Abstract
Background
Autism spectrum disorders (ASD) is a neurodevelopmental disorder with high incidence rate and difficult diagnosis. The purpose of this study was to explore whether salivary cortisol, dehydroepiandrosterone (DHEA) and pregnenolone can be used as biomarkers of ASD children.
Methods
The saliva samples of 55 boys with ASD were collected as the experimental group, and the saliva samples of 24 neurotypical boys were collected as the control group. The Child Behavior Checklist (CBCL), Autism Behavior Checklist (ABC), Social Responsiveness Scale (SRS), Repetitive Behavior Scale (RBS) were used to assess the severity of symptoms in boys with ASD. Cortisol, DHEA and pregnenolone concentrations in saliva were measured using an ABSSCIEX QTRAP® 6500 + LC/MS/MS system. SPSS 23.0 was used for statistical analysis. Comparisons between the two groups which conform to normal distribution were performed by T-test, and those which don’t conform to normal distribution were performed by Mann–Whitney U test. Correlation analysis between two variables was performed using Spearman's correlation analysis. Receiver operating characteristic curve (ROC) analysis was performed to evaluate the discriminatory sensitivity of each hormone between ASD and normal control groups. Logistic regression models were used to analyze whether DHEA and salivary pregnenolone can be used as a biomarker of ASD.
Results
There were no significant differences in age, and weight between the ASD group and the normal control group. The ABC, SRS, RBS and CBCL scale scores in the ASD group were significantly higher than those in the normal control group. The salivary DHEA and pregnenolone concentrations in the ASD group were significantly higher than those in the normal control group, but there was no significant difference in cortisol. Spearman's correlation analysis showed that only pregnenolone associated with ABC. Logistic regression model analysis suggested that pregnenolone in saliva was an independent predictor of ASD. ROC analysis found that pregnenolone had good discrimination sensitivity between ASD and normal controls.
Conclusion
Gave salivary preoperative a space for utilization as biomarker as number of cases are limited to this high expectation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
According to the 5th edition of the diagnostic and statistical manual of mental disorders (DSM-5), autism spectrum disorders (ASD) is a neurodevelopmental disorder characterized by social interaction and communication disorders, narrow interests, stereotyped behavior and abnormal perception [1]. Neurodevelopmental changes in ASD have been reported to affect not only cognitive abilities, social brain, and other neural structures but also other major physiological systems such as immune, endocrine, and gut microbiota systems [2]. Over the past few years, the incidence of ASD has increased markedly, with approximately 1 in 68 children suffering from ASD, severely affecting the quality of life of individuals as well as caregivers and families, resulting in a heavy psychological and economic burden on families and society [2,3,4]. The heterogeneity of the symptomatic presentation of ASD, the lack of biomarkers and the evolving diagnostic criteria all create unique challenges in the diagnosis of ASD [5, 6]. Therefore, finding better diagnostic tools is of great significance for ASD early diagnosis and treatment.
Several studies have shown that ASD is more common in males than in females, which may be caused by androgens [7]. Human and animal studies have shown that androgen exposure results in decreased social function [7, 8]. It has been reported that the risk of ASD may stem from increased exposure to androgens (e.g., testosterone) in the prenatal period [7, 9]. At the same time, studies have also reported that postpartum androgens have persistent but impermanent effects on the human brain and cognition [10]. For example, there is a trend toward increased salivary androgen (androsterone and its polar conjugates) levels in prepubertal ASD children [11]. Pregnenolone can be converted to androstenediol via HSD3B and androstenedion is further processed to testosterone using AKRIC3 [12]. In addition, pregnenolone is a precursor of glucocorticoids [13]. Studies have shown that pregnenolone is hydroxylated at the 17th position and enters the glucocorticoid series, and the cleavage of the glucocorticoid side chain can generate androgens [14]. Corticosterone is widely regarded as the major glucocorticoid produced in amphibians [15]. Therefore, pregnenolone, DHEA, and cortisol are key hormones in the synthesis of androgens. In addition, pregnenolone, dehydroepiandrosterone (DHEA) as well as cortisol were demonstrated to exist in the serum of children with ASD [12, 16, 17]. At present, the levels of pregnenolone, DHEA, and cortisol in the saliva of children with ASD and whether they could be a diagnostic marker for children with ASD need to be further explored.
The child behavior checklist (CBCL) is one of the screening scales for assessing children's emotional and behavioral problems and is widely used to assess, collect, and rate children's internalizing and externalizing behavioral problems [18, 19]. The Autism Behavior Checklist (ABC) is a 57-item scale commonly used in clinic to characterize the behavior of children aiming to screen and diagnose ASD [20, 21]. The social responsiveness scale (SRS) is a rating scale that assesses social, communication, and repetitive behaviors associated with ASD [22]. The repetitive behavior scale (RBS) is primarily used to assess repetitive stereotypic behaviors in individuals with ASD, which is not limited to the pediatric population, and involves a wide range of behavioral items. It is more clinically applicable [23] since it is relatively easy to assess ASD. These scales are all widely used in clinical studies and are a broad-spectrum assessment tool for ASD, which can more accurately assess common behavioral problems and severity in children with ASD with good reliability and validity [23,24,25,26]. In this study, we assessed ASD severity by CBCL, ABC, SRS, and RBS.
Serum is generally considered as the "gold standard" for steroid analysis because it is thought that it can detect more biologically active compounds than saliva. However, some studies have found that some salivary steroids, such as testosterone, are highly correlated with serum steroids [27]. In addition, saliva can be collected non-invasively and non-stressfully, which is especially important for children with autism who are very vulnerable to stress. It is well known that gender has a great influence on steroid hormones. Therefore, in this study, in order to exclude the influence of gender on the results, we collected saliva samples of boys as specimens to explore the differences of salivary cortisol, DHEA and pregnenolone levels between boys with or without ASD and the relationship between salivary cortisol, DHEA and pregnenolone levels and CBCL, ABC, SRS, and RBS scale scores. Also, we investigated whether salivary cortisol, DHEA, and pregnenolone can be biomarkers for ASD.
Methods
Design
The present study was a descriptive study that employed appropriate scales to assess the degree of validation in boys with ASD. ABSCIEX QTRAP® 6500 + LC/MS/MS was used to test cortisol, DHEA, and pregnenolone in saliva samples. A series of statistical methods were used to analyze whether salivary cortisol, DHEA, and pregnenolone could be diagnostic markers for ASD.
Experimental subjects
A total of 55 ASD boys as experimental group and 24 normal boys as control group. The boys with ASD were recruited from several rehabilitation institutions including the inpatient department of Jingyang maternal and child health hospital, Deyang, Sichuan, China, the outpatient clinic of Guangyuan Central Hospital, and the rehabilitation center for disabled people in Guangyuan from January 2020 to May 2022. All subjects were less than 8 years old. The control group was obtained from boys attending outpatient clinics of Guangyuan Central Hospital, Sichuan Province, and kindergartens of the organs of Guangyuan City. Inclusion criteria for control group: ① under 8 years old; ② No secondary sexual characteristics were present. Exclusion criteria: ① sexual dysplasia; ② Tumor; ③ Developmental delay, mental retardation, epilepsy and other neurological and psychiatric disorders. Inclusion criteria for boys with ASD: ①Boys diagnosed with ASD according to the DSM-5 diagnostic criteria and diagnosed by the Autism Diagnostic Observation Schedule 2nd revision (ADOS- 2). ②Boys with organic heart disease and abnormal sexual development were excluded; ③Boys with Informed consent. The study obtained the written informed consent from the boys’s parents or legal guardians. This study was carried out in accordance with the declaration of Helsinki, and reviewed and approved by the human ethics committee of Chengdu Third People's Hospital, Ethics No.: Chengdu Sanyuan Lun [2020] No. S-112.
CBCL scale
The CBCL scale is a short, standardized questionnaire which was designed to identify social, behavioral, and emotional problems in children with ASD [28]. The CBCL scale consists of depression, somatic complaints, social withdrawal, aggression, defiant, compulsive, schizotypal, and poor interaction scores [20]. The scale was filled by children’s parents for more than half a year until the next by professionals based on the child's performance for almost half a year. The scored items were scored additively to obtain a total score for behavioral problems [18].
ABC scale
The ABC scale consists of sensory, communicative, somatic, linguistic, physiological self-care scores. A total of 57 behavioral traits of children with autism were included. It can be used for people between 2 months and 28 years of age [20]. Completed by the parents based on the child's performance at the time of the physical examination. Scores of ≥ 67 were considered as ASD children.
SRS scale
The SRS scale consists of social perception, social cognition, social communication, social motivation, behavioral approach scores. A total of 65 items were included. Completed by the parents based on the child's performance at the time of the physical examination. The higher the total score, the more severe the social impairment.
RBS scale
The RBS scale consists of stereotypic behavior, self-injurious behavior, compulsive behavior, ritualistic behavior, fixed behavior, and restricted behavior scores. A total of 57 behavioral traits were included. It can be used as an autism screening scale for children between 2 and 14 years of age [29]. Completed by the parents based on the child's performance at the time of the physical examination. Higher scores represent more pronounced autistic features.
Physical examination and evaluation criteria
The height and weight of all enrolled boys were collected, and the weight-for-age Z-score (WAZ) and the height-for-age Z-score (HAZ) were calculated according to the 2006 World Health Organization's reference standards for height and weight for children aged 0–18 years. Z score = (measured value—median of reference standard) / standard deviation of reference standard.
Saliva sample collection and determination of hormone concentration
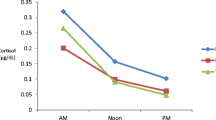
Saliva collection: all subjects refrained from consuming alcohol, coffee, beverages within 12 h before performing saliva collection. The following morning, brushing teeth at bedtime at night before collection, fasting in the morning, and about 2 ml of saliva was collected with a disposable saliva collector at resting state and put into -80℃ for freezing. Salivary hormone testing: Cortisol, DHEA and pregnenolone in saliva samples were detected by ABSSCIEX QTRAP® 6500 + LC/MS/MS system, data was collected by Analyst 1.6.2 software, and quantitative analysis was performed by Multiquant software.
Statistical analysis
Sample size was calculated using G-Power software. We aimed at 21 cases per group, as this would yield sufficient power (80%) at the α = 0.05 level to detect a large effect size (d = 0.8) in the Wilcoxon-Mann–Whitney test (two groups) [30]. The sample size we actually collected was 24 cases in the Ctrl group and 55 cases in the ASD group. Therefore, our sample size was reasonable.
All data in this paper were statistically analyzed using SPSS 23.0. The normality of the data distribution was assessed using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean ± standard deviation, while non-normally distributed data are presented as median and their respective 25% and 75% boundaries. The comparison between the two groups conformed to the normal distribution using the T test. The Mann–Whitney U test was used if the comparison which didn’t conform to the normal distribution. The correlation analysis was performed by Spearman correlation coefficient(r). ROC curve analysis discriminates sensitivity. Logistic regression models analyzed whether salivary pregnenolone could be a biomarker for ASD. Statistical difference was indicated by p < 0.05. The figure in this article is drawn using Graphpad Prism8.0.2.
Results
Demographic characteristics
Demographic comparison results showed that there was no significant difference in age, height and weight between the ASD group and the normal control group (all P > 0.05). The results are shown in Table 1.
The scores of each scale between the normal control group and the ASD group
All boys with ASD and normal control groups were evaluated by ABC, SRS, RBS and CBCL scales, and the score results are shown in Table 2. It was found that the CBCL, ABC, SRS and RBS scale scores of the ASD group were significantly higher than those of the normal control group ( All P < 0.05).
Comparison of hormone concentrations in saliva between normal control group and ASD group
Differences in the concentrations of cortisol, DHEA and pregnenolone in the saliva of ASD boys and normal control boys were analyzed by T-test or Mann–Whitney U test. In saliva, cortisol concentrations were not significantly different between ASD and normal controls, whereas DHEA and pregnenolone were significantly higher in ASD (Table 3, all P < 0.05).
Relationship between concentrations of each hormone and scores on each scale
The relationships between DHEA and pregnenolone concentrations and scores on each scale were tested by Spearman. The results showed that only pregnenolone associated with ABC (Table 4). We suggest that this phenomenon may be related to the complex etiology, genes, environment, and diet of ASD.
Boys with high pregnenolone have a higher risk of ASD
Since only pregnenolone was associated with ABC in each of the ASD rating scales, we used logistic regression models to test whether salivary pregnenolone could be a biomarker of ASD. The results showed that the association of the variable pregnenolone with ASD was statistically significant. Boys with higher pregnenolone had a higher risk of ASD (Table 5).
Pregnenolone in saliva has good discrimination sensitivity between ASD and normal controls
Further, we performed a ROC analysis of salivary pregnenolone, and the results showed that salivary pregnenolone had good discrimination sensitivity between ASD and normal controls (Fig. 1).
Discussion
ASD primarily occurs in early childhood, so it is called childhood autism. ages 2–5 years are the most obvious stage of ASD behavior, and the incidence of ASD in males is higher than in females [11, 31, 32]. In our study, we only investigate cortisol, DHEA and pregnenolone in saliva samples from boys to rule out gender effect on the results. Emotional dysregulation, irritability, anxiety, irritability, and aggressive behaviors or avoidant withdrawal are commonly observed during the development of ASD, severely impacting development, communication, socialization, and behavior, and life [33,34,35]. It is reported that only 10–35% of ASD cases have known major risk factors or established etiologies [36]. Because of the difficulty of early diagnosis of ASD, early treatment cannot be performed, leading to a poor prognosis of ASD. As early diagnosis improves the efficacy of behavioral therapies, molecular biomarkers represent an attractive approach to identify 'at-risk' populations and may contribute to the development of personalized therapies [37]. This prospect is becoming increasingly important given the rising rate of ASD diagnosis.
At present, there are no objective and reliable biomarkers for the diagnosis of ASD, which mainly rely on clinical diagnosis and standardized diagnostic scale evaluation [38, 39]. For example, Kim [40] et al. examined state and anxiety levels in adolescents with ASD by analyzing Pearson correlations between RRB, problem behavior variables, and the State/Trait Anxiety Inventory (STAI) and CBCL. Li [41] et al. used the ABC scale and the Childhood Autism Rating Scale to assess the severity of ASD. We used the CBCL, ABC, SRS and RBS scales to evaluate the behavioral problems of boys with ASD, and found that the CBCL, ABC, SRS and RBS scores in the ASD group were significantly higher than those in the normal control group.
Currently, in addition to genetics, non-genetic factors such as environment, metabolism, infection, gut microbiota, and endocrine have all been reported to be associated with the occurrence of ASD [29, 36, 42, 43]. Sex steroids, such as testosterone, are thought to be one of the biological factors associated with neurodevelopmental disorders including ASD [44]. During brain development, the expression of genes related to steroid biosynthesis increases [45]. It has been confirmed that high prenatal sex steroid levels are associated with autistic features in infants and children, meanwhile, increased circulating testosterone levels have also been shown in ASD patients during childhood or adulthood [46]. Majewska et al. [11] found increased levels of internal steroid hormones such as pregnenolone and DHEA in saliva of prepubertal children with ASD, but no significant differences in cortisol. It has also been shown that at night, cortisol levels are significantly higher in children with ASD [47, 48]. Our study found that salivary levels of dehydroepiandrosterone and pregnenolone were significantly increased in ASD patients, but cortisol was not significantly different, and only pregnenolone was significantly associated with the RBS score. The different results from previous study may be related to salivary collection time, complex etiological, genetic, environmental, and dietary differences in ASD.
Conclusions
Salivary levels of DHEA and pregnenolone were significantly increased in ASD cases, but cortisol was not significantly different. In addition, DHEA was not significantly associated with any of the scale scores. Pregnenolone was only significantly associated with RBS scores. The follow-up study found that boys with high pregnenolone had a higher risk of ASD than those with low pregnenolone, and it had good discriminatory sensitivity for ASD. Therefore, give salivary preoperative a space for utilization as biomarker as number of cases are limited to this high expectation.
Limitations
The small sample size, not enough variety of hormone assays, gender limitations and lack of diversity of samples are the main limitations of this paper. In addition, eating patterns, saliva collection times, and exercise among control and ASD children were not assessed and controlled, and it is unclear whether diet and sampling times have an effect on hormone levels. And the specific mechanism is still unclear. In later studies, we will continue to collect more saliva and blood samples, detect more hormone (e.g., testosterone, progesterone, etc.) levels, and further explore the specific mechanism.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASD:
-
Autism spectrum disorders
- DHEA:
-
Dehydroepiandrosterone
- CBCL:
-
Child Behavior Checklist
- ABC:
-
Autism Behavior Checklist
- SRS:
-
Social Responsiveness Scale
- RBS:
-
Repetitive Behavior Scale
- ROC:
-
Receiver operating characteristic curve
References
Zhang XC, Shu LQ, Zhao XS, et al. Autism spectrum disorders: autistic phenotypes and complicated mechanisms[J]. World J Pediatr. 2019;15(1):17–25.
Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder[J]. Cell Mol Life Sci. 2019;76(7):1275–97.
Roman P, Rueda-Ruzafa L, Cardona D, et al. Gut-brain axis in the executive function of austism spectrum disorder[J]. Behav Pharmacol. 2018;29(7):654–63.
Sharma AK, Gokulchandran N, Kulkarni PP, et al. Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: a clinical study[J]. Am J Stem Cells. 2020;9(5):89–100.
Baio J, Wiggins L, Christensen DL, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014[J]. MMWR Surveill Summ. 2018;67(6):1–23.
Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1–21.
Mckenna BG, Huang Y, Vervier K, et al. Genetic and morphological estimates of androgen exposure predict social deficits in multiple neurodevelopmental disorder cohorts[J]. Mol Autism. 2021;12(1):43.
Kuwahara N, Nicholson K, Isaacs L, et al. Androgen Effects on Neural Plasticity[J]. Androg Clin Res Ther. 2021;2(1):216–30.
Quartier A, Chatrousse L, Redin C, et al. Genes and Pathways Regulated by Androgens in Human Neural Cells, Potential Candidates for the Male Excess in Autism Spectrum Disorder[J]. Biol Psychiatry. 2018;84(4):239–52.
Auyeung B, Lombardo MV, Baron-Cohen S. Prenatal and postnatal hormone effects on the human brain and cognition[J]. Pflugers Arch. 2013;465(5):557–71.
Majewska MD, Hill M, Urbanowicz E, et al. Marked elevation of adrenal steroids, especially androgens, in saliva of prepubertal autistic children[J]. Eur Child Adolesc Psychiatry. 2014;23(6):485–98.
Janšáková K, Hill M, Čelárová D, et al. Alteration of the steroidogenesis in boys with autism spectrum disorders[J]. Transl Psychiatry. 2020;10(1):340.
Alherz FA, El Daibani AA, Abunnaja MS, et al. Effect of SULT2B1 genetic polymorphisms on the sulfation of dehydroepiandrosterone and pregnenolone by SULT2B1b allozymes[J]. Mol Cell Endocrinol. 2019;496: 110535.
Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions[J]. J Steroid Biochem Mol Biol. 2013;137:107–23.
Hopkins WA, Durant SE, Beck ML, et al. Cortisol is the predominant glucocorticoid in the giant paedomorphic hellbender salamander (Cryptobranchus alleganiensis)[J]. Gen Comp Endocrinol. 2020;285: 113267.
Bozkurt H, Şimşek Ş, Şahin S. Elevated levels of cortisol, brain-derived neurotropic factor and tissue plasminogen activator in male children with autism spectrum disorder[J]. Autism Res. 2021;14(10):2078–84.
Hassan MH, Desoky T, Sakhr HM, et al. Possible Metabolic Alterations among Autistic Male Children: Clinical and Biochemical Approaches[J]. J Mol Neurosci. 2019;67(2):204–16.
Rescorla LA, Winder-Patel BM, Paterson SJ, et al. Autism spectrum disorder screening with the CBCL/1½-5: Findings for young children at high risk for autism spectrum disorder[J]. Autism. 2019;23(1):29–38.
Chericoni N, Balboni G, Costanzo V, et al. A Combined Study on the Use of the Child Behavior Checklist 1½-5 for Identifying Autism Spectrum Disorders at 18 Months. J Autism Dev Disord. 2021;51(11):3829–42.
Zhao G, Liu SJ, Gan XY, et al. Analysis of Whole Blood and Urine Trace Elements in Children with Autism Spectrum Disorders and Autistic Behaviors[J]. Biol Trace Elem Res. 2023;201(2):627–35.
Hu C, Yang F, Yang T, et al. A Multi-Center Study on the Relationship Between Developmental Regression and Disease Severity in Children With Autism Spectrum Disorders[J]. Front Psychiatry. 2022;13: 796554.
Bardsley MZ, Kowal K, Levy C, et al. 47, XYY syndrome: clinical phenotype and timing of ascertainment[J]. J Pediatr. 2013;163(4):1085–94.
Martínez-González AE, Piqueras JA. Validation of the Repetitive Behavior Scale-Revised in Spanish-Speakers Participants with Autism Spectrum Disorder[J]. J Autism Dev Disord. 2018;48(1):198–208.
Nguyen PH, Ocansey ME, Miller M, et al. The reliability and validity of the social responsiveness scale to measure autism symptomology in Vietnamese children[J]. Autism Res. 2019;12(11):1706–18.
Guérin NA, Gabriels RL, Germone MM, et al. Reliability and Validity Assessment of the Observation of Human-Animal Interaction for Research (OHAIRE) Behavior Coding Tool[J]. Front Vet Sci. 2018;5:268.
Kat S, Xu L, Guo Y, et al. Reliability and Validity of the Simplified Chinese Version of the Aberrant Behavior Checklist in Chinese Autism Population[J]. Front Psychiatry. 2020;11:545445.
Tan DW, Maybery MT, Clarke MW, et al. No relationship between autistic traits and salivary testosterone concentrations in men from the general population[J]. PLoS ONE. 2018;13(6):e0198779.
Mills W, Kondakis N, Orr R, et al. Does Hydrotherapy Impact Behaviours Related to Mental Health and Well-Being for Children with Autism Spectrum Disorder? A Randomised Crossover-Controlled Pilot Trial. Int J Environ Res Public Health. 2020;17(2):558.
Jiang L, Tian L, Yuan J, et al. Associations Between Sex Hormone Levels and Autistic Traits in Infertile Patients With Polycystic Ovary Syndrome and Their Offspring[J]. Front Endocrinol (Lausanne). 2021;12:789395.
Panday DR, Rauniar GP. Effect of root-extracts of Ficus benghalensis (Banyan) in pain in animal models. J Neurosci Rural Pract. 2016;7(2):210–5.
Wu X, Li W, Zheng Y. Recent Progress on Relevant microRNAs in Autism Spectrum Disorders. Int J Mol Sci. 2020;21(16):5904.
Ferri SL, Abel T, Brodkin ES. Sex Differences in Autism Spectrum Disorder: a Review. Curr Psychiatry Rep. 2018;20(2):9.
Green J, et al. Commentary: Anxiety and behaviour in and beyond ASD; does the idea of “PDA” really help? - a commentary on Stuart et al (2020). Child Adolesc Ment Health 2020;25(2):74–6.
Oubrahim L, Combalbert N. Frequency and origin (reactive/proactive) of aggressive behavior in young people with intellectual disability and autism spectrum disorder[J]. Int J Dev Disabil. 2019;67(3):209–16.
Cheung Y, Man Kit Cheung A, Ho Yan Luk E, et al. An evaluation of a comprehensive training package for interventionists providing behavioral intervention for children with autism spectrum disorder. Int J Dev Disabil. 2020;66(5):358–69.
Gillberg C, Fernell E, Kočovská E, et al. The role of cholesterol metabolism and various steroid abnormalities in autism spectrum disorders: A hypothesis paper. Autism Res. 2017;10(6):1022–44.
Needham BD, Adame MD, Serena G, et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder[J]. Biol Psychiatry. 2021;89(5):451–62.
Wei QH, Xie XF, Dai JJ, et al. Value of autism screening checklists in the early identification of autism spectrum disorder. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23(4):343–9.
Pagan C, Delorme R, Callebert J, et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders[J]. Transl Psychiatry. 2014;4(11):e479.
Kim SY, Kim YA, Song DY, et al. State and Trait Anxiety of Adolescents with Autism Spectrum Disorders[J]. Psychiatry Investig. 2021;18(3):257–65.
Li HH, Wang CX, Feng JY, et al. A Developmental Profile of Children With Autism Spectrum Disorder in China Using the Griffiths Mental Development Scales[J]. Front Psychol. 2020;11:570923.
Kushak RI, Winter HS. Gut Microbiota and Gender in Autism Spectrum Disorders[J]. Curr Pediatr Rev. 2020;16(4):249–54.
Gasser BA, Buerki SF, Kurz J, et al. Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. Int J Mol Sci. 2021;22(22):12324.
Romano E, Cosentino L, Laviola G, et al. Genes and sex hormones interaction in neurodevelopmental disorders[J]. Neurosci Biobehav Rev. 2016;67:9–24.
Hu VW, Nguyen A, Kim KS, et al. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: altered pathways in neuronal development and steroid biosynthesis. PLoS ONE. 2009;4(6):e5775.
Simantov T, Pohl A, Tsompanidis A, et al. Medical symptoms and conditions in autistic women. Autism, 2021: 13623613211022091.
Muscatello RA, Corbett BA. Comparing the effects of age, pubertal development, and symptom profile on cortisol rhythm in children and adolescents with autism spectrum disorder. Autism Res. 2018;11(1):110–20.
Tomarken AJ, Han GT, Corbett BA. Temporal patterns, heterogeneity, and stability of diurnal cortisol rhythms in children with autism spectrum disorder. Psychoneuroendocrinology. 2015;62:217–26.
Acknowledgements
Thank all the families who participated in this study for their support. At the same time, we also thank all teachers for their valuable comments on this study.
Funding
The Science and technology planning project of Sichuan Provinc (2021YJ0170) and Project of Chengdu Science and Technology Bureau (2019-YF05-00498-SN).
Author information
Authors and Affiliations
Contributions
The corresponding author and the first author designed the experiment and critically revised the important knowledge content of the article. The first author analyzed the data and wrote a paper. All authors collected samples. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study obtained the consent of the boys's parents or legal guardians and signed written informed consent. And this study was carried out in accordance with the declaration of Helsinki, and was reviewed and approved by the Human Ethics Committee of f the third people’s Hospital. Ethics No.: Ethics of Chengdu Third Hospital [2020] s-112.
Consent for publication
Not applicable (No personal data published.).
Competing interests
All of the authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, Q., Wang, Y., Liu, Z. et al. Analysis of salivary steroid hormones in boys with autism spectrum disorder. BMC Psychiatry 23, 105 (2023). https://doi.org/10.1186/s12888-023-04586-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-04586-2