Abstract
Introduction
Nearly 40% of patients with Major Depressive Disorder (MDD) have been found to experience cognitive impairment in at least one domain. Cognitive impairment associated with MDD is disproportionately represented in patients that have not fully returned to psychosocial functioning. As awareness regarding cognitive dysfunction in MDD patients grows, so does the interest in developing newer treatments that specifically address these deficits.
Method
In the present study, we conduct a systematic review of controlled randomized clinical trials that used cognitive training and remediation interventions for improving cognitive functions and reducing symptom severity in adult patients with MDD. We selected studies published before March 2022 using search databases including PubMed, ScienceDirect, Scopus, and Google scholar. For conducting the meta-analysis, standard differences in means with the random effect model and with a 95% confidence interval of change in outcome measures from baseline to post-intervention between the cognitive rehabilitation and the control groups were calculated.
Results
The database search resulted in identifying 756 studies of interest, which ultimately 15 studies with 410 participants in the cognitive rehabilitation group and 339 participants in the control group were included. The meta-analysis of the data extracted from these studies, shows a moderate and significant effect on the executive function (d = 0.59 (95% CI, 0.25 to 0.93) p-value = 0.001, I2 = 15.2%), verbal learning (d = 0.45 (95% CI, 0.12 to 0.78) p-value = 0.007, I2 = 0.00%), and working memory (d = 0.41 (95% CI, 0.18 to 0.64) p-value < 0.001, I2 = 33%) of MDD patients. Although, there were no significant difference between intervention and control group in attention (d = 0.32 (95% CI, -0.01 to 0.66) p-value = 0.058, I2 = 0.00%) or depressive symptoms.
Conclusion
This systematic review and meta-analysis indicate that cognitive rehabilitation is an effective intervention for the executive function, verbal learning, and working memory of MDD patients. Due to the importance of these neuropsychological deficits in day-to-day life and the core symptoms of MDD, cognitive rehabilitation should be considered an important part of treating MDD. Further research in this area and concentrated on these particular deficits is warranted.
Similar content being viewed by others

Introduction
MDD is considered a chronic, disabling, and (in most cases) recurring psychiatric condition [1] which is characterized by depressed mood, reduced interest and pleasure in daily activities, weight fluctuation, sleep and psychomotor distress, fatigue, feelings of worthlessness, trouble concentrating, and suicidal ideation [2, 3].
Depression is a major public health concern, with worldwide estimates indicating that 10.8% of individuals suffer from this chronic condition at some point in their lives [4]. Based upon estimations from the World Health Organization (WHO), MDD is responsible for the greatest share of burden linked to non-fatal health outcomes and accounts for nearly 12% of total years lived with disability [5].
Cognitive impairment is a common and frequent symptom of MDD. Nearly 40% of people who have currently or formerly been diagnosed with depression have been found to experience cognitive impairment in at least one domain. Cognitive impairment associated with MDD is disproportionately represented in patients that have not fully returned to psychosocial functioning [6, 7] and cannot be considered an epiphenomenon entirely secondary to signs of low mood [8].
Impaired cognitive function in patients with MDD does not prove to be limited to the acute phase of depression but persists when MDD has remitted [9]. Deficits in selective attention, working memory, long-term memory, verbal and visuospatial memory, attention, and processing speed, executive functioning, and verbal fluency remain persistent in remission from a depression episode and the level of cognitive impairment appears to worsen with repeated episodes [10,11,12,13,14]. These cognitive symptoms seem to have a significant impact on patients’ function and quality of life, interfere with their ability to contribute actively to the society, by sustaining employment or schooling, and risk the recurrence of their depression [15, 16].
Little is known about how such deficits arise in MDD. Current theories indicate that neuroanatomic changes in MDD patients’ brains might be the cause of observed deficits [17]. Neuroimaging studies show abnormal physical changes in the hippocampus, amygdala, caudate nucleus, putamen, and frontal cortex [18] and postmortem studies have shown a reduction in synaptic proteins in subgenual and/or glia and neural size, orbital and dorsolateral prefrontal cortex and amygdala [19,20,21].
Many studies indicate the effect of antidepressant drugs on cognitive impairment symptoms (in MDD patients) such as processing speed, working memory, visuospatial skills, sustained attention, etc., to be very small or statically non-significant [22] and overall, the data show that most of the standard treatments for MDD result in improved cognition. Although, the evidence continues to be limited by the small number of studies on this matter and small sample sizes and can’t be considered conclusive [23].
Current evidence indicates that cognitive interventions that are generally defined as cognitive remediation, training, and rehabilitation can show significant, albeit modest, improvements in cognitive functions such as attention, problem-solving, and memory across a range of mental illnesses and have been beneficial to patients suffering from anxiety disorders, Schizophrenia, ADHD, etc. [24,25,26,27].
Unfortunately, despite the growing recognition of the clinical importance of cognitive impairment in MDD, a major lack of consensus regarding clinical monitoring strategies persists as a barrier to clinicians. As awareness regarding cognitive dysfunction in MDD grows, so does the interest in developing newer treatments that specifically address these deficits [23].
Fortunately, present approach to treatment of psychiatric disorders is not limited to symptom management and has a major focus on functional abilities [28]. Hence, therapeutic interventions for improving cognitive function, including cognitive rehabilitation, have been growing and trending over past decade [29]. As a result, various models of cognitive rehabilitation have been developed and have been widely used for psychiatric disorders, such as MDD. The goal of these interventions was primarily focused on improving cognitive functions and then generalized on symptoms severity and daily functioning [30]. Since cognitive rehabilitation interventions are extensively growing and the research on the effectiveness of them on psychiatric disorders (other than schizophrenia) are relatively recent [30, 31], review study to verify this matter seems necessary.
In the present study, we aimed to conduct a systematic review of research projects that used cognitive training and remediation interventions for improving cognitive functions and reducing symptom severity in adult patients with MDD. This study is designed to determine the quality of evidence and the effectiveness of cognitive rehabilitation in the treatment of various cognitive impairments and also reducing the severity of MDD symptoms.
Methods
Eligibility
This article follows the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [32].
Patients participating in studies involved in this systematic review and meta-analysis had at least 18 years of age and were clinically-defined current or lifetime history of MDD, using established criteria such as the Diagnostic and Statistical Manual of Mental Disorders 5th edition guidelines (DSM-5). No geriatric research projects were included in the study.
Eligible studies included patients without any established neurodegenerative disease (e.g., dementia, Multiple system atrophy, etc.) or neurological condition (e.g., Parkinson’s disease, traumatic brain injury, etc.) in other words; participants whom their cognitive impairment is only derived from depression. All articles recruiting participants diagnosed with psychiatric illnesses (e.g., Schizophrenia) or specific neuropsychiatric conditions (e.g., stroke) were also excluded. Regarding studies with mixed diagnostic samples (e.g., patients with MDD and another group of patients diagnosed with Obsessive Compulsive Disorder), or mixed samples of preclinical depression and MDD articles, the research was included in our study if only the data of the patients with only MDD diagnosis could be extracted from the reports of the study.
The research projects which evaluate the effect of “cognitive remediation”, “cognitive rehabilitation”, and “cognitive training” were included and researches which assess effect of any other pharmacological or non-pharmacological treatment (e.g., Cognitive Behavioral Therapy or group therapies) were excluded.
Eligible studies included controlled randomized clinical trials involving at least two groups of eligible participants, with measures in pre- and post-intervention on at least one of the mood or cognition domains.
Search strategy and study selection
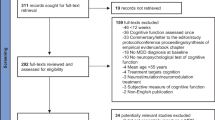
Using search databases including PubMed, ScienceDirect, Scopus, and Google scholar alongside with collaborating with an expert research librarian we selected controlled and randomized (either blinded or not) trials published before march 2022. The literature search and study selection procedure and the terms used in our systematic search are illustrated in Fig. 1.
Our search and the primary review were conducted by our librarian. Then the first and second author reviewed abstracts and full texts. In the case of disagreement, a third researcher (the corresponding author) made the decision.
Data analysis
For conducting the meta-analysis, we used Comprehensive Meta-Analysis Software (version 3) [33]. Standard differences in means with the random effect model and with a 95% confidence interval of change in outcome measures from baseline to post-intervention between the cognitive rehabilitation and the control groups were calculated.
Symptom severity of MDD and cognitive functions were included in the analysis as outcome measures. Standard tests for measuring MDD symptoms and objective standardized cognitive tests were eligible as the outcome. Cognitive functions were divided into standard cognitive domains and each domain used in more than two studies was included in the analysis.
Definition of weighted mean effect size were: 0.2–0.49 as small; 0.5–0.79 as medium; and > 0.8 as large [34]. The chi-square statistic and calculation of I2 were used to evaluate the heterogeneity across studies. I2 < 40% was defined as small, 30–60% moderate, 50–90% substantial and I2 > 75% as considerable heterogeneity [35]. Moreover, we applied sensitivity analysis and/or subgroup analysis in case of clinical heterogeneity.
Publication bias of studies was performed by inspecting funnel plots and we also evaluate the risk of bias in the studies for six main biases considering the Cochrane “Risk of bias” tool [36].
Results
Study characteristics
The database search identified 756 studies of interest, initially of which 182 duplications were discarded from them. From 574 remaining records, 464 studies were removed by evaluating the titles and abstracts and from 110 full texts of articles, 15 studies met the inclusion criteria and were eligible for the study (Fig. 2). These studies contain 410 participants in the cognitive rehabilitation group and 339 participants in the control group. Nine studies used cognitive training interventions and six studies evaluated cognitive remediation. The reported data and type of evaluation in these 15 studies consisted of 13 studies that reported MDD symptoms’ severity, five studies reported attention, five studies reported executive function, five studies reported verbal learning, and nine studies reported working memory. The studies’ details are described in Table 1.
MDD symptoms’ severity
Thirteen studies, with 302 patients in the cognitive intervention group and 310 in the control group, evaluated the severity of MDD symptoms and reported the needed data. The meta-analysis of these data did not show a significant difference between intervention and control group (d = 0.09 (95% CI, -0.06 to 0.25) p-value = 0.23, I2 = 0.00%). The forest plot of analyses of MDD symptoms’ severity is shown in Fig. 3.
Cognitive functions
After examining all included studies, we included attention, executive function, verbal learning, and working memory in the analyses. The meta-analysis of five studies that evaluated the attention of 93 patients in the cognitive intervention group and 72 patients in the control group showed an I2 = 62% and therefore we used the random effect model. The analysis showed a significant difference between the two groups with a moderate effect size (d = 0.61 (95% CI 0.07 to 1.16) p-value = 0.02). The forest plot of analyses of the attention is shown in Fig. 4 and the funnel plot of the analyses is shown in Fig. 5.
As it is evident in the plots, sensitivity analysis (remove-one analysis) showed that Bowie et al. study has the outlier data. The meta-analysis without this study did not show a significant difference between intervention and control group (d = 0.32 (95% CI, -0.01 to 0.66) p-value = 0.058, I2 = 0.00%). The forest plot of this analysis is shown in Fig. 6.
Five studies evaluated the executive function of 99 patients in the cognitive intervention group and 78 patients in the control group. The meta-analysis of these data showed a significant difference between the two groups with a moderate effect (d = 0.59 (95% CI, 0.25 to 0.93) p-value = 0.001, I2 = 15.2%). The forest plot of analyses of the executive function is shown in Fig. 7.
Five studies evaluated the verbal learning of 101 patients in the cognitive intervention group and 87 patients in the control group. The meta-analysis of these data showed an I2 = 83% and therefore we used the random effect model. The analysis showed a significant difference between the two groups with a large effect size (d = 0.94 (95% CI 0.15 to 1.73) p-value = 0.01). The forest plot of analyses of the verbal learning is shown in Fig. 8 and the funnel plot of the analyses is shown in Fig. 9.
As it is shown in the plots and sensitivity analysis (remove-one analysis) Bowie et al. study has the outlier data. The meta-analysis without this study showed a significant difference between intervention and control group (d = 0.45 (95% CI, 0.12 to 0.78) p-value = 0.007, I2 = 0.00%). The forest plot of this analysis is shown in Fig. 10.
Nine studies evaluated the working memory of 264 patients in the cognitive intervention group and 241 patients in the control group. The meta-analysis of these data showed a significant difference between the two groups (d = 0.41 (95% CI, 0.18 to 0.64) p-value < 0.001, I2 = 33%). The forest plot of analyses of the working memory is shown in Fig. 11.
Subgroup analysis
We performed a sub-group analysis on the severity of MDD symptoms to determine the effect of the model of intervention (cognitive training and cognitive remediation).
The sub-group analysis of severity of MDD symptoms did not show a significant difference between the cognitive intervention and the control group in four studies that evaluated the cognitive remediation (d = 0.21 (95% CI, -0.16 to 0.6) p-value = 0.26, I2 = 0.00%), and 11 studies that evaluated the cognitive training (d = 0.06 (95% CI, -0.12 to 0.25) p-value = 0.5, I2 = 11%).
Risk of bias
The evaluation of the risk of bias in the studies for six main biases showed that the quality of all the studies was relatively high. The result of the assessment of the main biases of the studies is shown in Fig. 12.
Discussion
We conducted this systematic review and meta-analysis to evaluate whether cognitive rehabilitation is effective in terms of symptom severity and cognition in adult patients with MDD.
In summary, our study indicates that cognitive rehabilitation is an effective intervention for the executive function, verbal learning, and working memory of MDD patients. However, current cognitive rehabilitation therapies are not effective for improving attention, and in reducing the severity of symptoms of MDD patients.
A systematic search strategy revealed a noticeably larger number of studies on this matter, although 15 articles were standard randomized clinical trials with a control group and on adult patients with clinical criteria of MDD. The exclusion of a large number of studies with subclinical depressed patients has shown that cognitive rehabilitation is not at the center of attention for treating clinically diagnosed MDD, even though cognitive impairment associated with MDD is disproportionately represented in patients that have not fully returned to psychosocial functioning [6, 7]and cannot be considered an epiphenomenon entirely secondary to signs of low mood [8].
Based on the findings of this review, current cognitive rehabilitation therapies are not effective in reducing the severity of symptoms of MDD patients. This finding is not different between cognitive training and cognitive remediation therapies. Our finding is not consistent with the latest meta-analysis conducted on cognitive remediation and cognitive training in MDD patients. Woolf et al. [52] performed a meta-analysis of cognitive training in adults with MDD and reported a moderate and statistically significant effect on the severity of depressive symptoms. This difference can be due to including non-randomized control trials, a smaller number of studies (up to 2016) and larger heterogeneity (I2 = 40.6%) in Woolf et al. study. Legemaat et al. [9] conducted a meta-analysis on cognitive remediation in MDD patients and reported a small and statistically significant effect on the severity of depressive symptoms. The inconsistency of our findings can be due to including geriatric patients, non-randomized clinical trials, and larger heterogeneity (I2 = 40%) in the study of Legamaat et al.
The findings of our study did not show a significant effect of cognitive rehabilitation on the attention of adults with MDD. We performed the random effect model analysis because of I2 = 62% and reached a significant difference between the two groups with a moderate effect size. Despite that, due to the asymmetry of one study in the funnel plot and the results of sensitivity analysis (remove-one analysis), we excluded one study with outlier data. The analyses without this study showed no significant differences. Our findings are not consistent with recent meta-analysis on this matter even though, no systematic review has been conducted on both cognitive training and cognitive remediation in adults with MDD. The study of Legamaat et al. in 2021 showed a small and significant effect of cognitive remediation in MDD patients. This inconsistency can be due to aforementioned differences between our study and Legammat’s.
We found a moderate and significant effect on the executive function, verbal learning, and working memory of patients with MDD. All of the studies that evaluated executive function and were included in our analysis have used cognitive remediation techniques. Despite this fact, our findings were consistent with Woolf et al. (that evaluated cognitive training), and not similar to Legammat et al. (that evaluated cognitive remediation) which can be because of the differences mentioned before. The studies included in our analysis of verbal learning and working memory were from both cognitive remediation and cognitive training methods. Our findings in this matter are with less heterogeneity but are consistent with the study of Legammat et al. (I2 = 64%).
The importance and magnitude of deficit in executive function among MDD patients has been shown in different studies. Multiple studies have shown that executive dysfunction is strongly associated with some well-known aspects of MDD characteristics such as deficits in emotion regulation [53], attentional bias for negative stimuli [54], and rumination [55, 56] and can even give rise to other cognitive dysfunctions such as attention and problem-solving impairments [57]. Furthermore, executive function impairment remains among individuals with MDD even in remission and in euthymic phase [8, 58, 59]. The cognitive deficits in executive function and memory domains have been shown to be associated with occupational, social and global functioning and quality of life of patients with MDD [60]. Moreover, executive function, verbal learning and memory have been shown to be treatment outcome predictors in MDD [61] and a recent study indicated that executive dysfunction can be risk factor for suicide attempt and suicide preventive interventions should concentrate on executive function rehabilitation [62]. Considering all these significant aspects of verbal learning, memory and particularly executive function in adults with MDD and the significant effect of cognitive rehabilitation on these domains, clinical focus on cognitive rehabilitation is warranted.
Even though included studies used different methods of cognitive remediation and cognitive training for MDD patients, some similar and related methods are worth mentioning:
Five of the included studies (more than half of the studies that used cognitive training interventions) evaluated the Cognitive Control Training’s (CCT) effect on cognitive functions and symptom severity of patients with MDD. It has been shown that cognitive control impairment and emotion regulation deficits have a role in depression vulnerability and it has been suggested that improving cognitive control can be beneficial for treatment outcome of MDD patients [63, 64]. CCT is a modified trainings focuses on working memory to improve cognitive control [65]. CCT contains different tasks in this matter which the most frequently used tasks are the adaptive Paced Auditory Serial Addition Task (aPASAT), and the sustained attention training [65]. The suggested mechanism of effect of these tasks is an increase in activity of the dorsolateral prefrontal cortex (DLPFC), which is known to be under-activated in depression [66, 67]. Three of the included studies that used CCT, only evaluated the symptoms severity of patients and two of them assessed both symptom severity and the working memory of participants. Based on the findings of our study, similar to other cognitive rehabilitation interventions, CCT has a beneficial effect on working memory of MDD patients but it is not effective for MDD’s symptoms severity (d = 0.02 (95% CI, -0.28 to 0.33) p-value = 0.87, I2 = 30%)).
All of the included studies that evaluated cognitive remediation therapies, used computer-based models. Two of these studies used a training program named CogniPlus® which focuses on divided and selective attention, working memory, planning, response inhibition and alertness with a personalized task difficulty. Another two researches used a computerized intervention package named RehaCom as a neurocognitive remediation therapy. This software has been assessed and used on patients with schizophrenia [68] and neurological problems [69]. In the depression model, RehaCom focuses on divided attention, figural memory, verbal memory, and planning (plan a day and shopping [47, 48]). The suggested effect mechanism of both these computer-based models is mobilizing brain neuroplasticity and improving the synaptic communication between neurons by simulating the environmental change and learning new skills by repetitive practices [42, 47, 48, 70]. This mechanism can be a possible rational for improvement of the cognitive functions of MDD patients but it was not effective on their symptom severity.
The evaluation of included studies showed relatively high quality. As we included only randomized and controlled trials, there are no biases in these areas and the majority of studies were conducted concealed and blinded. Considering the moderate effect size and low risk of bias of almost all of the studies, these results can be reliable and conclusive.
Even though the number of included studies and their quality were satisfactory, there are some limitations in this study that is notable to mention. Only three studies evaluated a follow-up measure. However, the duration of follow-up was not similar (one month, three month and one year) and hence not suitable for analysis. This matter is important for many reasons. Besides the obvious value of evaluating the persistency of the effect of cognitive rehabilitation through time, the follow-up measure can be helpful to distinguish different factors of the treatment effect. For example, the mood improvement that is reported by many of the studies can be due to the feel of self-efficacy and self-confidence generated by involving in cognitive rehabilitation interventions and passing the structures and levels of cognitive tasks and consequently it will not show in follow-up measurements.
Another important matter and limitation of our study is that we couldn’t analyze the effect of MDD severity and many of included studies did not provide the history of MDD treatments and duration of participants’ diagnosis and our analysis is not homogenous on this matter. It can be an important factor since the effect of interventions can be inconsistent in different levels of severity and state of treatment. For example, it is possible that severely depressed patients or individuals in first stages of antidepressant treatment cannot fully engage in cognitive demanding tasks or the helplessness of chronic and treatment resistant patients can affect the results.
Mentioned factors should be considered in future studies. Additionally, further research should focus on executive function, verbal learning and working memory to provide further evidence and be of service to clinical practice.
Conclusion
This systematic review and meta-analysis indicate that cognitive rehabilitation is an effective intervention for the executive function, verbal learning, and working memory of MDD patients. Due to the importance of these neuropsychological deficits in day-to-day life and the core symptoms of MDD, cognitive rehabilitation should be considered an important part of treating MDD. Further research in this area and concentrated on these particular deficits is warranted.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Araminia B, Shalbafan M, Mortezaei A, Shirazi E, Ghaffari S, Sahebolzamani E, et al. L-Carnosine combination therapy for major depressive disorder: A randomized, double-blind, placebo-controlled trial. J Affect Disord. 2020;267:131–6.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013.
Ghazizadeh-Hashemi M, Ghajar A, Shalbafan M-R, Ghazizadeh-Hashemi F, Afarideh M, Malekpour F, et al. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: A double-blind, randomized and placebo-controlled trial. J Affect Disord. 2018;232:127–33.
Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8(1):1–10.
Chiriţă AL, Gheorman V, Bondari D, Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Rom J Morphol Embryol. 2015;56(2 Suppl):651–8.
Culpepper L, Lam RW, McIntyre RS. Cognitive impairment in patients with depression: awareness, assessment, and management. J Clin Psychiatry. 2017;78(9):3185.
Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69(7):1122–30.
Rock PL, Roiser J, Riedel WJ, Blackwell A. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–40.
Legemaat AM, Semkovska M, Brouwer M, Geurtsen GJ, Burger H, Denys D, et al. Effectiveness of cognitive remediation in depression: a meta-analysis. Psychol Med. 2021;52:1–16.
Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):851–61.
Mattern M, Walter H, Hentze C, Schramm E, Drost S, Schoepf D, et al. Behavioral evidence for an impairment of affective theory of mind capabilities in chronic depression. Psychopathology. 2015;48(4):240–50.
Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81.
Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological function in young patients with unipolar major depression. Psychol Med. 1997;27(6):1277–85.
Austin M-P, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178(3):200–6.
Perini G, Ramusino MC, Sinforiani E, Bernini S, Petrachi R, Costa A. Cognitive impairment in depression: recent advances and novel treatments. Neuropsychiatr Dis Treat. 2019;15:1249–58.
Castaneda A, Suvisaari J, Marttunen M, Perälä J, Saarni S, Aalto-Setälä T, et al. Cognitive functioning in a population-based sample of young adults with a history of non-psychotic unipolar depressive disorders without psychiatric comorbidity. J Affect Disord. 2008;110(1–2):36–45.
Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39–48.
Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiat. 2000;48(8):791–800.
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci. 1996;93(9):3908–13.
Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53(1):545–74.
Rogers MA, Bradshaw JL, Pantelis C, Phillips JG. Frontostriatal deficits in unipolar major depression. Brain Res Bull. 1998;47(4):297–310.
Prado CE, Watt S, Crowe SF. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol Rev. 2018;28(1):32–72.
Chakrabarty T, Hadjipavlou G, Lam RW. Cognitive dysfunction in major depressive disorder: assessment, impact, and management. Focus. 2016;14(2):194–206.
Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171(5):510–22.
Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiat. 2010;68(11):982–90.
Kurtz MM. Cognitive remediation for schizophrenia: current status, biological correlates and predictors of response. Expert Rev Neurother. 2012;12(7):813–21.
Beck SJ, Hanson CA, Puffenberger SS, Benninger KL, Benninger WB. A controlled trial of working memory training for children and adolescents with ADHD. J Clin Child Adolesc Psychol. 2010;39(6):825–36.
Shah UR. Cognitive rehabilitation in psychiatry. Annals of Indian Psychiatry. 2017;1(2):68.
Anaya C, Aran AM, Ayuso-Mateos JL, Wykes T, Vieta E, Scott J. A systematic review of cognitive remediation for schizo-affective and affective disorders. J Affect Disord. 2012;142(1–3):13–21.
Kim EJ, Bahk Y-C, Oh H, Lee W-H, Lee J-S, Choi K-H. Current status of cognitive remediation for psychiatric disorders: a review. Front Psych. 2018;9:461.
Bakizadeh F, Mokhtari S, Saeed F, Mokhtari A, Akbari Koli P, Shalbafan M. Cognitive Rehabilitation for Improving Cognitive Functions and Reducing the Severity of Symptoms in adult Patients with Obsessive-Compulsive Disorder: A Systematic Review of Randomized Controlled Clinical Trials. Basic and Clinical Neuroscience.0-.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11.
Borenstein M, Hedges LV, Higgins JPT, Rothstein, editors. Comprehensive meta-analysis Version. 3rd ed. Englewood: Biosat Inc; 2013.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates; 1988. p. 20–6.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. West Sussex: Wiley; 2019.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28(366):l4898 PubMed PMID: 31462531. Epub 2019/08/30. eng.
Arean PA, Hallgren KA, Jordan JT, Gazzaley A, Atkins DC, Heagerty PJ, et al. The use and effectiveness of mobile apps for depression: results from a fully remote clinical trial. J Med Internet Res. 2016;18(12):e6482.
Au RW, Sezto HN, Lam VW, Wan Y, Poon L, Pang P, et al. Brief report: A randomized controlled trial of a compensatory cognitive training to improve prospective memory performance in people with schizophrenia or depression. Psychiatry Res. 2021;300:113914.
Bowie CR, Gupta M, Holshausen K, Jokic R, Best M, Milev R. Cognitive remediation for treatment-resistant depression: effects on cognition and functioning and the role of online homework. J Nerv Ment Dis. 2013;201(8):680–5.
Ferrari GR, Vanderhasselt M-A, Rinck M, Demeyer I, De Raedt R, Beisel S, et al. A cognitive control training as add-on treatment to usual care for depressed inpatients. Cogn Ther Res. 2021;45(5):929–43.
Hoorelbeke K, Koster EH. Internet-delivered cognitive control training as a preventive intervention for remitted depressed patients: Evidence from a double-blind randomized controlled trial study. J Consult Clin Psychol. 2017;85(2):135.
Klojčnik M, Bakracevic K. The effectiveness of computerized cognitive remediation therapy (CCRT) for deficits in attention and executive functions in depression: A pilot study. Applied Neuropsychology: Adult. 2021:1–9.
Iacoviello BM, Wu G, Alvarez E, Huryk K, Collins KA, Murrough JW, et al. Cognitive-emotional training as an intervention for major depressive disorder. Depress Anxiety. 2014;31(8):699–706.
Listunova L, Kienzle J, Bartolovic M, Jaehn A, Grützner TM, Wolf RC, et al. Cognitive remediation therapy for partially remitted unipolar depression: A single-blind randomized controlled trial. J Affect Disord. 2020;276:316–26.
Moshier SJ, Molokotos EK, Stein AT, Otto MW. Assessing the effects of depressed mood and cognitive control training on memory confidence and accuracy following repeated checking. Int J Cogn Ther. 2015;8(3):206–21.
Moshier SJ, Otto MW. Behavioral activation treatment for major depression: A randomized trial of the efficacy of augmentation with cognitive control training. J Affect Disord. 2017;210:265–8.
Semkovska M, Lambe S, Lonargáin DÓ, McLoughlin DM. Neurocognitive remediation therapy for depression: a feasibility study and randomized controlled pilot protocol testing. J Nerv Ment Dis. 2015;203(8):609–16.
Semkovska M, Ahern E. Online neurocognitive remediation therapy to improve cognition in community-living individuals with a history of depression: A pilot study. Internet Interv. 2017;9:7–14.
Trapp W, Engel S, Hajak G, Lautenbacher S, Gallhofer B. Cognitive remediation for depressed inpatients: Results of a pilot randomized controlled trial. Aust N Z J Psychiatry. 2016;50(1):46–55.
Wanmaker S, Hopstaken J, Asselbergs J, Geraerts E, Franken I. Decreasing dysphoric thoughts by a working memory training: A randomized double-blind placebo-controlled trial. J Depress Anxiety. 2014;3(165):1–8.
Wanmaker S, Geraerts E, Franken IH. A working memory training to decrease rumination in depressed and anxious individuals: a double-blind randomized controlled trial. J Affect Disord. 2015;175:310–9.
Woolf C, Lampit A, Shahnawaz Z, Sabates J, Norrie L, Burke D, et al. A systematic review and meta-analysis of cognitive training in adults with major depressive disorder. Neuropsychology review. 2022;32:419–37. https://doi.org/10.1007/s11065-021-09487-3.
Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cogn Emot. 2010;24(2):281–98.
Everaert J, Grahek I, Koster EH. Individual differences in cognitive control over emotional material modulate cognitive biases linked to depressive symptoms. Cogn Emot. 2017;31(4):736–46.
Zetsche U, D’Avanzato C, Joormann J. Depression and rumination: Relation to components of inhibition. Cogn Emot. 2012;26(4):758–67.
Whitmer AJ, Gotlib IH. Switching and backward inhibition in major depressive disorder: the role of rumination. J Abnorm Psychol. 2012;121(3):570.
Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cogn Ther Res. 2007;31(2):211–33.
Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–26.
Wang Y, Zhang A, Yang C, Li G, Sun N, Liu P, et al. Enhanced Functional Connectivity Within Executive Function Network in Remitted or Partially Remitted MDD Patients. Front Psych. 2021;11:538333.
Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: A systematic review. Psychiatry Res. 2018;269:157–71.
Groves SJ, Douglas KM, Porter RJ. A systematic review of cognitive predictors of treatment outcome in major depression. Front Psych. 2018;9:382.
Fernández-Sevillano J, Alberich S, Zorrilla I, González-Ortega I, López MP, Pérez V, et al. Cognition in Recent Suicide Attempts: Altered Executive Function. Frontiers in psychiatry. 12:701140. https://doi.org/10.3389/fpsyt.2021.701140.
Joormann J, D’Avanzato C. Emotion regulation in depression: Examining the role of cognitive processes: Cognition & Emotion Lecture at the 2009 ISRE Meeting. Cogn Emot. 2010;24(6):913–39.
Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cogn Ther Res. 2007;31(2):235–62.
Koster EH, Hoorelbeke K, Onraedt T, Owens M, Derakshan N. Cognitive control interventions for depression: A systematic review of findings from training studies. Clin Psychol Rev. 2017;53:79–92.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–9.
Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88(2–3):233–42.
d’Amato T, Bation R, Cochet A, Jalenques I, Galland F, Giraud-Baro E, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Schizophr Res. 2011;125(2–3):284–90.
Friedl-Francesconi H, Binder H. Training in cognitive functions in neurologic rehabilitation of craniocerebral trauma. Z Exp Psychol. 1996;43(1):1–21.
Kolb B, Teskey GC, Gibb R. Factors influencing cerebral plasticity in the normal and injured brain. Front Hum Neurosci. 2010;4:204.
Acknowledgements
Not applicable
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Iranian Cognitive Sciences and Technologies Council (Grant no: 10684).
Author information
Authors and Affiliations
Contributions
Conceptualization and design: SM, AlM and MS; Data collection: SM, AsM and FB; Initial draft preparation: SM, AsM and MS; Editing & review: All authors.The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Because of the type of article (Review), there was no need for obtaining ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mokhtari, S., Mokhtari, A., Bakizadeh, F. et al. Cognitive rehabilitation for improving cognitive functions and reducing the severity of depressive symptoms in adult patients with Major Depressive Disorder: a systematic review and meta-analysis of randomized controlled clinical trials. BMC Psychiatry 23, 77 (2023). https://doi.org/10.1186/s12888-023-04554-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-04554-w