Abstract
Background
This study was designed to examine the possible efficacy of the probiotic strain Lactobacillus acidophilus LB (Lacteol Fort) on attention-deficit/hyperactivity disorder (ADHD) symptomatology and evaluate its influence on cognition function.
Methods
In this randomized controlled trial, 80 children and adolescents with ADHD diagnosis, aged 6–16 years, were included. The participants were randomly assigned to two groups: one group received probiotics plus atomoxetine, whereas the other group received atomoxetine only. ADHD symptomatology was assessed using the Conners Parent Rating Scale–Revised Long Version (CPRS-R-L) and Child Behavioral Checklist (CBCL/6–18). The participants were evaluated for their vigilance and executive function using Conner’s Continuous Performance Test (CPT) and Wisconsin Card Sort Test (WCST). Both groups were assessed at the beginning of the study and the end of the twelve weeks.
Results
The probiotic group comprised 36 patients, whereas the control group comprised 40 patients in the final analysis after four patients dropped out of the trial. After 3 months of probiotic supplementation, a significant improvement in the CPRS-R-L and CBCL total T scores was observed compared with those in the control group (p = 0.032, 0.024, respectively). Additionally, the probiotic group demonstrated improved focus attention (target accuracy rate and omission errors;p = 0.02, 0.043, respectively) compared with the control group. An analysis of the Wisconsin Card Sorting Test (WCST) performance demonstrated that the probiotic group had significantly lower perseverative (p = 0.017) and non-perseverative errors (p = 0.044) but no significant differences compared to the control group.
Conclusion
Lactobacillus acidophilus LB supplementation combined with atomoxetine for 3 months had a beneficial impact on ADHD symptomology and a favorable influence on cognitive performance. As a result, the efficacy of probiotics as an adjunctive treatment for managing ADHD may be promising.
Trial registration
ClinicalTrials.gov (identifier: NCT04167995). Registration date: 19–11-2019.
Similar content being viewed by others
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common early-onset neurodevelopmental disorder in the pediatric population, affecting 7.2% of school-age children globally. It is characterized by deficits in the cognitive functioning pattern, with hyperactivity, impulsivity, and attention problems that are developmentally inappropriate and significantly impairing symptoms [1]. The pathophysiological mechanism that underlies ADHD remains being investigated. Nevertheless, research highlights the complex interplay of genetic and environmental risk factors, which may underlie the observed clinical symptom heterogeneity among individuals with ADHD [2]. It has been determined that neurotransmitter dysregulation, particularly norepinephrine, serotonin, and dopamine, may play a fundamental role in ADHD pathogenesis [3]. This is supported by evidence of the abnormal gene expression linked to those neurotransmitters in children with ADHD [4]. Therefore, targeting monoaminergic systems underpins most ADHD treatments [5].
Recently, a growing interest has been directed to the bidirectional pathway between the gut and brain—the gut–brain axis (GBA). Alterations and imbalances in the gut microbiota may play a role in developing and progressing neurodevelopmental disorders like ADHD. [6, 7]. Mounting evidence hypothesized that through this pathway, neurotransmitters released by the bacteria in the intestinal lumen may stimulate epithelial cells to release hormones and cytokines. These substances may then modify neural circuitry within the enteric nervous system, thus regulating brain activity and behavior [8]. Related to this, brain diseases, such as ADHD, caused by neurotransmitter dysregulation may benefit from targeting the gut microbiota as a therapeutic approach. Detecting abnormalities in the functioning of the gastrointestinal system, altered composition in gut microbes, and an increased prevalence of inflammatory problems in children with ADHD provided further support for the involvement of the GBA in the etiology of ADHD [9,10,11].
Furthermore, several reports showed an association between ADHD and low levels of brain-derived neurotrophic factor (BDNF), which is essential for neuronal development, suggesting that BDNF contributes to its pathophysiology [12,13,14]. There is clear evidence that short-chain fatty acids (SCFAs) produced during microbial fermentation have been positively correlated with BDNF levels [12]. This suggests that the gut microbiota indirectly impacts BDNF levels, which could be modified to benefit those with ADHD [15].
Probiotics are bacteria that benefit the host body [16]. Because probiotics have various health benefits for the host, high intestinal adhesion abilities [17], and few adverse effects, they are widely applied as dietary supplements. The research results are varied, investigating the potential benefits of probiotic supplementation in ADHD regarding their type, dose, and duration and the different assessment methods throughout conducted trials. Interestingly, it has been demonstrated that probiotics could reduce the risk of later neurodevelopmental disorders in children supplemented with Lactobacillus rhamnosus GG (LGG) early in life [18]. Lactobacillus rhamnosus and Bifidobacterium species are the most researched strains, and their neurobehavioral impacts have been reported [19, 20]. Another probiotic bacterium, Lactobacillus acidophilus LB, reduces cholesterol levels and has physiological and pharmaceutical benefits in preventing and treating certain disorders [21]. However, the potential therapeutic benefits of the Lactobacillus acidophilus strain in children with ADHD have not yet been investigated. Accordingly, the primary objective of this randomized controlled trial was to examine the potential effects of Lactobacillus acidophilus LB supplementation combined with atomoxetine on the core clinical symptoms of ADHD. The secondary objective was to investigate whether 12-week probiotic supplementation could improve cognitive functions in children with ADHD.
Patients and methods
Study design
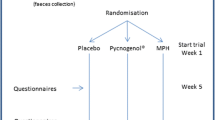
This study was a 12-week randomized controlled trial set as a prospective, parallel, open-label study conducted from June 2020 to October 2021 on pediatric and adolescent outpatients with ADHD. The trial was registered at ClinicalTrials.gov (identifier: NCT04167995, on 19/11/2019). The study’s reporting complies with the Consolidated Standards of Reporting Trials 2010 statement [22]. The study was approved by the Ethics Committee of Ain-Shams University Hospitals (Ethical Committee No. FMASU 158) and was conducted following the Helsinki Declaration of 1975. The legal guardians of the participants signed the informed consent form after being provided with a thorough explanation of the procedures, the study’s purpose, and assurances of confidentiality.
Participants
Eighty children and adolescents aged 6–16 were recruited from the Developmental and Behavioral Pediatrics Clinic Children’s Hospital and Psychiatry Institute, Faculty of Medicine, Ain-Shams University, Cairo, Egypt. The participants fulfilled the diagnostic criteria for ADHD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [23] criteria and Conner’s Parent Rating Scales-Revised (CRS-R), which a psychiatrist established before the study. The exclusion criteria were as follows: (a) individuals with an estimated intelligence quotient (IQ) less than 80% based on the Arabic version of the Wechsler Intelligence Scale for Children (WISC-III) [24]; (b) those with any significant medical or other neurodevelopmental disorders, such as autism; (c) those who have received medications for ADHD less than 8 weeks before the study; and (d) those who have taken antibiotics or probiotics recently.
Randomization
A statistician, independent from the study investigators, randomly allocated the enrolled participants into the probiotic group (n = 40) or the control group (n = 40) using a random number generator from a computer-based randomization software. The allocation was concealed using opaque, sealed, sequentially numbered envelopes. After obtaining informed consent, the opaque sealed envelopes were unwrapped, and the participants were enrolled in the relevant group. Only the researchers collecting and analyzing the data were blinded to the study groups (assessor-blinded).
Intervention
The participants with ADHD randomized to the study (Probiotic) group received a probiotic preparation (Lacteol Fort®; lyophilized heat-killed Lactobacillus acidophilus LB, sachets containing 10 billion colony-forming units, manufactured by Rameda Pharmaceutical Company, Egypt, under the license of Axcan Pharma S.A, France) in a dose of two sachets disintegrated in 50-mL freshwater, twice daily, from the first day of the study until 3 months. Additionally, each parent received a daily text message reminding them to let their children take their supplements as directed and to report any side effects. The control (no probiotic) group did not receive any probiotics. All participants were on a stable pharmacological treatment for ADHD (atomoxetine) with a consistent dosage throughout the study (1.2 mg/kg/day).
Instruments and measures
Conners Parent Rating Scale-Revised Long Version (CPRS-R-L) [25] (Arabic version [26]).
The CPRS-R-L is an 80-item behavior rating scale that identifies children at risk of ADHD and assesses the severity of their ADHD symptoms. On a 4-point Likert scale, from 0 to 3, parents’ responses to their children’s behavior over the previous month were rated, with 0 indicating “not at all” and scores of 1–3 indicating “just a little” to “severely affected,” respectively. The raw scores were interpreted using T-values, with scores above 60 considered moderately elevated and those above 70 considered significantly high. The scale included seven subscales, three DSM-IV Symptom Indices, an ADHD Index, and three Conners’ Global Indices. Our study targeted the three DSM-IV subscales (Inattentive, Hyperactive/impulsive, and Total score).
Child Behavioral Checklist (CBCL/6–18)
The CBCL/school-aged (6–18 years) is a parent-rated questionnaire containing 113 items subdivided into three dimensions, noted as internalizing, externalizing, and total behavior problems, quantitatively assessing and providing dimensional insights concerning children’s psychopathology and behavioral functioning [27]. Responses to the CBCL were rated on a 3-point rating scale, from 0 to 2, with 0 indicating “not true” and scores of 1–2 indicating “somewhat” or “sometimes true” to “very true” or “often true,” respectively. Test results were interpreted using T-values, and children with scores ≥ 65 are more likely to have behavior problems with clinical relevance. The assessment was made using the Arabic version of the CBCL/6–18, provided by the Achenbach System of Empirically Based Assessment Foundation (Burlington, USA) after signing a license agreement with them.
Psychology Experiment Building Language (PEBL) version 2.0 of the Conners Continuous Performance Test (CPT) [28, 29]
PEBL version 2.0 of the CPT measures sustained attention and impulsivity in a 14-min computerized task. Participants are instructed to hit the button whenever any alphabet letter other than the X letter is shown. The test measures selective inattentiveness (missing target stimuli: omission errors), impulsivity (false responding to non-target stimuli: commission errors), and sustained attention (reaction time and reaction time variability).
PEBL version 2.0 of the Wisconsin (Berg) Card Sort Test (WCST) [30]
PEBL version 2.0 of the WCST measures executive functioning, cognitive flexibility, and set-shifting abilities. It comprises two sets of cards: 64 reaction cards and four stimulus cards. Based on the patterns present on the cards, participants were instructed to categorize them. The rule for properly sorting the stimuli shifts regularly, and the ability to change strategies that vary according to the stimuli's color, number, or shape is recorded. The participant should first choose the proper sorting principle and stick with it throughout the test to perform successfully. Shifting the matching rule to another category occurs after ten successively correct matches in one category (e.g., matching numbers). The main outcome parameters are the correct responses, categories completed, perseverative errors, non-perseverative errors, total errors, and failure to maintain a set. Executive dysfunction is assumed to be reflected in preservative and non-preservative errors [31].
Primary and secondary outcomes
Both groups were assessed at baseline and follow-up at twelve weeks. The primary outcomes were changes in the severity of ADHD symptoms and associated behavioral problems assessed using the CPRS-R-L (Inattentive, Hyperactive/impulsive, and Total score) and CBCL (Syndrome scale and Total score), respectively. Secondary outcomes were improvements in sustained and focused attention, impulsivity, executive functioning, and set-shifting abilities based on the CPT and WSCT tasks.
Statistical analysis
Using G*Power, the alpha error and study power were set at 5% and 80%, respectively. Assuming an effect size of 0.7 (Cohen’s d), a sample size of 40 cases per group was required, considering a dropout rate of 20%.
The collected data were revised, coded, tabulated, and introduced to a personal computer using Statistical Package for the Social Sciences, version 25.
Student’s t-test was used to evaluate the statistical significance of the mean difference between the two study groups. The chi-square test was used to compare the two study groups. The Mann–Whitney U-test was used to assess the statistical significance of the difference in baseline and follow-up changes between the two study groups. The Wilcoxon signed-rank test was applied to evaluate the statistical significance of the difference in scores measured twice for the same group.
Results
Of 100 children and adolescents, 80 (40 in each group) met the eligibility criteria and were willing to participate in the study. However, only 36 of the 40 probiotic group participants completed the study as we failed to follow up with four children who were reluctant to continue the investigation (Fig. 1).
As shown in Table 1, the mean ages did not differ between the two groups (8.72 ± 2 years in the probiotics group and 8.48 ± 1.5 years in the control group; p = 0.543), and most participants were males (24 in the probiotics group and 27 in the control group; p = 0.938). Moreover, no significant difference in the IQ measurements was observed between the two groups.
Primary outcomes.
From baseline to 12 weeks at the end of the trial, the probiotics group showed a reduction in CPRS-R-L scores relative to the control group. Significant differences in the mean changes in the CPRS-R-L subscale T scores, DSM Inattentive, DSM hyperactive-impulsive, and DSM Total (-6.7 ± 10, -6.8 ± 8.7, and -6.11 ± 8, respectively, vs. -2.5 ± 6.7, -2.6 ± 12, and -2.5 ± 9.5; p < 0.001) were observed over 12 weeks (Table 2, Fig. 2). Similarly, the probiotics group showed a considerable improvement in the overall behavioral problems as measured using the CBCL over 12 weeks compared with the control group, with significant mean change differences in the CBCL subscale T scores on the syndrome scale internalizing, externalizing, and the total score; p = 0.001 (Table 3, Fig. 3).
Secondary outcomes.
Table 4 shows the CPT parameters of the children under study. Twelve weeks of intervention increased the correct responding rate during the entire number of trials (target accuracy rate) in the probiotics group compared with the control group with a significant mean change difference (p = 0.02). The absence of the required response (omission errors) for attention selectiveness was less in the probiotics group than in the control group, with a significant mean change difference between the two groups (p = 0.043). Although the probiotics group’s mean score decreased from baseline with a mean change over 12 weeks in reaction time (RT) on correct trials in ms (− 3.9 ± 96.56) and the standard deviation of RT (variability) (− 7.3 ± 134.7) compared with those of the control group (0.58 ± 130.13 and 1.31 ± 115.06, respectively), this difference did not reach statistical significance. Furthermore, the two groups did not differ in the mean and standard deviation of the error RT (p > 0.05). Addressing impulsivity, we found no significant difference between the two groups over 12 weeks in the inhibited RT during the entire number of trials (foil accuracy rate) (p = 0.606) and in the false required response (commission errors) (p = 0.559).
Regarding WCST performance, a significant difference over 12 weeks was found in the probiotics group in the following indices: correct responses, perseverative responses, perseverative error (response when the old rule is still applied), and non-perseverative errors (attentional inability to inhibit distraction within the same perceptual category),(p = 0.016, p = 0.027, p = 0.017, and p = 0.044, respectively), but not in the control group. However, the Mann–Whitney U-test analysis of the mean change difference between the two groups in these indices revealed no significant differences (p > 0.05). The remaining index, including failure to maintain the set, did not significantly improve for either group (p > 0.05) (Table 5).
Adverse events
An analysis of the medication’s potential adverse effects revealed no notable undesirable symptoms in either group. Seven participants (three in the probiotics group and four in the control group) reported decreased appetite. One patient from the probiotics group reported having diarrhea, which cleared up after a few days.
Discussion
As knowledge of the GBA has risen with emerging research highlighting this bidirectional relationship with a theoretical translation of animal models to human analyses, the core mechanism and which probiotics have a promising or negative result remain ambiguous. To the best of our knowledge, this study is the first randomized controlled trial to use a Lactobacillus acidophilus LB strain as supplementation added to a weight-dependent dose of atomoxetine to examine its impact on the core symptoms, behavior, and cognition of children and adolescents with ADHD.
Twelve-week supplementation with L. acidophilus LB combined with a weight-dependent dose of atomoxetine could improve the symptoms and behavioral problems of ADHD, according to the parental reports of the CPRS-R-L and CBCL, respectively, relative to the control group.
Our findings support the results of a recent trial by Ghanaatgar et al. [32]. The study showed that taking a multispecies probiotic capsule containing 14 bacterial strains, including L. acidophilus, for 8 weeks alongside Ritalin medication positively affected the severity of ADHD symptoms. This was measured by improved scores on the Clinical Global Impression–Severity scale (CGI–S) and the Revised Conners Parent Rating Scale–Short Version (CPRS–RS).
Another study investigated the effects of probiotics on the psychological health of children with ADHD. The study found that probiotic treatment for 8 weeks, using four bacterial strains, including Lactobacillus acidophilus, significantly improved the severity of ADHD symptoms and anxiety compared to a placebo. The improvement was measured using the ADHD and Hamilton Anxiety Rating scales. However, probiotics did not have an impact on depression. [33]. Of note, a recent Taiwanese study found that using the oral probiotic Bifidobacterium bifidum-688 (Bf-688) for 8 weeks reduced the clinical symptoms of patients with ADHD while increasing their body weights and body mass index. The Bf-688 supplement also markedly changed the composition of the gut flora [20].
Contradictory to our findings, Kumperscak et al. [34] evaluated the influence of LGG on treatment-naive children and adolescents with ADHD and found no considerable improvement in the core symptoms or mental health problems compared with those who received a placebo after 3 months. However, the authors found that the probiotic group significantly outperformed the placebo group in the Child Self-Report measure of quality of life and suggested including patients receiving stable pharmacotherapy in future trials to identify more significant changes. The fact that probiotic benefits can differ according to the strain employed, dose, duration, and methodological variations may help explain the variable outcomes between trials.
Available reviewing research has broadened our understanding of the link between probiotic supplementation and its impact on ADHD clinical symptoms. However, their findings remain inconclusive to formulate any clinical recommendation or approaches. As the underlying etiopathogenesis of ADHD is still unclear, investigating the role of the complex messaging system between the microbiota, gut, and brain has drawn much attention [35, 36]. Furthermore, researchers considered these pathways as an area that seems amenable to change in treating neurodevelopmental disorders, such as ADHD, possibly without adverse effects.
Considering that the GBA hypothesis has been linked to the pathophysiological pathways underlying ADHD [36], it may be reasonable to view probiotic supplementation's ability to address gut dysbiosis as a possible treatment target for ADHD. Moreover, probiotics' effectiveness in treating ADHD symptoms may be due to their ability to prevent an inflammatory reaction [37]. A microbial imbalance is associated with a breakdown of the immune system’s homeostasis by a boost in potentially inflammatory bacteria, which disrupts intestinal permeability and can increase the movement of pathogenic bacteria’s metabolites into the systemic circulation, which may lead to an inflammatory process [12, 13]. Hence, this may affect the permeability of the blood–brain barrier, contributing to neuroinflammation in neurodevelopmental disorders such as ADHD [14, 15]. Probiotics can improve gut integrity, preventing metabolite leakage and inhibiting the inflammatory cascade [37, 38].
Regarding the secondary outcomes under study, our current analysis found that the probiotic group revealed improvement in the target accuracy rate and omission errors compared with the control group (medium effect size), suggesting that Lactobacillus acidophilus LB could have a favorable effect on focused attention as indicated by the CPT. However, we did not find a comparable improvement in impulsivity.
Mounting evidence suggests that the cholinergic and dopaminergic systems contribute to the pathophysiology of selective attention [39]. An interesting study found that the psychobiotic strain Lactobacillus PS128 improved CPT measures of ADHD in children with Tourette syndrome [40]. The authors attributed the improvements to PS128’s potential to regulate serotonergic and dopaminergic signaling in mouse brains, as demonstrated by experimental findings from animal models [41].
Our results showed improvement in the executive functions in the probiotic group reflected in both perseverative and non-perseverative errors on WCST performance (small-medium effect size); however, this did not reach a significant level of differences between the two groups.
Impairments in executive functions (i.e., cognitive flexibility, working memory, sustained attention, inhibitory control, and planning) are considered a core deficit in the cognitive function of ADHD, which may play a crucial role in the challenging adaptation of ADHD [42, 43].
The cognitive regulation of behavior and reward perception is modulated via the norepinephrine and dopamine circuits, which connect to the prefrontal cortex and striatum, and these pathways are considered fundamental in the pathophysiology of ADHD [44].
Until now, limited randomized trials have investigated the impact of probiotic supplementation on cognitive performance [45]. Although a questionable probiotic strain influences cognition, one study employing Lactobacillus rhamnosus supplements demonstrated a favorable influence on cognitive function and a lowered risk of developing ADHD [18].
The linkage between ADHD and the microbiota can be understood in terms of how neurotransmitters function in cognition. A recent study has provided insights into the GBA and introduced a new strain that improves cognitive function through this axis. Using healthy mice, Jeon et al. [46] investigated the effects of three probiotic groups on cognitive function: Lactobacillus acidophilus EG004, Lacticaseibacillus rhamnosus, and Lacticaseibacillus paracasei. The three probiotic-fed testing groups demonstrated better cognitive function; however, the L. acidophilus-fed group was superior to the other two groups and scored the highest on cognitive–behavioral assessments. Focusing on understanding how the changed microbial diversity affects the brain, a 16S-23S rRNA sequencing of the gut microbiome of the L. acidophilus group was performed. It was found that the L. acidophilus group had an elevated proportion of L. acidophilus presence, suggesting that a good proportion of L. acidophilus can be adequately ingested without being harmed by the digestive juices. Researchers hypothesized that an increase in L. acidophilus in the intestines modifies neurotransmitters and neurotrophic factors, including dopamine, noradrenaline, gamma-aminobutyric acid (GABA), and serotonin, affecting an animal’s nervous system. Interestingly, they found that ingesting L. acidophilus increased SCFAs in the gut of experimental mice, indicating L. acidophilus’s ability to produce SCFAs, which may positively impact brain function. The microbially fermented compounds SCFAs, such as acetate, propionate, and butyrate, stimulate indirect signaling in the brain by modifying and inducing neurotransmitters and neurotrophic factors, such as GABA and BDNF [47, 48]. Considering this information, the authors reported the inability to fully identify the changed metabolites from the animal body, which is required to understand the mechanism underlying the improved cognitive ability.
In the present study, most participants were males. The finding aligns with previous studies indicating that ADHD is more common in boys [49, 50]. However, evidence displayed a comparable clinical profile in boys and girls [51, 52].
Limitations
This study is an open-label study without a placebo intervention. Given that the parents were knowledgeable of the treatment their child had experienced, this potentially may have affected how they responded to the questionnaires. However, parallel to the parent responses, we also included various objective measure evaluations (i.e., the CPT and WCST), and the results showed the advantage of adding probiotics to the standard treatment alone. While our findings showed that a 3-month probiotic intervention was beneficial, there were no observable changes in some cognitive functions measured by the neuropsychological assessment battery, leading us to believe that the study’s duration was insufficient to track the improvements. Therefore, further research with a longer intervention time is needed. In this study, we did not investigate the socioeconomic status of the groups; however, SES should be assessed in microbiome studies, given that it can be an influential confounding variable that impacts the analysis of the study results [53]. Finally, the homogeneity of the sample’s ethnic and geographic distribution, its small size, and its recruitment from two similar referral centers may restrict the generalizability of our findings.
Conclusion
In conclusion, this study has demonstrated that 3 months’ supplementation of oral probiotics, such as Lactobacillus acidophilus LB (Lacteol Fort) added to a weight-dependent dose of atomoxetine improved the severity of symptoms, sustained attention, and executive functions in children and adolescents with ADHD. Considering this information, Lactobacillus acidophilus LB may be a desirable supplementary therapy for children with ADHD without side effects. Future research is recommended to verify the treatment impacts of the probiotic Lactobacillus acidophilus LB on the core symptoms and cognitive functions of ADHD.
Availability of data and materials
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Abbreviations
- ADHD:
-
Attention-deficit/hyperactivity disorder
- BDNF:
-
Brain-derived neurotrophic factor
- Bf-688:
-
Bifidobacterium bifidum-688
- CBCL:
-
Child Behavioral Checklist
- CPRS-R-L:
-
Conners Parent Rating Scale–Revised Long Version
- CPT:
-
Conners Continuous Performance
- GBA:
-
Gut–brain axis
- SCFAs:
-
Short-chain fatty acids
- HPA:
-
Hypothalamic–pituitary–adrenal
- LGG:
-
Lactobacillus rhamnosus GG
- L. acidophilus:
-
Lactobacillus acidophilus
- PEBL:
-
Psychology Experiment Building Language
- WISC-III:
-
Wechsler Intelligence Scale for children
- WCST:
-
Wisconsin (Berg) Card Sort Test
References
Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics. 2015;135:e994-1001.
Dias TGC, Kieling C, Graeff-Martins AS, Moriyama TS, Rohde LA, Polanczyk GV. Developments and challenges in the diagnosis and treatment of ADHD. Rev Bras Psiquiatr. 2013;35(SUPPL. 1):40–50.
Stewart A, Davis GL, Gresch PJ, Katamish RM, Peart R, Rabil MJ, et al. Serotonin transporter inhibition and 5-HT 2C receptor activation drive loss of cocaine-induced locomotor activation in DAT Val559 mice. Neuropsychopharmacology. 2019;44:994–1006.
Kim JI, Yoo JH, Kim D, Jeong B, Kim BN. The effects of GRIN2B and DRD4 gene variants on local functional connectivity in attention-deficit/hyperactivity disorder. Brain Imaging Behav. 2018;12:247–57.
Sharma A, Couture J. A Review of the Pathophysiology, Etiology, and Treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Ann Pharmacother. 2014;48:209–25.
Sukmajaya AC, Lusida MI, Soetjipto, Setiawati Y. Systematic review of gut microbiota and attention-deficit hyperactivity disorder (ADHD). Ann Gen Psychiatry. 2021;20:1–12.
Boonchooduang N, Louthrenoo O, Chattipakorn N, Chattipakorn SC. Possible links between gut–microbiota and attention-deficit/hyperactivity disorders in children and adolescents. Eur J Nutr. 2020;59:3391–403.
Luczynski P, Neufeld KAMV, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016;19:1–17.
Jurek L, Sevil M, Jay A, Schröder C, Baghdadli A, Héry-Arnaud G, et al. Is there a dysbiosis in individuals with a neurodevelopmental disorder compared to controls over the course of development? A systematic review. Eur Child Adolesc Psychiatry. 2021;30:1671–94.
Kedem S, Yust-Katz S, Carter D, Levi Z, Kedem R, Dickstein A, et al. Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults. World J Gastroenterol. 2020;26:6626–37.
Chang JPC, Mondelli V, Satyanarayanan SK, Chiang YJ, Chen HT, Su KP, et al. Cortisol, inflammatory biomarkers and neurotrophins in children and adolescents with attention deficit hyperactivity disorder (ADHD) in Taiwan. Brain Behav Immun. 2020;88:105–13.
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609.
Corominas-Roso M, Ramos-Quiroga JA, Ribases M, Sanchez-Mora C, Palomar G, Valero S, et al. Decreased serum levels of brain-derived neurotrophic factor in adults with attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2013;16:1267–75.
Tsai SJ. Attention-deficit hyperactivity disorder may be associated with decreased central brain-derived neurotrophic factor activity: Clinical and therapeutic implications. Med Hypotheses. 2007;68:896–9.
Cenit MC, Nuevo IC, Codoñer-Franch P, Dinan TG, Sanz Y. Gut microbiota and attention deficit hyperactivity disorder: new perspectives for a challenging condition. Eur Child Adolesc Psychiatry. 2017;26:1081–92.
Sanders ME. Probiotics: Definition, sources, selection, and uses. Clin Infect Dis. 2008;46(SUPPL):2.
McFarland L V. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: A systematic review. BMJ Open. 2014;25;4(8):e005047.
Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr Res. 2015;77:823–8.
McVey Neufeld KA, O’Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, et al. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2019;22:425–34.
Wang LJ, Yang CY, Kuo HC, Chou WJ, Tsai CS, Lee SY. Effect of Bifidobacterium bifidum on Clinical Characteristics and Gut Microbiota in Attention-Deficit/Hyperactivity Disorder. J Pers Med. 2022;7;12(2):227.
María Remes Troche J, Coss Adame E, Ángel Valdovinos Díaz M, Gómez Escudero O, Eugenia Icaza Chávez M, Antonio Chávez-Barrera J, et al. Lactobacillus acidophilus LB: a useful pharmabiotic for the treatment of digestive disorders. Therap Adv Gastroenterol. 2020;13:1–15.
Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18.
American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). 5th ed. Washington, DC, USA: American Psychiatric Publishing; 2013.
Ismail MEML. Wechsler Intelligence Scale for children: Arabic manual. 7th ed. Cairo: El-Nahda Press; 1999.
Conners CK. The Conners Rating Scales – Revised manual. North Towanda, NY: Multi-health Systems; 1997.
Al-Behairy A, Aglaan A. Conner’s’ rating scales. Egypt: Dar El Nahda; 2009.
Achenbach TMRL. Manual for the ASEBA School-Age Forms & Profiles. VT: ASEBA Burlington; 2001.
Mueller STPB. The Psychology Experiment Building Language (PEBL) and PEBL test battery. J Neurosci Methods. 2014;222:250–9.
Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;31(5):555–62.
Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22.
Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–99.
Ghanaatgar M, Taherzadeh S, Ariyanfar S, Razeghi Jahromi S, Martami F, Mahmoudi Gharaei J, et al. Probiotic supplement as an adjunctive therapy with Ritalin for treatment of attention-deficit hyperactivity disorder symptoms in children: a double-blind placebo-controlled randomized clinical trial. Nutr Food Sci. 2022. https://doi.org/10.1108/NFS-12-2021-0388.
Sepehrmanesh Z, Shahzeidi A, Mansournia M, Ghaderi A, Ahmadvand A. Clinical and metabolic reaction to probiotic supplement in children suffering attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled experiment. Int Arch Heal Sci. 2021;8:90.
Kumperscak HG, Gricar A, Ülen I, Micetic-Turk D. A Pilot Randomized Control Trial With the Probiotic Strain Lactobacillus rhamnosus GG (LGG) in ADHD: Children and Adolescents Report Better Health-Related Quality of Life. Front Psychiatry. 2020;11:181.
Sandgren AM, Brummer RJM. ADHD-originating in the gut? The emergence of a new explanatory model. Med Hypotheses. 2018;120:135–45.
Dam SA, Mostert JC, Szopinska-Tokov JW, Bloemendaal M, Amato M, Arias-Vasquez A. The Role of the Gut-Brain Axis in Attention-Deficit/Hyperactivity Disorder. Gastroenterol Clin North Am. 2019;48:407–31.
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front Immunol. 2021;12:578386.
Mennigen R, Bruewer M. Effect of probiotics on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:183–9.
Noudoost B, Moore T. The role of neuromodulators in selective attention. Trends Cogn Sci. 2011;15:585–91.
Wu CC, Wong LC, Hsu CJ, Yang CW, Tsai YC, Cheng FS, et al. Randomized controlled trial of probiotic ps128 in children with tourette syndrome. Nutrients. 2021;13(11):3698.
Liu WH, Chuang HL, Te HY, Wu CC, Chou GT, Wang S, et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res. 2016;298:202–9.
Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94.
Wåhlstedt C, Thorell LB, Bohlin G. ADHD symptoms and executive function impairment: Early predictors of later behavioral problems. Dev Neuropsychol. 2008;33:160–78.
Kalenik A, Kardaś K, Rahnama A, Sirojć K, Wolańczyk T. Gut microbiota and probiotic therapy in ADHD: A review of current knowledge. Prog Neuro-Psychopharmacology Biol Psychiatry. 2021;30;110:110277.
Rianda D, Agustina R, Setiawan EA, Manikam NRM. Effect of probiotic supplementation on cognitive function in children and adolescents: A systematic review of randomised trials. Benef Microbes. 2019;10:873–82.
Jeon S, Kim H, Kim J, Seol D, Jo J, Choi Y, et al. Positive Effect of Lactobacillus acidophilus EG004 on Cognitive Ability of Healthy Mice by Fecal Microbiome Analysis Using Full-Length 16S–23S rRNA Metagenome Sequencing. Microbiol Spectr. 2022;10:e0181521.
Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–7.
Barichello T, Generoso JS, Simões LR, Faller CJ, Ceretta RA, Petronilho F, et al. Sodium Butyrate Prevents Memory Impairment by Re-establishing BDNF and GDNF Expression in Experimental Pneumococcal Meningitis. Mol Neurobiol. 2015;52:734–40.
Bauermeister JJ, Shrout PE, Chávez L, Rubio-Stipec M, Ramírez R, Padilla L, et al. ADHD and gender: Are risks and sequela of ADHD the same for boys and girls? J Child Psychol Psychiatry Allied Discip. 2007;48:831–9.
Willcutt EG. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics. 2012;9:490–9.
Biederman J, Kwon A, Aleardi M, Chouinard VA, Marino T, Cole H, et al. Absence of gender effects on attention deficit hyperactivity disorder: Findings in nonreferred subjects. Am J Psychiatry. 2005;162:1083–9.
Levy F, Hay DA, Bennett KS, McStephen M. Gender differences in ADHD subtype comorbidity. J Am Acad Child Adolesc Psychiatry. 2005;44:368–76.
Nobre JG, Alpuim Costa D. ”Sociobiome”: How do socioeconomic factors influence gut microbiota and enhance pathology susceptibility? - A mini-review. Front Gastroenterol. 2022;1:1–6.
Acknowledgements
We would like to thank all children and adolescents who participated in the study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
RE and HE were responsible for the determination of the study topic and the design of the study. RA contributed to the first draft of the manuscript writing. RE and HE revised the manuscript. RA and HM were responsible for the research activity planning and execution. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Ain-Shams University Hospitals (Ethical Committee No. FMASU 158). All methods were carried out in accordance with the Declaration of Helsinki.
Informed consent was obtained from the legal guardians of the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elhossiny, R.M., Elshahawy, H.H., Mohamed, H.M. et al. Assessment of probiotic strain Lactobacillus acidophilus LB supplementation as adjunctive management of attention-deficit hyperactivity disorder in children and adolescents: a randomized controlled clinical trial. BMC Psychiatry 23, 823 (2023). https://doi.org/10.1186/s12888-023-05324-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-05324-4