Abstract
Objective
Investigate the sleep characteristics of patients with obstructive sleep apnea syndrome (OSAS) comorbidity with panic disorder (PD), exploring its potential association with serum C-reactive protein (CRP) levels.
Patients and methods
Fifty-four patients (25 OSAS patients with PD and 29 without PD) and 25 healthy controls (HCs) were included. The Self-rating anxiety scale (SAS), self-rating depression scale (SDS), and Pittsburgh sleep quality index (PSQI) were used to assess the mood and sleep quality of the subjects. All patients had circulating CRP levels and polysomnography was performed.
Results
OSAS with PD had higher SAS, SDS, PSQI than the OSAS without PD. Compared to OSAS without PD, OSAS with PD had higher percentage of non- rapid eye movement sleep 1 and 2 (N1 and N2%), sleep latency, and a lower percentage of rapid eye movement sleep (REM%). Respiratory-related microarousal index, AHI, and time below 90% oxygen saturation (T90) were low, and the lowest oxygen saturation (LO2) was high. Serum CRP levels in OSAS patients with PD were lower than that in OSAS patients without PD, but higher than that in HCs. In OSAS patients with PD, serum CRP levels were negatively correlated with wake time after sleep onset and SAS scores but positively correlated with sleep efficiency and N2%. Serum CRP levels were positively correlated with T90 and negatively correlated with LO2.
Conclusion
OSAS patients with PD had worse sleep quality, less severe OSAS, and low serum CRP levels. Serum CRP levels in OSAS patients with PD were associated with poorer sleep quality and duration of hypoxia rather than AHI.
Similar content being viewed by others
Introduction
Obstructive sleep apnea–hypopnea syndrome (OSAS) is a common sleep-disordered breathing disorder, with an estimated 425 million adults suffering from moderate-to-severe OSAS worldwide [1]. OSAS is characterized by recurrent closing or narrowing of the upper airway, resulting in intermittent hypoxia, sleep fragmentation, and recurrent nocturnal awakenings [2]. OSAS is a heterogeneous syndrome characterized by various pathophysiological mechanisms, clinical manifestations, and consequences of respiratory events [3]. Notably, the efficacy of OSAS treatments may also differ due to these characteristics. Therefore, identifying specific comorbidities can aid in developing more targeted diagnostic and therapeutic strategies [4].
Studies have found that OSAS is closely related to endocrine and cardiovascular diseases and can induce mood disorders [5]. Untreated OSAS may lead to the emergence of depressive and anxious emotions in patients, while prolonged emotional disorders can promote the progression of OSAS. Lee observed that more than half of the patients with OSAS have accompanying emotional problems [6]. Panic disorder (PD) is an extreme manifestation of anxiety disorders. Symptoms of nocturnal repetitive panic attacks in PD are similar to those of repetitive respiratory pauses in a patient with OSAS [7]. OSAS combined with PD patients often results in misdiagnosis or missed diagnoses. However, few studies have examined the relationship between OSAS and PD [8].
A follow-up study involving 8,704 OSAS patients found that the risk ratio for PD was 2.17 times higher in patients with sleep apnea [8]. In addition, a randomized crossover trial found that continuous positive airway pressure can not only reduce AHI, but also significantly reduce the number of panic attacks in OSAS patients with PD [9]. This indicates a close link between obstructive sleep apnea syndrome and PD. OSAS patients with PD may present with frequent arousal, racing heart, and breathlessness at night. Sleep complaints in OSAS patients with PD often depend on self-observed measures. In contrast, objective measures, including polysomnography, have not been observed in the literature on OSAS patients with PD.
Emerging evidence indicates that inflammation plays a significant role in the pathophysiology of OSAS and PD [10, 11]. Li et al. measured serum CRP levels in 156 patients with OSA and 110 healthy controls. Univariate logistic regression analysis showed that CRP levels were associated with the presence and severity of obstructive sleep apnea syndrome, and that the serum CRP levels of patients with severe OSA were significantly higher than those of patients with moderate OSA, suggesting that CRP may be part of the pathological mechanism of OSAS [12]. A recent meta-analysis found similar results [13]. Some studies have also found that CRP levels are not related to the degree of upper airway obstruction in OSA patients [11]. In addition, although several studies have shown that there is no difference in CRP levels between patients with PD and healthy controls, a meta-analysis involving 152 PD patients and 156 controls showed that the CRP levels of PD patients were significantly higher than those of the control group [14, 15]. There is also increasing evidence that immune-mediated inflammation is involved in the pathophysiological processes of psychiatric disorders. Investigating the levels of inflammation in the comorbid state of OSAS and PD may shed light on the critical pathophysiological connections between these two disorders.
This study investigated differences in sleep structure, the severity of respiratory events, and serum CRP levels between OSAS patients with and without PD. Furthermore, we explored the association between changes in serum CRP levels, poor sleep quality, and respiratory disturbances in OSAS patients with PD, thus providing the basis for clinical diagnosis and treatment.
Methods
Subjects
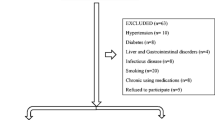
The patients came from the Department of Sleep Disorder, the Affiliated Chaohu Hospital of Anhui Medical University (China). The patients were diagnosed with OSAS following the Chinese Adult Obstructive Sleep Apnea Diagnosis and Treatment Guidelines [16]. Twenty-five individuals with PD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria were enrolled [17]. The following conditions need to be met: recurrent unexpected panic attacks. After an attack, 1 or 2 of the following symptoms occurred and lasted for more than 1 month: a. Constantly worried or worried about the recurrence of a panic attack or its consequences. b. There were significant adverse changes in the behavior associated with panic attacks. This disorder cannot be attributed to a substance or drug. This disorder cannot be better explained by other mental disorders. The Structured Clinical Interview for DSM-5 Disorders, Clinical Version (SCID-5-CV) was administered to verify the diagnosis [18]. The exclusion criteria were if the patient (1) suffered from severe somatic diseases (including cardiovascular diseases, diabetes, hypertension, liver and kidney dysfunction, and infections, etc.), (2) met other diagnostic criteria for mental disorders according to the DSM-5, (3) had received systematic OSAS treatment (including positional changes, weight loss, and the use of a breathing machine, etc.), (4) was pregnant or lactating, and (5) exclude nocturnal panic attacks. A total of 25 healthy controls(HCs) undergoing physical examination in our hospital during the same period were included as controls: (a) without any mental disorders according to the DSM-5, (b) without somatic disease(including cardiovascular diseases, diabetes, hypertension, liver and kidney dysfunction, and infections, etc.), (c) without complaints related to sleep disorders, and (d) exclude pregnant or lactating women.
Liver and renal function tests, blood glucose, blood routine, urine routine, electrolytes, chest X-ray, and electrocardiogram were performed for all participants to exclude inflammation, infection or other organic diseases. This study was approved by the Ethics Committees of the Affiliated Chaohu Hospital of Anhui Medical University (Number KYXM-202108–005). All subjects signed a written informed consent form.
General data collection
The general demographics of the patients were collected, including sex, age, and body mass index (BMI). Subjective sleep quality was assessed using The Pittsburgh Sleep Quality Index (PSQI). The scale includes seven dimensions: sleep onset time, sleep duration, sleep efficiency, sleep disturbance, subjective sleep quality, hypnotic drugs and daytime dysfunction. The threshold for sleep disturbance is 7. Higher total scores indicate poorer sleep quality [19]. The anxiety and depression levels of the participants were assessed using the Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) [20, 21]. The SDS scale consists of 20 items, and according to the Chinese standard, scores between 53 and 62 indicate mild depression, scores between 63 and 71 indicate moderate depression, and scores of 72 or higher indicate severe depression. The SAS scale also consists of 20 items, and according to the Chinese norm, the cutoff values for SAS standard scores are 50, with scores between 50 and 59 indicating mild anxiety, scores between 60 and 69 indicating moderate anxiety, and scores of 70 or higher indicating severe anxiety. The SAS and SDS scales have been widely used in China, and their reliability and validity have been evaluated in multiple studies [22, 23]. Furthermore, the assessment has been standardized for the Chinese population [24, 25].
C-reactive protein
Serum CRP levels were measured in fasting venous blood in the morning using a fully automated biochemical analyzer (Siemens, Germany) in the morning after polysomnography was completed. All subjects had their blood samples collected at 7 o'clock in the morning.
Polysomnography
All participants underwent one night of polysomnography (PSG) in the hospital's sleep monitoring to acclimatize to the novel environment, followed by two nights of PSG to collect the study data. The recording assessed sleep continuity and structure [26]. The former was measured as total sleep time (TST), wake time after sleep onset (WASO), sleep efficiency (SE), sleep onset latency (SL), percentages of stage 1, stage 2, stage 3, and REM sleep (N1, N2, N3, and REM%). Respiratory parameters, including apnea hypopnea index (AHI), time below 90% oxygen saturation (T90), and lowest oxygen saturation (LO2), respiratory event-related arousals (RA), REM sleep-related respiratory event-related arousals (REM-RA), NREM-related respiratory event-related arousals (NREM-RA), and average heart rate (HR) were recorded.
Sample sizes
OSAS and healthy human serum CRP levels are based on the study of Bhushan et al. There are few studies on patients with OSAS combined with PD [27]. We calculated the average serum CRP level of the five initially enrolled patients. Sample size was estimated using GPower 3.1 Assuming a statistical power of 95%, significance level of 0.05, and effected difference of 1.07, it was estimated that at least 18 participants would be needed to show a statistically significant difference in among three groups [28].
Statistical analysis
All data were analyzed using SPSS®20.0 for Windows (IBM Corp., Armonk, NY, USA). Normally distributed data are expressed as mean ± standard deviation (SD). Mann–Whitney U–tests were performed to analyze non-parametric data expressed as the 25th, 50th, and 75th percentiles (P25, P50, and P75, respectively). The Kruskal–Wallis H-test was used for three group (HC, OSAS with and without PD) comparisons by adjusting the significance level to P < 0.0167 (Bonferroni correcting: 0.05/3). Spearman correlation was used to investigate relationships between the quantitative variables. Two-tailed P values ≤ 0.05 were considered statistically significant.
Results
General data and CRP levels of healthy controls and OSAS patients with and without PD
There were no significant differences in sex, age, or BMI between the three groups (Ps > 0.05). There was statistical significance in differences in SAS, SDS, and PSQI scores among the three groups. Compared to the HCs and patients without PD, patients with PD had significantly higher SAS, SDS, and PSQI scores (Ps < 0.001). Meanwhile, there were no significant differences in SAS, SDS, and PSQI scores between the OSAS patients without PD and HCs (Table 1). Serum CRP levels in OSAS patients with PD were lower than that in OSAS patients without PD (P < 0.001)., but higher than that in HCs (P < 0.05). (Fig. 1).
Sleep parameters of healthy controls and OSAS patients with and without PD
There was a statistical significance in differences in TST, WASO, SE, N1%, N2%, N3%, and REM% among the three groups. Compared to the HCs group, OSAS patients with PD had significantly higher WASO, SL, N1%, and significantly lower TST, SE, N3% and REM%. Patients with OSAS with PD had significantly higher SL, N1%, N2%, and significantly lower REM% than the OSAS without PD group (Table 2).
Respiratory parameters of healthy controls and OSAS patients with and without PD
There were statistically significant differences in AHI, LO2, T90, REM-RA, NREM-RA, RA, and HR among the three groups. OSAS patients with PD had significantly higher AHI, T90, REM-RA, NREM-RA, RA, and HR and significantly lower LO2 compared to HCs group. OSAS patients with PD had significantly lower AHI, T90, and REM-RA and higher LO2 than OSAS patients without PD (Table 3).
Correlations among serum CRP, sleep parameters, and respiratory parameters in OSAS patients with PD
In OSAS patients with PD, serum CRP levels were negatively correlated with WASO (r = − 0.445, P < 0.05) and SAS score (r = − 0.471, P < 0.05) but positively correlated with SE (r = 0.473, P < 0.05) and N2% (r = 0.493, P < 0.05). In terms of respiratory parameters, serum CRP levels were positively correlated with T90 (r = 0.457, P < 0.05) and negatively correlated with LO2 (r = − 0.476, P < 0.05) in OSAS patients with PD. However, there was no correlation between serum CRP levels and AHI (P > 0.05) in the OSAS patients with PD (Fig. 2).
Correlation analysis revealed significant associations between CRP and sleep parameters, and respiratory parameters in OSAS patients with PD: A SAS score (r = − 0.471, P < 0.05), B WASO (r = − 0.445, P < 0.05), C SE (r = 0.473, P < 0.05), D N2% (r = 0.493, P < 0.05), E T90 (r = 0.457, P < 0.05), F LO2 (r = − 0.476, P < 0.05). Abbreviations: SAS, self-rating anxiety scale; WASO, wake time after sleep onset; SE, sleep efficiency; N2%, percentages of stage 2; T90, time with blood oxygen saturation < 90%; LO2, the lowest SpO2
Discussion
OSAS is a systemic disease with multiple organ involvement. It is considered an independent risk factor for the development of cardiovascular diseases [29]. However, some studies have ignored the significant correlation between OSAS and psychiatric disorder. PD and OSAS are both well-known and common diseases. Therefore, it can challenge the co-occurrence of two diseases in clinical decision making. Panic attacks can be mistaken for arousal caused by nocturnal hypoxemia and upper airway collapse in patients with OSAS, thus delaying treatment [30]. Still, using anxiolytics to control panic attacks is dangerous before excluding OSAS by PSG in patients with PD. This is because anxiolytics can aggravate airway collapse, exacerbating OSAS [31]. Therefore, identifying patients with coexisting OSAS and PDs would help optimize treatment and diagnosis for patients with OSAS with PD [32].
The current study represents the first to evaluate sleep-breathing characteristics and CRP levels in OSAS patients with PD. Our study shows that OSAS patients with PD had worse sleep quality, lower AHI values, and lower serum CRP levels compared to OSAS patients without PD. Serum CRP levels were associated with poorer sleep quality and duration of hypoxia in OSAS patients with PD.
OSAS patients with PD had more worse sleep quality and severe mood disorders
Currently, there are only a few studies about objective sleep architecture in OSAS patients with PD. All patients in this study group underwent PSG for two nights, which can objectively reflect the actual sleep state of patients at night, and has specific clinical value. Compared to patients without PD, OSAS patients with PD have increased sleep latency, light sleep, and decreased sleep time in the REM phase. Combined with the PSQI score, it is clear that patients with OSAS with PD have poor subjective sleep quality, and their objective sleep quality is significantly worse than that of patients with OSAS without PD.OSAS patients with PD have hypothalamic⁃pituitary⁃adrenocortical axis hyperfunction and increased alertness due to worry and anxiety, causing an increase in the duration/ratio of light sleep (i.e., increased duration/ratio of sleep in N1, N2, or both phases) [33]. Studies have shown that abnormal noradrenergic neurons in the brain are associated with the pathogenesis of PD and that neurological dysfunction of norepinephrine and 5-hydroxytryptamine leads to nocturnal panic attacks and central respiratory dysfunction [34, 35]. In turn, norepinephrinergic neurons in the brain are a critical factor in maintaining REM sleep, which may also contribute to reduced REM sleep in OSAS patients with PD [36]. Surprisingly, the PSG results showed a reduction in TST time in OSAS patients with PD compared to OSAS patients without PD. Still, there was no statistical difference between the two groups, which may be explained by the small sample size.
Additionally, anxiety symptoms were more pronounced in OSAS patients with PD, as seen in SAS scores. Anxiety also reduces sleep quality at night in OSAS patients with PD. Therefore, OSAS patients with PD had more severe anxiety and poorer sleep quality offset upper airway collapse, resulting in atypical nocturnal OSAS symptoms in OSAS patients with PD. This leads to their apnea events being masked in the initial stages of the disease, thus ignoring OSAS. Apnea hypoventilation events in OSAS patients with PD are of concern only when they develop further and worsen. However, OSAS may have caused severe damage to the organism at this time. Therefore, PSG should be perfected in patients with PD, especially those with significant nocturnal seizures, to prevent underdiagnosis of OSAS.
OSAS patients with PD had had milder OSAS symptoms
Regarding respiratory parameters, OSAS patients without PD have increased AHI due to recurrent complete or partial upper airway obstruction during sleep, apnea, and hypoventilation, resulting in recurrent hypoxia. However, both apnea and hypoxemia were less severe in the OSAS patients with PD than in the OSAS patients without PD, suggesting a lesser degree of apnea and hypoxic events in OSAS patients with PD. This may be due to the lower arousal threshold in patients with PD [37]. Although the relationship between OSAS and upper airway obstruction was previously believed to be primarily related to anatomical factors, in recent years, the role of non-anatomical factors has received increasing attention from researchers [38]. Arousal is one of the most studied non-anatomical factors [39]. During obstructive breathing, patients gradually increase their inspiratory effort. When it exceeds the threshold for arousal, the brain wakes up from sleep, which can reopen the airway, restore oxygen saturation, and relieve hypercapnia in patients with OSAS [40]. The merger of panic disorder results in a decrease in the arousal threshold and an increase in vigilance in patients. This makes them more prone to awakening from pathological events such as airway obstruction, leading to a shorter duration of hypoxia in patients [41]. On the other hand, previous studies have suggested that an essential mechanism for the occurrence of panic symptoms is increased sensitivity to carbon dioxide (CO2) in patients [42]. High sensitivity may cause patients with comorbid PD to overreact to minor changes in CO2, leading to the early termination of respiratory events [43].
This study also found that the respiratory-related microarousal index was higher in the REM phase in OSAS patients with PD compared to OSAS patients without PD. This may be due to a more significant decrease in mandibular electromyographic activity in the REM phase compared to the awake and NREM phases, more severe apnea, and more respiratory-related arousals [44, 45]. Unexpectedly, although sleep disturbances were more severe in the OSAS patients with PD than in the OSAS patients without PD, there were no significant differences between the OSAS patients with PD and without PD in the total number of respiratory-related microarousals and the mean nocturnal heart rate.
Lower inflammation levels in OSAS patients with PD are associated with sleep and OSAS symptoms
The present study is the first to explore CRP serum concentrations in OSAS patients with PD. The level of CRP, which is synthesized in the liver, is a commonly used inflammatory biomarker [46]. According to recent meta-analyses, OSAS patients without PD had higher serum CRP levels than those in the control group [47]. Li also found that patients with severe OSA had significantly higher serum CRP levels than those with moderate OSAS [12]. In our study, although serum CRP levels were higher for OSAS patients with PD than for healthy people, they were lower for OSAS patients without PD. This may be because OSAS severity is milder in OSAS patients with PD. Our study also found that serum CRP levels in OSAS patients with PD were positively correlated with LO2and negatively correlated with T90. This indicates that the decrease in inflammation levels is related to the reduction of hypoxia symptoms in OSAS patients without PD. As mentioned above, PD may be reduce hypoxic events by lowering the threshold of awakening of the patient [48]. Patients with combined PD exhibit milder hypoxia, which reduces inflammation levels. Contrary to our expectations, serum CRP levels in OSAS patients without PD were not correlated with AHI. This suggests that alterations in OSAS-related inflammation may be mainly due to hypoxemia rather than AHI-induced changes.
Regarding limitations, first, as a cross-sectional study, this research cannot identify cause-and-effect relationships between OSAS and PD. There is a close association between OSAS and PD, but the specific mechanism remains unclear. Also, this study is a single-center study with a small overall sample size, so its results need to be further confirmed by large-scale clinical studies. Although we have recognized the impact of nocturnal panic attacks on sleep architecture, it is important to note that this study did not analyze such patients as a specific subgroup.
At present, there are few studies on OSAS patients with PD. Furthermore, the coexistence of these two conditions often leads to misdiagnosis and underdiagnosis. When encountering patients with complaints such as nocturnal snoring and awakening from sleep, physicians in both internal medicine in general hospitals tend to primarily consider a diagnosis of OSAS, neglecting the evaluation of the patients' psychiatric symptoms. Similarly, psychiatrists who come across patients with recurrent nocturnal awakenings accompanied by symptoms such as palpitations and sweating often think of panic disorder, thereby overlooking the occurrence of OSAS. Therefore, clinicians should pay attention to improving polysomnography and mood assessment to determine the possibility of comorbidity and achieve rational diagnosis and treatment.
Conclusion
In conclusion, this study revealed that patients suffering from OSAS combined with PD demonstrate notably diminished sleep quality compared to OSAS without PD. The excessive worry in patients with combined PD leads to increased light sleep and decreased arousal threshold. They are more prone to awaken from airway obstruction, resulting in shorter duration of hypoxia compared to the pure OSAS group. Additionally, we have found lower levels of CRP in OSAS patients with PD, which is associated with the duration of hypoxia rather than AHI. This study provides valuable insights into the understanding of the relationship between PD and OSAS and may serve as a basis for future research on potential therapeutic interventions.
Availability of data and materials
The datasets are available from the corresponding author (geyijun1984@126.com) at any request.
Abbreviations
- OSAS:
-
Obstructive sleep apnea syndrome
- PD:
-
PD
- TST:
-
Total sleep time
- WASO:
-
Wake time after sleep onset
- SE:
-
Sleep efficiency
- SL:
-
Sleep onset latency
- N1, N2, N3, and REM%:
-
Percentages of stage 1, stage 2, stage 3, and REM sleep; arousal index, T90, time with blood oxygen saturation < 90%
- LO2:
-
The lowest SpO2
- REM-RA:
-
REM sleep-related respiratory event-related arousals
- NREM-RA:
-
NREM-related respiratory event-related arousals
- RA:
-
Respiratory event-related arousals
- HR:
-
Average heart rate
References
Yi M, Zhao W, Tan Y, et al. The causal relationships between obstructive sleep apnea and elevated CRP and TNF-α protein levels. Ann Med. 2022;54:1578–89. https://doi.org/10.1080/07853890.2022.2081873.
Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–400. https://doi.org/10.1001/jama.2020.3514.
Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med. 2017;13:81–8. https://doi.org/10.5664/jcsm.6394.
Vanek J, Belohradova Minarikova K, Prasko J, et al. Obstruction sleep apnoea and panic disorder. Neuro Endocrinol Lett. 2023;44(2):86–96.
Lee SA, Im K, Seo JY, et al. Association between sleep apnea severity and symptoms of depression and anxiety among individuals with obstructive sleep apnea. Sleep Med. 2023;101:11–8. https://doi.org/10.1016/j.sleep.2022.09.023.
Lee W, Lee SA, Ryu HU, et al. Quality of life in patients with obstructive sleep apnea: relationship with daytime sleepiness, sleep quality, depression, and apnea severity. Chron Respir Dis. 2016;13:33–9. https://doi.org/10.1177/1479972315606312.
Edlund MJ, McNamara ME, Millman RP. Sleep apnea and panic attacks. Compr Psychiatry. 1991;32:130–2. https://doi.org/10.1016/0010-440x(91)90004-v.
Su VY, Chen YT, Lin WC, et al. Sleep apnea and risk of panic disorder. Ann Fam Med. 2015;13:325–30. https://doi.org/10.1370/afm.1815.
Takaesu Y, Inoue Y, Komada Y, et al. Effects of nasal continuous positive airway pressure on panic disorder comorbid with obstructive sleep apnea syndrome. Sleep Med. 2012;13:156–60. https://doi.org/10.1016/j.sleep.2011.10.016.
Herhaus B, Thesing G, Conrad R, et al. Alterations in heart rate variability and pro-inflammatory cytokine TNF-alpha in individuals with panic disorder. Psychiatry Res. 2023;322: 115107. https://doi.org/10.1016/j.psychres.2023.115107.
Korkmaz M, Korkmaz H, Kucuker F, et al. Evaluation of the association of sleep apnea-related systemic inflammation with CRP, ESR, and neutrophil-to-lymphocyte ratio. Med Sci Monit. 2015;21:477–81. https://doi.org/10.12659/MSM.893175.
Li W, Yu Z, Jiang C. Association of serum YKL-40 with the presence and severity of obstructive sleep apnea syndrome. Lab Med. 2014;45:220–5. https://doi.org/10.1309/LMS98AKCXRSL2XOR.
Li K, Wei P, Qin Y, et al. Is C-reactive protein a marker of obstructive sleep apnea?: a meta-analysis. Medicine (Baltimore). 2017;96(19): e6850. https://doi.org/10.1097/MD.0000000000006850.
Choi KW, Jang EH, Kim AY, et al. Predictive inflammatory biomarkers for change in suicidal ideation in major depressive disorder and panic disorder: a 12-week follow-up study. J Psychiatr Res. 2021;133:73–81. https://doi.org/10.1016/j.jpsychires.2020.12.011.
Liu CH, Hua N, Yang HY. Alterations in peripheral c-reactive protein and inflammatory cytokine levels in patients with panic disorder: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2021;17:3539–58. https://doi.org/10.2147/NDT.S340388.
中国医师协会睡眠医学专业委员会. 成人阻塞性睡眠呼吸暂停多学科诊疗指南. 中华医学杂志, 2018,98: 1902–1914. (in Chinese).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5 (R)). Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013. p. 280.
First MB. Structured Clinical Interview for the DSM (SCID). The Encyclopedia of Clinical Psychology. 2015. p. 1–6.
Pilz LK, Keller LK, Lenssen D, et al. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep. 2018;41:1–8. https://doi.org/10.1093/sleep/zsy029.
Thurber S, Snow M, Honts CR. The Zung Self-Rating Depression Scale: convergent validity and diagnostic discrimination. Assessment. 2002;9(4):401–5. https://doi.org/10.1177/1073191102238471.
Dunstan DA, Scott N. Norms for Zung’s Self-rating Anxiety Scale. BMC Psychiatry. 2020;20(1):90. https://doi.org/10.1186/s12888-019-2427-6.
Luo D, Tan L, Shen D, et al. Characteristics of depression, anxiety, impulsivity, and aggression among various types of drug users and factors for developing severe depression: a cross-sectional study. BMC Psychiatry. 2022;22(1):274. https://doi.org/10.1186/s12888-022-03933-z.
Chen K, Wei L, Zhang Y, et al. Work stress in nurses returning to tertiary a general hospitals in China after the delivery of their second child: a cross-sectional study. BMC Health Serv Res. 2022;22(1):492. https://doi.org/10.1186/s12913-022-07912-8.
Jin T, Zhang LJ. Introduction and application of self-rating depression scales in China. J Neurosci Mental Health. 2017;17(5):366–9. https://doi.org/10.3969/j.issn.1009-6574.2017.05.016.
Li Q, Yang J, He Y, et al. Investigation of the psychological health of first-year high school students with myopia in Guangzhou. Brain Behav. 2020;10(4): e01594. https://doi.org/10.1002/brb3.1594.
Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. 2012;8:597–619. https://doi.org/10.5664/jcsm.2172.
Bhushan B, Guleria R, Misra A, et al. Obstructive sleep apnoea correlates with C-reactive protein in obese Asian Indians. Nutr Metab Cardiovasc Dis. 2009;19(3):184–9. https://doi.org/10.1016/j.numecd.2008.06.008.
Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. https://doi.org/10.3758/bf03193146.
Baratta F, Pastori D, Fabiani M, et al. Severity of OSAS, CPAP, and cardiovascular events: a follow-up study. Eur J Clin Invest. 2018;48: e12908. https://doi.org/10.1111/eci.12908.
Trajanovic NN, Rasool MS, Voloh I, et al. Sleep-disordered breathing, cardiac arrhythmia, and panic disorder. J Clin Sleep Med. 2005;1:288–9.
Hsu TW, Chen HM, Chen TY, et al. The Association between use of benzodiazepine receptor agonists and the risk of obstructive sleep apnea: a nationwide population-based nested case-control study. Int J Environ Res Public Health. 2021;18:9720. https://doi.org/10.3390/ijerph18189720.
McNicholas WT, Pevernagie D. Obstructive sleep apnea: transition from pathophysiology to an integrative disease model. J Sleep Res. 2022;31(4): e13616. https://doi.org/10.1111/jsr.13616.
Masdrakis VG, Legaki EM, Papageorgiou C, et al. Pituitary-adrenal axis hormones in early-onset versus late-onset panic disorder. Int J Psychiatry Clin Pract. 2022;26:72–8. https://doi.org/10.1080/13651501.2020.1828929.
Duman RSBDNF. 5-HT, and Anxiety: Identification of a critical periadolescent developmental period. Am J Psychiatry. 2017;174:1137–9. https://doi.org/10.1176/appi.ajp.2017.17101084.
Sobanski T, Wagner G. Functional neuroanatomy in panic disorder: status quo of the research. World J Psychiatry. 2017;7:12–33. https://doi.org/10.5498/wjp.v7.i1.12.
Adamantidis AR, Gutierrez Herrera C, Gent TC. Oscillating circuitries in the sleeping brain. Nat Rev Neurosci. 2019;20:746–62. https://doi.org/10.1038/s41583-019-0223-4.
de Carvalho MR, Velasques BB, Freire RC, et al. Frontal cortex absolute beta power measurement in Panic Disorder with Agoraphobia patients. J Affect Disord. 2015;184:176–81. https://doi.org/10.1016/j.jad.2015.05.055.
Eckert DJ. Phenotypic approaches to obstructive sleep apnoea—New pathways for targeted therapy. Sleep Med Rev. 2018;37:45–59. https://doi.org/10.1016/j.smrv.2016.12.003.
Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest. 2020;157:403–20. https://doi.org/10.1016/j.chest.2019.09.002.
Lee JJ, Sundar KM. Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung. 2021;199:87–101. https://doi.org/10.1007/s00408-021-00426-w.
Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169(5):623–33. https://doi.org/10.1164/rccm.200307-1023OC.
Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. https://doi.org/10.1164/rccm.201303-0448OC.
Amaral JM, Spadaro PT, Pereira VM, et al. The carbon dioxide challenge test in panic disorder: a systematic review of preclinical and clinical research. Braz J Psychiatry. 2013;35:318–31. https://doi.org/10.1590/1516-4446-2012-1045.
Joosten SA, Landry SA, Wong AM, et al. Assessing the physiologic endotypes responsible for REM- and NREM-Based OSA. Chest. 2020;159:1998–2007. https://doi.org/10.1016/j.chest.2020.10.080.
Rishi AR, Rishi MA. Rapid eye movement-related obstructive sleep apnea: Where do we stand? Respir Investig. 2021;59:589–95. https://doi.org/10.1016/j.resinv.2021.06.006.
Ji SR, Zhang SH, Chang Y, et al. C-reactive protein: the most familiar stranger. J Immunol. 2023;210:699–707. https://doi.org/10.4049/jimmunol.2200831.
Imani MM, Sadeghi M, Farokhzadeh F, et al. Evaluation of blood levels of c-reactive protein marker in obstructive sleep apnea: a systematic review, meta-analysis, and meta-regression. Life. 2021;11:362. https://doi.org/10.3390/life11040362.
Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol. 2014;116:302–13. https://doi.org/10.1152/japplphysiol.00649.2013.
Acknowledgements
We also would like to thank Charlesworth for the English language editing.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Funding was provided by the Research Fund of Anhui Institute of transnational medicine (NO. 2022zhyx-C57) and Scientific research project of Anhui Provincial Health Commission (NO.AHWJ2022b027).
Author information
Authors and Affiliations
Contributions
Yijun Ge:Supervision, Funding acquisition, Conceptualization, Writing—Review & Editing; Shuai Yang: Methodology, Formal analysis, Writing-Original Draft; Xiaoyi Kong: Data Curation, Resources.; Xueyan Li: Data collection. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the Declaration of Helsinki and relevant regulations. This study was approved by the Ethics Committees of the Affiliated Chaohu Hospital of Anhui Medical University (Number KYXM-202108–005). All subjects signed a written informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Kong, X., Li, X. et al. Serum C-reactive protein level and sleep characteristics in obstructive sleep apnea syndrome comorbid with panic disorder: a preliminary study. BMC Psychiatry 23, 859 (2023). https://doi.org/10.1186/s12888-023-05376-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-05376-6