Affiliations
Contributions
RH and QL were involved in the study's conceptualization, design, and manuscript writing. YL, JL and HL managed the database. Statistical analysis was conducted by YZ, XW, and ZZ. The initial manuscript draft was prepared by RH. All authors participated in manuscript revisions, reviewed, and approved the final submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate: the study was approved by the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval Number: 2023–203). An independent ethical advisor, appointed by the Research Ethics Committee, fulfills the role of the subject group representative. We complied to the Declaration of Helsinki. Written informed consent was obtained from all participants and their legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Informed Consent Form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, R., Li, J., Lu, Y. et al. The effect of transcranial direct current stimulation (tDCS) on cognitive function recovery in patients with depression following electroconvulsive therapy (ECT): protocol for a randomized controlled trial. BMC Psychiatry 24, 130 (2024). https://doi.org/10.1186/s12888-024-05567-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05567-9