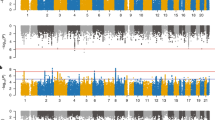
Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can invade both the peripheral and central nervous systems and impact the function of the brain. Therefore, it is necessary to evaluate the mutual influences between COVID-19 outcomes and childhood mental disorders.
Methods
We examined genetic correlations and potential causalities between three childhood mental disorders and three COVID-19 phenotypes by genetically proxied analyses. The three mental disorders included attention-deficit/hyperactivity disorder (ADHD, N = 292,548), Tourette’s syndrome (TS, N = 14,307), and autism spectrum disorder (ASD, N = 46,350). The three COVID-19 traits included SARS-CoV-2 infection (N = 2,597,856), hospitalized COVID-19 (N = 2,095,324), and critical COVID-19 (N = 1,086,211). Literature-based analysis was used to build gene-based pathways connecting ADHD and COVID-19.
Results
ADHD was positively correlated with the three COVID-19 outcomes (Rg: 0.22 ~ 0.30). Our Mendelian randomization (MR) analyses found that ADHD confers a causal effect on hospitalized COVID-19 (odds ratio (OR): 1.36, 95% confidence interval (CI): 1.10–1.69). TS confers a causal effect on critical COVID-19 (OR: 1.14, 95% CI: 1.04–1.25). Genetic liability to the COVID-19 outcomes may not increase the risk for the childhood mental disorders. Pathway analysis identified several immunity-related genes that may link ADHD to COVID-19, including CRP, OXT, IL6, PON1, AR, TNFSF12, and IL10.
Conclusions
Our study suggests that both ADHD and TS may augment the severity of COVID-19 through immunity-related pathways. However, our results did not support a causal role of COVID-19 in the risk for the childhood mental disorders.
Similar content being viewed by others
Introduction
COVID-19 has created a worldwide pandemic. It has been documented that the SARS-CoV-2 virus is neurotropic and neuroinvasive. In addition to the core symptoms from the respiratory system, neuropsychiatric manifestations are also common in COVID-19 patients [1,2,3,4]. Therefore, COVID-19 can adversely impact the brain function of patients, especially those with neuropsychiatric disorders [5,6,7,8,9,10].
With the ongoing spread of the pandemic, a significant proportion of infected individuals developed a variety of post-COVID symptoms, collectively known as “long COVID”. Among the various post-COVID complications, it has been reported that psychiatric patients are rising after the pandemic [11, 12], suggesting that coronavirus contributes to psychiatric symptoms and mental disorders through its ability to induce damage to neuron-glia homeostasis [13]. On the other hand, individuals with brain diseases may be more vulnerable to the impact of coronavirus, leading to severe outcomes after the infection.
A vital concern arises for children affected by the infection, considering the ongoing neurodevelopment and resultant vulnerability to the disturbances of the central nervous system (CNS) [14]. Earlier evidence showed that COVID-19 is less common in children and presents milder symptoms after the infection [15]. Despite this, COVID-19 severely affected children’s and adolescents’ mental health [16].
A recent antibody survey found that most (2/3) US children and adolescents aged 1–17 were exposed to the coronavirus [17]. The infection rate in children aged 1–4 exceeds those observed in adults during the Omicron wave [18]. Mental disorders, including those that occurred in childhood, are caused by a variety of neuroendocrine alterations, which may adversely influence the outcome of COVID-19. Autism spectrum disorder (ASD), Tourette’s syndrome (TS), and attention-deficit/hyperactivity disorder (ADHD) are three severe neurodevelopmental disorders that occur in children and possess a high heritability [19, 20]. Collectively, they may account for 15 ~ 30% of the disability-adjusted life-years, causing a substantial disability in this age group [21, 22]. They also have substantial phenotypic overlaps and shared genetic underpinnings with one another [23,24,25,26,27,28].
So far, evidence supporting associations between mental disorders and COVID-19 chiefly came from observational studies [29]. The causality between mental disorders and COVID-19 has yet to be explored. The Mendelian randomization (MR) framework infers potential causative associations between risk factors (exposures) and diseases (outcomes) by using genetic variants associated with exposure as instrumental variables [30]. The MR analysis is a widely used method to test causality between two traits [31, 32].
It’s not known whether the pathophysiological changes in children’s brains may exacerbate the process of COVID-19, or if childhood mental disorders could be triggered by outcomes of COVID-19. In this study, our objective was to assess the potential genetic connections between COVID-19 and three childhood mental disorders: ADHD, ASD, and TS. We hypothesize that specific genetic ties connect these mental health conditions and phenotypes of COVID-19. Gaining insights into these connections may contribute to improving both the management of COVID-19 and the care of individuals affected by these disorders.
Methods
GWAS summary datasets
Publicly available GWAS summary results on COVID-19 and three childhood mental disorders were used in this study. The three mental disorders included ADHD (38,691 cases and 275,986 controls) [33], ASD (18,381 cases and 27,969 controls) [34], and TS (4,819 cases and 9,488 controls) [35]. The summary GWAS datasets of COVID-19 were downloaded from the COVID-19 Host Genetics Initiative (HGI) (release on April 8, 2022), including critical COVID-19 (13,769 critically ill patients and 1,072,442 controls), hospitalized COVID-19 (32,519 hospitalized patients and 2,062,805 controls), and SARS-CoV-2 infection (122,616 virus-positive cases and 2,475,240 controls) [36]. All participants in the datasets were of European origin. Both critical COVID-19 and hospitalized COVID-19 were called “severe COVID-19” in this study.
Genetic correlation analysis
The genetic correlations between the three mental disorders and the COVID-19 outcomes were assessed via linkage disequilibrium (LD) score regression [37, 38]. P values were adjusted by the false discovery rate (FDR < 0.05).
MR analysis
The MR analysis has three assumptions on an instrumental variable (IV): (1) it needs to be associated with the exposure; (2) it cannot be associated with confounding factors influencing the exposure and the outcome; (3) it can only indirectly influence the outcome by its effect on the exposure [39]. Causal effects were inferred by three models in the TwoSampleMR package (version 0.5.6) [40], including the inverse variance weighting (IVW) model, the weighted median (WM) model, and the MR-Egger model, as complementary measures ensuring sensitivity [40, 41]. The IVW model operates under the assumption of zero intercepts and offers reliable estimates of causality via a fixed-effects meta-analysis approach. On the other hand, the MR-Egger model assumes that pleiotropic effects are independent and employs weighted linear regression to analyze outcome coefficients in relation to exposure coefficients. The pleiotropy is assessed by the intercepts of the MR-Egger regression [42]. If the MR-Egger intercepts significantly deviate from zero, it suggests that not all instrumental variables (IVs) are effective. The heterogeneity was evaluated by Cochran’s Q test and I2 statistics (P < 0.05 and I2 > 0.25). P values of the causal associations between COVID-19 and the mental disorders were adjusted by FDR (< 0.05). For each exposure phenotype, genome-wide significant (P < 5 × 10 –8) SNPs (single nucleotide polymorphisms) associated with the exposure were pruned by a clumping r2 value of 0.01 within a 10 Mb window and used as IVs. When the IVs were less than 10, a relatively relaxed threshold of 1 × 10− 5 was used to pick IVs [43]. For the ASD and TS GWAS datasets, the threshold of 1 × 10− 5 was employed for IV selection.
Literature-based analysis
To explore biological connections between ADHD and COVID-19, we utilized the Pathway Studio (www.pathwaystudio.com) environment to conduct literature-based data mining to create molecular pathways linking ADHD with COVID-19 [8, 44]. The Pathway Studio platform curated > 40 million scientific references, containing > 14 million unique associations. Initially, we identified the downstream targets and upstream regulators associated with both ADHD and COVID-19. Subsequently, we conducted a manual review of the references and each of the related sentences to ensure the quality of each extracted relationship. Relationships lacking polarity or those indirectly related to either COVID-19 or ADHD were eliminated. The remaining relationships were then utilized to construct a molecular pathway map that illustrates the connections between ADHD and COVID-19.
Results
Genetic correlation analysis
Our genetic correlation analyses showed that ADHD has significant positive genetic correlations with SARS-CoV-2 infection (rg = 0.22 ± 0.05, P = 1.86E-06), hospitalized COVID-19 (rg = 0.23 ± 0.04, P = 1.20E-07), and critical COVID-19 (rg = 0.30 ± 0.05, P = 3.09E-09). ASD and TS did not display genetic correlations with the COVID-19 outcomes (Table 1).
MR analysis
In the causal effect analysis of the mental disorders on the COVID-19 phenotypes, 26 IVs were yielded for ADHD (P < 5 × 10–8), 56–58 IVs for ASD (P < 1 × 10–5), and 36 IVs for TS (P < 1 × 10–5). The three COVID-19 datasets had different numbers of SNPs and the IVs were selected from the shared variants between an exposure and an outcome. Therefore, the numbers of IVs may vary across the three MR analyses, depending on a given exposure.
We found that ADHD confers a causal effect on hospitalized COVID-19 (OR: 1.36, 95% confidence interval (CI): 1.10–1.69). TS confers a causal effect on critical COVID-19 (OR: 1.14, 95% CI: 1.04–1.25) (Table 2; Fig. 1A). However, genetic liability to ASD did not have causal effects on the COVID-19 outcomes.
In the causal effect analysis of the COVID-19 conditions on the childhood mental disorders, we extracted 21–23 IVs for SARS-CoV-2 infection, 32–38 IVs for hospitalized COVID-19, and 32–37 IVs for critical COVID-19. We found that SARS-CoV-2 infection, critical COVID-19, and hospitalized COVID-19 have no causal effects on any of the mental disorders (Table 3; Fig. 1B).
The sensitivity analyses with different models indicated that the causal effects had the same directions across the three methods (Supplementary Tables 1–2). The MR-Egger result did not detect the pleiotropy in the MR analysis (MR-Egger intercept ≤ 0.011, P > 0.05). We found minimal evidence supporting the existence of heterogeneity, especially for the significant causal associations (Cochran’s P > 0.05 or I2 < 0.25).
Literature-based analysis
Literature-based data mining and construction of the molecular pathways revealed a total of seven genes connecting ADHD with COVID-19, including CRP, PON1, AR, OXT, IL6, TNFSF12, and IL10. (Fig. 2). Among these connections, ADHD exerts a promotion effect on COVID-19 through the inhibition of OXT and PON1 and the promotion of CRP, AR, and TNF.
Discussion
Previous studies suggested that mental disorders and COVID-19 are mutual risk factors for one another, with the underlying mechanisms being largely unknown [45,46,47]. We carried out bidirectional MR analyses to detect causal connections between COVID-19 and three childhood mental disorders.
Our MR analysis provides robust evidence for the causal role of ADHD in the risk of COVID-19. Our results indicated that genetically determined ADHD was associated with a 36% enhanced risk for COVID-19 hospitalization. To date, only one MR study reported the causal influence of ADHD on COVID-19 hospitalization (OR: 1.297 [1.029–1.634], P = 0.028) [48]. Our results corroborated the previous finding with more robust associations using the largest ADHD dataset and the largest COVID-19 datasets. The positive genetic correlations between ADHD and the COVID-19 phenotypes provide additional evidence for their close relationship. It was suggested that ADHD seems to constitute a behavioral risk factor both for SARS-CoV-2 infection and for severe outcomes of COVID-19 [49].
For TS, our findings showed that TS was associated with a 14% increased risk for critical COVID-19. Our results support that ADHD and TS may exacerbate the pathophysiology of COVID-19. Although TS has the highest comorbidity rate with ADHD [25], studies on the TS-COVID-19 connection were sparse. Therefore, we focused on ADHD for further discussion.
In the case of ASD, a 40% longer SARS-CoV-2-related mean hospital stays were noted [50], and a recent study also showed that COVID-19 has had psychological effects and led to increased difficulties among children with ASD [51]. However, our study did not support causal associations between ASD and COVID-19 outcomes in the context of genetic underpinning. ADHD and ASD co-occur commonly and have phenotypic overlaps and shared genetic components [24, 28, 52,53,54]. The two disorders also have different genetic properties, including their opposite genetic correlations with intelligence [55].
As a vital adverse exposure, COVID-19 pandemics aggravated individual life trajectories both in patients infected with SARS-CoV-2 and in virus-naïve bystanders exposed to pandemic-related stress, likely contributing to the accumulation of mental diagnoses in the general population. The unfavorable influences of COVID-19 on ADHD and ASD have been well documented [56,57,58]. In this study, our study indicated that COVID-19 may not be associated with the risk for the childhood mental disorders. Therefore, the increased symptoms of ADHD and ASD associated with COVID-19 may presumably be due to socio-psychological or environmental aspects of the pandemic [56].
To explore possible mechanisms underlying their connection, we created gene-based pathways linking ADHD and COVID-19. The constructed pathways support this MR analysis result at the molecular level. SARS-CoV2 infection has been shown to promote tumor necrosis factor-alpha (TNF-α) expression and secretion [59]. In addition, C-reactive protein (CRP) was frequently found elevated in COVID-19 patients [60], including children with severe MIS-C [61]. Studies have shown that increased CRP levels are positively correlated with the severity of COVID-19 [62]. Interestingly, non-critical COVID-19 patients were found with a significantly increased level of serum adrenocorticotropic hormone (ACTH) [63]. Notably, the levels of ACTH differentiate non-critical COVID-19 patients from non-critical ones [63, 64], possibly pointing at pre-existing or developing adrenal insufficiency associated with a severe form of COVID-19 [65]. On the other hand, in ADHD patients, the HPA axis may be under-reactive, thus, predisposing them to the severity of the coronavirus disease. Moreover, recombinant ACTH has been reported to improve symptom severity in ADHD [66].
Patients with ADHD commonly present with significantly decreased concentrations of oxytocin (encoded by OXT) [67]. Coupaye et al’s work showed that the administration of oxytocin may impede the progression to severe COVID-19 by suppressing cytokine storm and blocking viral invasion [68]. Therefore, oxytocin has been suggested as a candidate for treating COVID-19 [69]. By the depressing expression of OXT, ADHD may increase the propensity of an individual to develop severe COVID-19 and to be hospitalized [70]. In addition, the role of CRP in ADHD was also suggested [71]. This implies that ADHD-related increases in systemic CRP concentrations [72] may play a role in pulmonary fibrosis in COVID-19 [73]. To sum up, the investigation of molecular pathways supported our MR-facilitated inference of the causal effects of genetic liability to ADHD on severe COVID-19. These conclusions are consistent with the clinical observation that ADHD was associated with poorer outcomes after the SARS-CoV-2 infection [49].
A limitation of this study is the omission of the records of medication, potentially introducing unaccounted variability into the dataset. For a comprehensive understanding, the results of the current MR study should be taken into account alongside available clinical evidence. An additional constraint is that MR analysis solely investigates the causal relationship at the genetic level. To comprehend the comprehensive connections between COVID-19 and mental health disorders, it is essential to consider psychosocial factors and environmental variables as potential mediators. Lastly, direct laboratory data is essential to corroborate the literature-based molecular pathway.
Conclusions
In summary, our study showed that both ADHD and TS may aggravate the severity of COVID-19, while COVID-19 may not directly contribute to the risk of the childhood mental disorders.
Data availability
All de-identified data, including individual participant data, are publicly available. The COVID-19 datasets were available in the COVID-19 Host Genetics Initiative (https://www.covid19hg.org/results/r7/). The datasets for the three mental disorders were available in the Psychiatric Genomics Consortium (https://pgc.unc.edu/for-researchers/download-results/).
References
Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of Coronavirus Disease 2019: a review. JAMA Neurol. 2020;77(8):1018–27.
Murata F, Maeda M, Ishiguro C, Fukuda H. Acute and delayed psychiatric sequelae among patients hospitalised with COVID-19: a cohort study using LIFE study data. Gen Psychiatr. 2022;35(3):e100802.
Sahin BE, Celikbilek A, Kocak Y, Ilanbey B, Saltoglu GT, Konar NM, Hizmali L. Neurological symptoms and neuronal damage markers in acute COVID-19: is there a correlation? A pilot study. J Med Virol. 2023;95(1):e28240.
Dai X, Cao X, Jiang Q, Wu B, Lou T, Shao Y, Hu Y, Lan Q. Neurological complications of COVID-19. QJM 2023, 116(3):161–180.
Merza MA, Almufty HB, Younis HA, Rasool SO, Mohammed SA. Memory impairment among recovered COVID-19 patients: the prevalence and risk factors, a retrospective cohort study. J Med Virol. 2023;95(2):e28459.
Baranova A, Zhao Y, Cao H, Zhang F. Causal associations between major depressive disorder and COVID-19. Gen Psychiatr. 2023;36(2):e101006.
Zandy M, El Kurdi S, Samji H, McKee G, Gustafson R, Smolina K. Mental health-related healthcare service utilisation and psychotropic drug dispensation trends in British Columbia during COVID-19 pandemic: a population-based study. Gen Psychiatr. 2023;36(1):e100941.
Baranova A, Cao H, Teng S, Su KP, Zhang F. Shared genetics and causal associations between COVID-19 and multiple sclerosis. J Med Virol. 2023;95(1):e28431.
Cao H, Baranova A, Song Y, Chen JH, Zhang F. Causal associations and genetic overlap between COVID-19 and intelligence. QJM. 2023;116(9):766–73.
Baranova A, Cao H, Zhang F. Causal effect of COVID-19 on Alzheimer’s Disease: a mendelian randomization study. J Med Virol. 2023;95(1):e28107.
Dragioti E, Li H, Tsitsas G, Lee KH, Choi J, Kim J, Choi YJ, Tsamakis K, Estrade A, Agorastos A, et al. A large-scale meta-analytic atlas of mental health problems prevalence during the COVID-19 early pandemic. J Med Virol. 2022;94(5):1935–49.
Chow CM, Schleyer W, DeLisi LE. The prevalence of psychiatric symptoms and their correlates as part of the long-COVID syndrome. Psychiatry Res. 2023;323:115166.
Savelieff MG, Feldman EL, Stino AM. Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 Infection. Neurobiol Dis. 2022;168:105715.
Cost KT, Crosbie J, Anagnostou E, Birken CS, Charach A, Monga S, Kelley E, Nicolson R, Maguire JL, Burton CL, et al. Mostly worse, occasionally better: impact of COVID-19 pandemic on the mental health of Canadian children and adolescents. Eur Child Adolesc Psychiatry. 2022;31(4):671–84.
Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, Zhang J, Dong C, Na R, Zheng L, et al. A systematic review and meta-analysis of children with coronavirus Disease 2019 (COVID-19). J Med Virol. 2021;93(2):1057–69.
Bai MS, Miao CY, Zhang Y, Xue Y, Jia FY, Du L. COVID-19 and mental health disorders in children and adolescents (review). Psychiatry Res. 2022;317:114881.
Clarke KEN, Kim Y, Jones J, Lee A, Deng Y, Nycz E, Iachan R, Gundlapalli A, MacNeil A, Hall AJ. Pediatric infection-Induced SARS-CoV-2 Seroprevalence Estimation using commercial laboratory specimens: how Representative is it of the General U.S. Pediatric Population? SSRN: https://ssrncom/abstract=4092074 2022.
Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Incidence rates and clinical outcomes of SARS-CoV-2 Infection with the Omicron and Delta Variants in children younger than 5 years in the US. JAMA Pediatr 2022.
Brainstorm C, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Escott-Price V, Falcone GJ, Gormley P et al. Analysis of shared heritability in common disorders of the brain. Science 2018, 360(6395).
Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47(7):702–9.
Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, Rohde LA, Srinath S, Ulkuer N, Rahman A. Child and adolescent mental health worldwide: evidence for action. Lancet. 2011;378(9801):1515–25.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, et al. Global burden of Disease attributable to mental and substance use disorders: findings from the global burden of Disease Study 2010. Lancet. 2013;382(9904):1575–86.
Pettersson E, Lichtenstein P, Larsson H, Song J, Attention Deficit/Hyperactivity Disorder Working Group of the iPsych-Broad-Pgc Consortium ASDWGoti-B-PGCCBDWG, Tourette Syndrome Working Group of the Pgc SCSUDWGotPGC, Agrawal A, Borglum AD, Bulik CM, Daly MJ et al. Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychol Med 2019, 49(7):1166–1173.
Zablotsky B, Bramlett MD, Blumberg SJ. The Co-occurrence of Autism Spectrum Disorder in Children with ADHD. J Atten Disord. 2020;24(1):94–103.
Robertson MM. Mood disorders and Gilles De La Tourette’s syndrome: an update on prevalence, etiology, comorbidity, clinical associations, and implications. J Psychosom Res. 2006;61(3):349–58.
Baranova A, Wang J, Cao H, Chen JH, Chen J, Chen M, Ni S, Xu X, Ke X, Xie S, et al. Shared genetics between autism spectrum disorder and attention-deficit/hyperactivity disorder and their association with extraversion. Psychiatry Res. 2022;314:114679.
Rao S, Baranova A, Yao Y, Wang J, Zhang F. Genetic relationships between Attention-Deficit/Hyperactivity disorder, Autism Spectrum Disorder, and intelligence. Neuropsychobiology. 2022;81(6):484–96.
Cao H, Wang J, Baranova A, Zhang F. Classifying major mental disorders genetically. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110410.
Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL. Neuropsychiatric Complications of COVID-19. Curr Psychiatry Rep. 2021;23(5):25.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Baranova A, Chandhoke V, Cao H, Zhang F. Shared genetics and bidirectional causal relationships between type 2 Diabetes and attention-deficit/hyperactivity disorder. Gen Psychiatr. 2023;36(2):e100996.
Baranova A, Cao H, Teng S, Zhang F. A phenome-wide investigation of risk factors for severe COVID-19. J Med Virol. 2023;95(1):e28264.
Demontis D, Walters GB, Athanasiadis G, Walters R, Therrien K, Nielsen TT, Farajzadeh L, Voloudakis G, Bendl J, Zeng B, et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet. 2023;55(2):198–208.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, Pallesen J, Agerbo E, Andreassen OA, Anney R, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431–44.
Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, Illmann C, Osiecki L, Darrow SM, Hirschtritt ME, et al. Interrogating the genetic determinants of Tourette’s syndrome and other Tic disorders through genome-wide Association studies. Am J Psychiatry. 2019;176(3):217–27.
Initiative C-HG. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. 2020;28(6):715–8.
Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric, Genomics C, Patterson N, Daly MJ, Price AL, Neale BM. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–5.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, ReproGen C, Psychiatric Genomics C, Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control, Duncan C. An atlas of genetic correlations across human Diseases and traits. Nat Genet. 2015;47(11):1236–41.
Swerdlow DI, Kuchenbaecker KB, Shah S, Sofat R, Holmes MV, White J, Mindell JS, Kivimaki M, Brunner EJ, Whittaker JC, et al. Selecting instruments for mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol. 2016;45(5):1600–16.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Cao H, Baranova A, Wei X, Wang C, Zhang F. Bidirectional causal associations between type 2 Diabetes and COVID-19. J Med Virol. 2023;95(1):e28100.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, Mujagic Z, Masclee AAM, Jonkers D, Oosting M, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic Diseases. Nat Genet. 2019;51(4):600–5.
Nikitin A, Egorov S, Daraselia N, Mazo I. Pathway studio–the analysis and navigation of molecular networks. Bioinformatics. 2003;19(16):2155–7.
Dehghani A, Zokaei E, Kahani SM, Alavinejad E, Dehghani M, Meftahi GH, Afarinesh MR. The potential impact of Covid-19 on CNS and psychiatric sequels. Asian J Psychiatr. 2022;72:103097.
Vai B, Mazza MG, Delli Colli C, Foiselle M, Allen B, Benedetti F, Borsini A, Casanova Dias M, Tamouza R, Leboyer M, et al. Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry. 2021;8(9):797–812.
Rhoades R, Solomon S, Johnson C, Teng S. Impact of SARS-CoV-2 on host factors involved in Mental disorders. Front Microbiol. 2022;13:845559.
Liu N, Tan JS, Liu L, Wang Y, Hua L, Qian Q. Genetic predisposition between COVID-19 and Four Mental illnesses: a bidirectional, two-sample mendelian randomization study. Front Psychiatry. 2021;12:746276.
Merzon E, Weiss MD, Cortese S, Rotem A, Schneider T, Craig SG, Vinker S, Golan Cohen A, Green I, Ashkenazi S, et al. The Association between ADHD and the severity of COVID-19 Infection. J Atten Disord. 2022;26(4):491–501.
Koyama AK, Koumans EH, Sircar K, Lavery A, Hsu J, Ryerson AB, Siegel DA. Severe outcomes, readmission, and length of Stay among COVID-19 patients with Intellectual and Developmental Disabilities. Int J Infect Dis. 2022;116:328–30.
Huang S, Sun T, Zhu Y, Song S, Zhang J, Huang L, Chen Q, Peng G, Zhao D, Yu H, et al. Impact of the COVID-19 pandemic on children with ASD and their families: an online survey in China. Psychol Res Behav Manag. 2021;14:289–97.
Brookman-Frazee L, Stadnick N, Chlebowski C, Baker-Ericzen M, Ganger W. Characterizing psychiatric comorbidity in children with autism spectrum disorder receiving publicly funded mental health services. Autism. 2018;22(8):938–52.
Liu S, Rao S, Xu Y, Li J, Huang H, Zhang X, Fu H, Wang Q, Cao H, Baranova A, et al. Identifying common genome-wide risk genes for major psychiatric traits. Hum Genet. 2020;139(2):185–98.
Wu Y, Cao H, Baranova A, Huang H, Li S, Cai L, Rao S, Dai M, Xie M, Dou Y, et al. Multi-trait analysis for genome-wide association study of five psychiatric disorders. Transl Psychiatry. 2020;10(1):209.
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912–9.
Behrmann JT, Blaabjerg J, Jordansen J, Jensen de Lopez KM. Systematic review: investigating the impact of COVID-19 on Mental Health outcomes of individuals with ADHD. J Atten Disord. 2022;26(7):959–75.
Bitan DT, Krieger I, Weinstein O. Challenges of the COVID-19 pandemic among individuals with Autism Spectrum Disorder. JAMA Psychiatry. 2022;79(5):389–90.
Baweja R, Brown SL, Edwards EM, Murray MJ. COVID-19 pandemic and impact on patients with Autism Spectrum Disorder. J Autism Dev Disord. 2022;52(1):473–82.
Lee CY, Huang CH, Rastegari E, Rengganaten V, Liu PC, Tsai PH, Chin YF, Wu JR, Chiou SH, Teng YC et al. Tumor Necrosis Factor-Alpha Exacerbates Viral Entry in SARS-CoV2-Infected iPSC-Derived Cardiomyocytes. Int J Mol Sci 2021, 22(18).
Cao L, Guo Q, Chen Y, Chen N, Liu M, Tian D. Management of gastrointestinal endoscopy unit during post covid-19 endemic outbreak: a report from Wuhan epicenter. Am J Infect Control. 2021;49(3):361–5.
Zhao Y, Yin L, Patel J, Tang L, Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: a meta-analysis. J Med Virol. 2021;93(7):4358–69.
Luo Z, Chen W, Xiang M, Wang H, Xiao W, Xu C, Li Y, Min J, Tu Q. The preventive effect of Xuebijing injection against cytokine Storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur J Integr Med. 2021;42:101305.
Gu WT, Zhou F, Xie WQ, Wang S, Yao H, Liu YT, Gao L, Wu ZB. A potential impact of SARS-CoV-2 on pituitary glands and pituitary neuroendocrine tumors. Endocrine. 2021;72(2):340–8.
Ekinci I, Hursitoglu M, Tunc M, Kazezoglu C, Isiksacan N, Yurt S, Akdeniz E, Eroz E, Kumbasar A. Adrenocortical System hormones in Non-critically Ill COVID-19 patients. Acta Endocrinol (Buchar). 2021;17(1):83–9.
Vakhshoori M, Heidarpour M, Bondariyan N, Sadeghpour N, Mousavi Z. Adrenal Insufficiency in Coronavirus Disease 2019 (COVID-19)-Infected Patients without Preexisting Adrenal Diseases: A Systematic Literature Review. Int J Endocrinol 2021, 2021:2271514.
Hempel A, Frost M, Agarwal N. Language and behavioral outcomes of treatment with pulse-dose prednisone for electrical status epilepticus in sleep (ESES). Epilepsy Behav. 2019;94:93–9.
Taurines R, Schwenck C, Lyttwin B, Schecklmann M, Jans T, Reefschlager L, Geissler J, Gerlach M, Romanos M. Oxytocin plasma concentrations in children and adolescents with autism spectrum disorder: correlation with autistic symptomatology. Atten Defic Hyperact Disord. 2014;6(3):231–9.
Coupaye M, Laurier V, Benvegnu G, Poitou C, Faucher P, Mosbah H, Diene G, Pinto G, Briceno LG, Merrien C, et al. Paradoxical low severity of COVID-19 in Prader-Willi syndrome: data from a French survey on 647 patients. Orphanet J Rare Dis. 2021;16(1):325.
Wang SC, Wang YF. Cardiovascular protective properties of oxytocin against COVID-19. Life Sci. 2021;270:119130.
Diep PT. Is there an underlying link between COVID-19, ACE2, oxytocin and vitamin D? Med Hypotheses. 2021;146:110360.
Namjoo I, Alavi Naeini A, Najafi M, Aghaye Ghazvini MR, Hasanzadeh A. The relationship between antioxidants and inflammation in children with attention deficit hyperactivity disorder. Basic Clin Neurosci. 2020;11(3):313–21.
Muehsam D, Lutgendorf S, Mills PJ, Rickhi B, Chevalier G, Bat N, Chopra D, Gurfein B. The embodied mind: a review on functional genomic and neurological correlates of mind-body therapies. Neurosci Biobehav Rev. 2017;73:165–81.
Torzewski J, Heigl F, Zimmermann O, Wagner F, Schumann C, Hettich R, Bock C, Kayser S, Sheriff A. First-in-Man: Case Report of selective C-Reactive protein apheresis in a patient with SARS-CoV-2 Infection. Am J Case Rep. 2020;21:e925020.
Acknowledgements
We thank members of the COVID-19 Host Genetic Initiative, the Psychiatric Genomics Consortium, and other teams who generously shared the GWAS data.
Funding
None.
Author information
Authors and Affiliations
Contributions
FZ conceived the study and performed the analyses; FC, HC, AB, QZ, and FZ wrote the manuscript. All authors contributed to revising the work and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, F., Cao, H., Baranova, A. et al. Causal associations between COVID-19 and childhood mental disorders. BMC Psychiatry 23, 922 (2023). https://doi.org/10.1186/s12888-023-05433-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-05433-0