Abstract
Introduction
Sexual violence is one of the most severe traumatic events. It is associated with a higher risk for post-traumatic stress disorder (PTSD) development. Sleep disturbances such as insomnia are frequently reported by PTSD patients and play a key role in the development and course of the disorder. Sleep disturbances are associated with higher levels of pro-inflammatory cytokines emphasizing the importance of sleep studies in individuals with PTSD.
Objectives
To investigate the association between subjective and objective sleep measurements and PTSD symptoms with inflammatory markers in women with PTSD following sexual assault.
Methods
In this longitudinal study fifty-seven women with PTSD were evaluated for sleep measurements and inflammatory markers. Participants completed the Clinician-Administered PTSD Scale, the Beck Depression Inventory, the Pittsburgh Sleep Quality Index (PSQI), and the Insomnia Severity Index. In addition, patients underwent full in-lab polysomnography and serum levels of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP) measurement. All assessments were performed at baseline and after one year. Patients received pharmacological and/or psychological interventions between baseline and one-year follow-up.
Results
Despite improving PTSD symptoms severity and sleep quality (expressed in PSQI), we found an increase in the inflammatory markers IL-1β, TNF-α, IL-6 and CRP after one year of follow-up. These findings suggest that neurobiological processes may advance independently of PTSD symptoms. We found a significant increase in the levels of IL-1β and TNF-α associated with decreased slow-wave sleep (p = 0.019 and p = 0.018 respectively), IL-6 associated with arousal index (p = 0.024), and CRP associated with insomnia severity (p = 0.012), and sleep duration longer than 6 h per night (p < 0.001).
Conclusions
Sleep impairments in PTSD may be associated with a gradual and persistent alteration in the immune system, resulting in a progressive inflammatory process. Our results suggest that sleep mechanisms are involved in this incident inflammatory process in young women with PTSD.
Similar content being viewed by others
Background
Nightmares and insomnia are common symptoms and are considered the hallmark of PTSD [1]; also, a growing body of evidence suggests that sleep disturbances are a component of the pathophysiology of PTSD. Sleep disturbances immediately after trauma are a risk factor for PTSD development [2, 3], and improved sleep quality has been associated with a reduction in the severity of PTSD symptomatology [4, 5]. Moreover, sleep disruption preceding traumatic exposure also predicts PTSD development [6, 7].
Sleep is a crucial physiological process controlled by several regulatory systems, including a complex system of neuroendocrine, neurotransmitters, and neuropeptides that can be influenced by inflammatory biomarkers in a bidirectional manner [8]. Evidence suggests an association between disturbed sleep and inflammatory cytokines [8,9,10,11]. A study demonstrated that acute total and short-term partial sleep deprivation elevated C-reactive protein (CRP) concentrations in healthy adult subjects [10]. A meta-analysis evaluating sleep disturbance, sleep duration, and inflammation in population-based samples found an association between sleep disturbance and long sleep duration (> 8 h/night) with increases in inflammatory biomarkers [11]. Moreover, studies suggest that the association between sleep disturbances and pro-inflammatory cytokines is more robust in women than in men [5, 13]. Suarez (2008) found a correlation between poor sleep quality and greater frequency of disturbed sleep symptoms with elevated levels of IL-6 and CRP in women, while such an association was not observed in men, demonstrating sex differences in the association between sleep and cytokines [14,15,16].
Research suggests an association between disturbed sleep and inflammatory cytokines [8,9,10,11] however, few studies have investigated inflammatory biomarkers in PTSD-related sleep disturbances. Heinzelmann et al. (2014) showed that military personnel with insomnia who reported sleep restoration after deployment had reduced CRP concentrations, decreased depression severity, and tended toward fewer PTSD symptoms [17]. Nevertheless, another study did not find a significant correlation between insomnia severity and inflammatory markers in women with PTSD [18]. Understanding the magnitude of this association, in which potential inflammation might be involved, has critical clinical applications for reducing PTSD symptom severity and physical comorbidities. Elevated levels of systemic pro-inflammatory cytokines are a risk factor for the development of cardiovascular disease, metabolic syndrome, and dementia observed in chronic PTSD and are better described among male veterans [19,20,21].
Therefore, we aimed to study the relationship between sleep quality and inflammatory markers in women with PTSD. We investigated sexually assaulted women with PTSD by measuring changes in sleep measurements and inflammatory biomarkers over one year.
Methods
Fifty-seven women with PTSD were evaluated between October 2015 and October 2018. Inclusion criteria were age between 18 and 45 years, a history of sexual assault six months before study enrollment, and a diagnosis of PTSD according to DSM-5 criteria. Exclusion criteria were menopausal symptomatology, pregnancy, corticosteroid use, history of human immunodeficiency virus infection, acute or unstable clinical conditions, neurological disorders, and a lifetime history of bipolar, psychotic, or substance dependence or abuse (not in remission in the previous six months). Participants undergoing any psychological or psychiatric treatment were also excluded. The ethics committee of the Universidade Federal de Sao Paulo (UNIFESP) approved all clinical procedures (reference number 3.115.325). All participants provided written informed consent prior to the assessments.
The current study is part of a more extensive study protocol entitled “Post-traumatic stress disorder and neuroprogression in women following sexual assault: protocol for a randomized clinical trial evaluating allostatic load and aging process acceleration.” Detailed methods have been described previously [22]. The clinical trial of this study was registered at the Brazilian Clinical Trials Registry (registration number: RBR-3z474z, registration date: 28/09/2017).
Trained psychiatrists and psychologists clinically assessed participants and underwent polysomnography (PSG) and blood collection at baseline and one-year follow-up. Participants were randomized to treatment with sertraline or interpersonal psychotherapy adapted to PTSD (IPT-PTSD) and were followed for 14 weeks. Sertraline dosage ranged from 50 to 200 mg/daily, depending on clinical presentation and tolerance. IPT-PTSD was delivered in 14-weekly 50-min sessions. Participants were clinically evaluated at baseline, week 2, week 4, week 8, and week 14. After 14 weeks, participants received treatment as usual: antidepressants, antipsychotics, hypnotics, and/or psychotherapy until completing one year of follow-up (Fig. 1).
Measures
The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) is a structured diagnostic interview containing a 30-item questionnaire capable of assessing the frequency and intensity of PTSD symptoms and the variables associated with the trauma using a frequency/severity scale varying from 0 to 4. A large-scale psychometric study showed strong evidence of its validity and reliability as a measure for the symptoms of PTSD. A validated Brazilian Portuguese language version was used [23, 24].
The Beck Depression Inventory–Second Edition (BDI-II) is a self-reported instrument composed of a 21-item questionnaire measuring clinical depression. The participant must evaluate each question regarding depression symptoms on a 0–4 severity scale. The score consists of the sum of the individual items classifying the severity of the depression as minimum 0–13, mild 14–19, moderate 20–28, or severe 29–63. A validated Portuguese language version was used [25, 26].
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-reported measure that assesses seven components of sleep quality, including sleep latency, duration, efficiency, disturbances, use of sleep medication, and daytime dysfunction. Each component is rated on a 0–3 scale referring to the frequency of each disturbance, and the sum of the components provides a global score ranging from 0 to 21. A PSQI global score higher than 5 indicates clinical sleep disturbance. A validated Portuguese language version was used [27, 28].
The Insomnia Severity Index (ISI) is a 7-item self-report questionnaire measuring the patient’s self-perception of insomnia. The ISI targets the subjective symptoms and consequences of insomnia and assesses the severity of sleep onset, sleep maintenance, early morning awakening problems, sleep dissatisfaction, interference of sleep difficulties with daytime functioning, noticeability of sleep problems by others, and distress caused by sleep difficulties. The total score ranges from 0 to 28; 0–7 indicates the absence of insomnia, 8–14 is subthreshold, 15–21 is moderate, and 22–28 is severe [29, 30].
Polysomnography
Polysomnography is the gold standard for objectively measuring sleep. Participants underwent one night of polysomnography recording at baseline and one night at the one-year follow-up. PSG recordings were conducted in the Instituto do Sono-AFIP at the Universidade Federal de Sao Paulo. Recordings were conducted using an Embla (Embla N7000, Embla Systems, Inc., Broomfield, CO, USA), and all recordings included a six-channel electroencephalogram (EEG) (F4-M1, C4-M1,02-M1, F3-M2, C3-M2, and 01-M2), a two-channel electrooculogram, a four-channel electromyograph (EMG) (electrodes submental and tibial) and a one-channel electrocardiograph (D2-modified). Airflow was detected using a thermocouple and nasal cannula. Respiratory effort was assessed using inductance plethysmography belts. Snoring, body position, blood oxygen saturation, and pulse were evaluated using Embla® sensors (Embla Systems Inc.). All PSGs were performed and scored by two technicians following sleep studies guidelines and were reviewed by a sleep medicine physician. Sleep stages, EEG arousals, and leg movements were scored according to established criteria [31]. Apnea was defined as complete or close to complete airflow cessation for ≥ 10 s, and hypopnea was identified as an evident reduction in the breathing amplitude (at least 30% below the baseline) for ≥ 10 s accompanied by either an EEG arousal or a blood oxygen saturation drop of ≥ 3% [32, 33].
The following sleep variables were determined: REM sleep latency, sleep onset latency; total sleep time, wake after sleep onset, sleep efficiency, and the percentages of total sleep composed of N1, N2, N3, REM sleep, arousal index (number of arousals per hour), periodic limb movements index with and without arousal, number of limb movements per hour with and without arousal, apnea-hypopnea index, number of apneas, and hypopneas per hour. In our study, we included the following variables in the analysis: total sleep time (TST), arousal index (AI), and slow-wave sleep (N3%) since research on sleep and inflammation have associated sleep duration, sleep fragmentation, and slow wave sleep with inflammation [8,9,10,11,12]. We categorized each of the sleep variables into TST (< and > 6 h), N3% (< and > 20), and AI (< and > 10) to assess the effect of sleep architecture on inflammatory markers.
Blood collection and cytokine assays
Blood samples were collected at approximately 7 AM on the day following the PSG recordings. The inflammatory markers were assayed from overnight fasting serum samples. The serum was stored at − 80 ºC. The Milliplex Map® panel based on the Luminex Map® technology was used to measure interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, and highly sensitive enzyme-linked immunosorbent was used to measure the C-reactive protein (CRP) levels. All procedures were performed following the manufacturers’ guidelines. After completing one year in the study, participants repeated blood collection and analysis procedures.
The minimum detectable concentrations for IL-1β, IL-6, TNF-α, and CRP were 0.8 pg/ml, 0.9 pg/ml, 0.7 pg/ml, and 0.9 ng/ml, respectively. Samples were sent for analysis at baseline (T1) and one-year follow-up (T2). All analyses were performed in duplicate. Samples were kept for a maximum of two and a half years for the first analysis and three years for the second analysis. All procedures were performed following the manufacturers’ guidelines.
Statistical analysis
Statistical analyses were performed using SPSS software version 24.0, with an alpha level of 0.05 for all analyses. The Kolmogorov-Smirnov test was used to analyze continuous data distributions. Log transformation was applied if the data did not fit the normality assumption. A generalized linear model was used to analyze clinical characteristics between groups (PTSD group at baseline and after one-year follow-up). Variables were expressed as means and standard deviations. Fisher exact test or Chi-Square test was used to compare frequencies. Multiple linear regression analysis was used to estimate the association of inflammatory markers’ delta values (T2-T1) with clinical and sleep assessments’ delta values (T2-T1). The final model was obtained from preliminary analysis using bivariate Pearson correlations. The best-fit model was chosen using the Wald method. The Durbin-Watson test was used for autocorrelations, and the variance inflation factor (VIF) and tolerance were performed to determine the presence of multicollinearity. We calculated tolerance and VIF values to evaluate multicollinearity between variables, with tolerance > 0.2 and VIF < 10 indicating no collinearity among the independent variables. Regarding the extreme (outliers) values of the concentrations of the cytokines, we used Z-score to determine outliers. The cutoff values for finding outliers were a Z-score of +/- 3 or more. Participants with outliers’ values were excluded from the analysis. The generalized estimating equation (GEE) was used for data analysis at baseline (T1) and after a one-year (T2) follow-up.
Results
Clinical and sleep assessments, PSG parameters, and inflammatory markers levels collected at baseline and at one-year follow-up are displayed in Table 1. The mean age of the sample was 24.5 ± 6.8 years, and the mean body mass index was 24.5 ± 4.8 kg/m2. The mean time between the sexual assault and baseline evaluation was 2.4 ± 1.7 months.
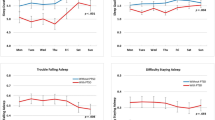
The patients showed a significant increase in the levels of inflammatory markers IL-1β (p < 0.001), TNF-α (p < 0.001), and CRP (p < 0.001), despite improvement in PTSD (p < 0.001) and depression (p < 0.001) symptoms from baseline through one year follow-up. Regarding sleep assessments, there was an improvement in sleep quality (PSQI, p = 0.001) and insomnia severity (p < 0.001).
Regression analysis revealed a significant association between arousal index (AI) and IL-6 score change from T2 - T1. Patients with greater arousal index values were associated with higher IL-6 values (Table 2). This finding suggests that sleep fragmentation is associated with IL-6 enhancement.
Regarding CRP (Table 2), there was a significant association between insomnia severity (ISI) and CRP score change from T2-T1, indicating that patients with worse insomnia showed higher CRP levels. We found no association between clinical and sleep measurements and IL-1β and TNF-α levels (Table 3). None of the inflammatory markers were associated with PTSD symptoms (CAPS-5), depressive symptoms (BDI), and sleep quality (PSQI).
GEE analysis
Inflammatory markers and total sleep time (TST): TST < and > six hours (Table 4)
We found a significant interaction between sleep duration and CRP levels. Participants who slept more than 6 h per night showed greater CRP levels when compared to women who slept less than 6 h after controlling for body mass index, depression, and PTSD severity.
Inflammatory markers and slow-wave sleep (SWS): N3% < 20 and > 20 (Table 4)
There were significant interaction effects between SWS and IL-1β and TNF-α levels. The N3% < 20 group showed increased levels of IL-1β and TNF-α than the N3% > 20 groups throughout one year of follow-up. Participants with lower SWS had higher IL-1β and TNF-α levels after adjustment for confounders. Regarding CRP levels, we found a trend toward significance in the association with lower SWS (p = 0.06) after adjustment.
Inflammatory markers and arousal index (AI): AI < 10 and > 10 (Table 4)
No significant interaction effects existed between sleep fragmentation (expressed in AI) categories and inflammatory levels throughout the one-year study.
Discussion
The current study examined subjective and objective sleep measurements and levels of inflammatory cytokines following one year of PTSD treatment in a sample of young women. Our results showed a significant increase in the pro-inflammatory markers despite PTSD symptoms improvement after one year of follow-up, suggesting that neurobiological processes may advance independently of clinical improvement. Nevertheless, we found a significant association between greater sleep fragmentation and IL-6, decreased SWS and TNF-α, and greater insomnia severity, sleep duration, and CRP.
These results are consistent with some previous studies on inflammation and sleep. Two studies have found an association between sleep and IL-6. Vgontzas and colleagues (2019) reported that IL-6 levels had a negative association with the amount and depth of sleep; sleep deprivation was associated with increased daytime IL-6 levels, while good sleep quality was associated with decreased IL-6 [34]. Hong and colleagues (2005) evaluated the association of IL-6 with sleep architecture in healthy subjects and suggested that daytime IL-6 levels were negatively associated with sleep efficiency and slow-wave sleep [35]. Furthermore, in a study that investigated the effects of two hours of sleep deprivation per night found increased TNF-α in healthy men but not women [36].
Previous investigations of sleep duration and CRP have produced mixed results. Some studies have found an association between increased CRP and short sleep duration [10, 37], while others have found increased CRP and long sleep duration [38, 39]. In our study, levels of CRP were significantly higher in women who slept more than 6 h per night, compared to women who slept less than 6 h. Consistent with our findings, studies showed that each additional hour of sleep duration was associated with an 8% increase in CRP levels [39] and longer sleep duration was associated with higher levels of CRP and IL-6. These associations remained after adjustment for waist circumference, diabetes, heart disease, and depressive symptoms [40]. A large-scale study found higher CRP levels in women who slept 5 h or less but no association between sleep duration and CRP was observed in men [41]. Indeed, some evidence suggests that sex may influence the inflammatory response to sleep impairments [41,42,43].
The mechanisms that might explain the relationship and directionality between sleep and inflammation are complex and are not fully understood. Physiological sleep is associated with a decline in circulating catecholamines, with lower levels of noradrenaline and adrenaline during sleep [44]. Brief sleep deprivation or fragmentation is usually associated with mild and temporary increased sympathetic activity. However, chronic sleep deprivation can promote the activation of the stress systems, and a progressive and gradual increase in catecholamine levels [45]. These neurobiological changes may not be immediately evident; however, studies have shown that chronic sleep deprivation can gradually affect the reactivity of the neuroendocrine system, increasing vulnerability or maintenance of stress-related disorders [46]. Therefore, chronic sleep impairments in PTSD can cause gradual changes in the central nervous system and affect neuroendocrine system reactivity, and pro-inflammatory response over time. These neurobiological processes may advance independently of PTSD symptoms. In the reverse direction, increased levels of inflammatory mediators may act directly on neurons in the brain to further interrupt sleep [47].
The present study has limitations. Because our participants were sexually assaulted women and had severe PTSD symptoms, the high dropout rate observed in the follow-up may be due to feelings of shame, fear of stigma, or avoidance of exposure to painful memories, which is one of the symptoms of PTSD. An additional limitation, the objective sleep measures were depicted from one night of PSG assessments, which might not represent the participants’ sleep duration. However, an epidemiological study examining risk factors for chronic insomnia based on one night of PSG showed that their results were consistent with other epidemiological studies regarding objective sleep duration [48]. Another limitation is that neither socioeconomic status nor randomized treatment (sertraline vs. psychotherapy) was adjusted for possible confounders. Lastly, we examined inflammatory markers based on a single blood sample drawn at enrollment and after one year. Multiple and sequential blood testing could better demonstrate the trend toward the increase in inflammatory markers.
Our strengths include a longitudinal study design in young women with PTSD following sexual assault, subjective sleep measurements, in-laboratory PSG monitoring, and measures of several pro-inflammatory markers.
Conclusion
In summary, our results suggest that chronic sleep restriction and fragmentation may be associated with a gradual and persistent alteration in the immune mediators, resulting in a progressive inflammatory process, which, in turn, may be a risk factor for several pathologies such as cardiovascular and neurodegenerative diseases in PTSD women.
Prospective studies investigating the association between the immune, neuroendocrine, and central nervous systems in PTSD are essential for better understanding of sleep disorders’ pathophysiology. Our findings suggest that improving the assessment and treatment of sleep disturbances in PTSD women may reduce morbidity risk and PTSD symptoms severity.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- AI:
-
Arousal index
- BAI:
-
Beck Anxiety Inventory
- BDI:
-
Beck Depression Inventory
- BMI:
-
Body mass index
- CAPS-5:
-
Clinician-Administered PTSD Scale
- CRH:
-
Corticotropin-releasing hormone
- CRP:
-
C-reactive protein
- DSM-5:
-
Diagnostic and statistical manual of mental disorders
- EEG:
-
Electroencephalogram
- ELISA:
-
Enzyme-Linked-Immuno-Sorbent Assay
- EMG:
-
Electromyograph
- GEE:
-
Generalized estimating equation
- GLM:
-
Generalized linear model (GLM)
- HPA:
-
Hypothalamic-pituitary-adrenal
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- IPT-PTSD:
-
Interpersonal psychotherapy adapted to PTSD
- ISI:
-
Insomnia Severity Index
- M:
-
Mean
- MDD:
-
Major Depressive Disorder
- MINI:
-
Mini-International Neuropsychiatric Interview
- N3%:
-
Percentage of total sleep composed of N3
- NF-kB:
-
Nuclear factor kappa B
- PSG:
-
Polysomnography
- PSQI:
-
Pittsburgh Sleep Quality Index
- PTSD:
-
Post-traumatic stress disorder
- SD:
-
Standard deviation
- SE:
-
Standard Error
- SWS:
-
Slow-wave sleep
- TST:
-
Total sleep time
- TNF-α:
-
Tumor necrosis factor α
- VIF:
-
Variance inflation factor
References
Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12(3):185–95.
Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–7.
Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240–58.
Galovski TE, Harik JM, Blain LM, Elwood L, Gloth C, Fletcher TD. Augmenting cognitive processing therapy to improve sleep impairment in PTSD: a randomized controlled trial. J Consult Clin Psychol. 2016;84(2):167–77.
Pruiksma KE, Cranston CC, Rhudy JL, Micol RL, Davis JL. Randomized controlled trial to dismantle exposure, relaxation, and rescripting therapy (ERRT) for trauma-related nightmares. Psychol Trauma. 2018;10(1):67–75.
Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69–74.
Gehrman P, Seelig AD, Jacobson IG, Boyko EJ, Hooper TI, Gackstetter GD, Ulmer CS, Smith TC. Predeployment sleep duration and insomnia symptoms as risk factors for New-Onset Mental Health Disorders following Military Deployment. Sleep. 2013;36(7):1009–18.
Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210.
Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–8.
Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–83.
Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, Sleep Duration, and inflammation: a systematic review and Meta-analysis of Cohort Studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52.
Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, Kumari M, Davey Smith G, Shipley MJ. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II study. Am J Epidemiol. 2013;178(6):956–61.
Pruiksma KE, Taylor DJ, Wachen JS, Mintz J, Young-McCaughan S, Peterson AL, Yarvis JS, Borah EV, Dondanville KA, Litz BT, et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol Trauma. 2016;8(6):697–701.
Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22(6):960–8.
Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36(5):769–779E.
Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24(1):54–7.
Heinzelmann M, Lee H, Rak H, Livingston W, Barr T, Baxter T, Scattergood-Keepper L, Mysliwiec V, Gill J. Sleep restoration is associated with reduced plasma C-reactive protein and depression symptoms in military personnel with sleep disturbance after deployment. Sleep Med. 2014;15(12):1565–70.
Imai R, Hori H, Itoh M, Lin M, Niwa M, Ino K, Ogawa S, Ishida M, Sekiguchi A, Matsui M, et al. Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J Psychiatr Res. 2018;102:192–200.
Beristianos MH, Yaffe K, Cohen B, Byers AL. PTSD and risk of Incident Cardiovascular Disease in Aging Veterans. Am J Geriatr Psychiatry. 2016;24(3):192–200.
Edmondson D, von Kanel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320–9.
Mohlenhoff BS, O’Donovan A, Weiner MW, Neylan TC. Dementia risk in posttraumatic stress disorder: the relevance of sleep-related abnormalities in Brain structure, amyloid, and inflammation. Curr Psychiatry Rep. 2017;19(11):89.
Coimbra BM, Yeh M, D’Elia AT, Maciel MR, Carvalho CM, Milani AC, Mozzambani A, Juruena M, Belangero SI, Jackowski AP, et al. Posttraumatic stress disorder and neuroprogression in women following sexual assault: protocol for a Randomized Clinical Trial evaluating allostatic load and aging process acceleration. JMIR Res Protoc. 2020;9(11):e19162.
Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, Marx BP. The clinician-administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383–95.
Oliveira-Watanabe TT, Ramos-Lima LF, Santos RC, Mello MF, Mello AF. The clinician-administered PTSD scale (CAPS-5): adaptation to brazilian portuguese. Braz J Psychiatry. 2019;41(1):92–3.
Beck AT, Steer RA, Brown G. Beck Depression Inventory–II (BDI-II). 1996.
Gomes-Oliveira MH, Gorenstein C, Lotufo Neto F, Andrade LH, Wang YP. Validation of the brazilian portuguese version of the Beck Depression Inventory-II in a community sample. Braz J Psychiatry. 2012;34(4):389–94.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, de Barba ME, Barreto SS. Validation of the brazilian portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12(1):70–5.
Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8.
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619.
Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, Lux L, Harris RP. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(4):415–33.
American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89.
Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84(8):2603–7.
Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19(2):165–72.
Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–26.
Matthews KA, Zheng H, Kravitz HM, Sowers M, Bromberger JT, Buysse DJ, Owens JF, Sanders M, Hall M. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: study of Women’s Health across the Nation sleep study. Sleep. 2010;33(12):1649–55.
Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30(5):1233–40.
Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4.
Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a taiwanese population. Ann Epidemiol. 2011;21(11):799–806.
Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, Marmot MG, Cappuccio FP. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;37:857–64.
Irwin MR, Wang M, Ribeiro D, Cho H, Olmstead E, Breen E, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40.
Lainonen M, Meyer-Rochow B, Timonen M. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 birth cohort study. Psychosom Med. 2007;69:756–61.
Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84(6):1979–85.
Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210.
Roman V, Hagewoud R, Luiten PGM, Meerlo P. Altered serotonergic and corticotropin releasing hormone regulation of the hypothalamo-pituitary-adrenal stress system in chronic partial sleep deprivation. The Netherlands: University of Groningen; 2007.
Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr OpinPulm Med. 2005;11(6):481–4.
Singareddy R, Vgontzas AN, Fernandez-Mendoza J, Liao D, Calhoun S, Shaffer ML, Bixler EO. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13(4):346–53.
Acknowledgements
None.
Funding
This study was funded by grants from FAPESP (2014/12559-5) and CNPq (303389/2016-8). There was financing in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and Associação Fundo de Incentivo à Pesquisa (AFIP).
Author information
Authors and Affiliations
Contributions
MSLY contributed to data acquisition and collection and writing of the manuscript; DP contributed to the data analysis and interpretation and drafting and revision of the manuscript; MFM contributed to the design of the study, research preparation, data curation, and manuscript revision; BMC contributed to recruitment, research organization, and manuscript revision. AFM contributed to the research preparation, data collection and curation, and manuscript revision; ATD contributed to the research preparation, data collection, data curation, and revision of the manuscript; ST contributed to data acquisition and curation and manuscript revision. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent and were informed in detail about the nature and purpose of the study. The Universidade Federal de Sao Paulo Committee Board approved the study (CAAE: 84772918.0.0000.5505) following receipt of a statement of compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Consent for publication
Not applicable.
Competing interests
Marcelo F. Mello, Mary Sau Ling Yeh, Ana Teresa D’ Elia, Bruno M. Coimbra and Andrea F. Mello, Sergio Tufik, and Dalva Poyares declare they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yeh, M.S.L., Poyares, D., D’Elia, A.T.D. et al. Sleep characteristics and inflammatory markers in women with post-traumatic stress disorder. BMC Psychiatry 23, 273 (2023). https://doi.org/10.1186/s12888-023-04765-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-04765-1