Abstract
Objective
The purpose of this study is to investigate the possible link between dietary theobromine intake and symptoms of depression.
Materials and methods
These results are based on the responses of 3637 people who took part in the National Health and Nutrition Examination Survey in 2017–2018. Participants' daily theobromine intake was determined using a 24-h food questionnaire from the 2017–2018 cycle. Presence of depression was defined as a score of 5 or above on the Patient Health Questionnaire. Association between theobromine intake and depression was examined using a multivariate logistic regression adjusting for several relevant sociodemographic, lifestyle and health-related factors.
Results
A total of 6903 participants were included in the study. The results of multivariate logistic regression showed a correlation between depressive symptoms and theobromine intake (OR:1.17, 95%CI:1.02–1.34).
Conclusions
Our cross-sectional population based study suggests that increased theobromine intake is associated with increased risk for depression. Nevertheless, more investigations are needed to confirm our findings.
Similar content being viewed by others
Introduction
Depression is a serious condition affecting 246 millions of individuals worldwide [1]. Depression is the most common cause of disability and the fourth most common contribution to the overall illness burden in the world [2]. Antidepressant drugs, psychotherapies, and a variety of brain stimulation methods are all validated therapy options for depression [3]. Antidepressants are one of the most often recommended groups of psychotropic drugs for adolescents in the United States [4]. However, patient adherence was quite poor, with as many as half of patients interrupting their therapy in the first six weeks [5]. Increasing data also shows that dietary factors have an impact on depression symptoms [6, 7]. Previous studies have reported a protective effect of chocolate against depression [5]. In this regard, one of the primary ingredient of chocolate, theobromine, has been show to protect cognitive function by regulating neurotransmitter signaling [8]. Despite this, few population-based studies have investigated the link between theobromine in the diet and depression. Therefore, we aimed at examining the association between theobromine consumption and depressive symptoms taking advantage of a large population-based cohort in the United States.
Materials and methods
Study population
The National Health and Nutrition Examination Survey, also known as NHANES, is an ongoing survey that is carried out on a rolling basis in order to collect cross-sectional data from the civilian population in the United States that does not reside in institutions [9]. Since 1999, the NHANES has been conducting a survey of a nationally representative, complicated, stratified, multi-stage probability sample of the US population [10]. Each wave of the survey has included a different participant. The assessment procedures include a household interview and a physical examination at a mobile examination center (MEC) [11]. In this study, we obtained data from 2017–2018. In NHANES, depressive symptoms were only assessed in patients aged 18 years; therefore, we only included data from this age group.
Measures
Exposure: Theobromine intake
Participants in the NHANES were asked to take part in an in-person household interview as well as a health examination at a MEC, which included a recall of their dietary intake over the previous 24 h [12]. The Automated Multiple Pass Method, which was utilized in NHANES in order to collect dietary data, has been successfully validated [13]. More details are available at www.ars.usda.gov/ba/bhnrc/fsrg. Based on the distribution of theobromine intake in NHANES, we defined increased theobromine intake as values above the third quartile (Q3), ie 43 mg/day [8].
Outcome: depressive symptoms
The Patient Health Questionnaire (PHQ-9) is a validated 9-item depression screener that was used to evaluate depressive symptoms. The questions on this screener enquire about the duration and severity of depressive symptoms during the last two weeks [14]. For each question, the score ranged from 0 to 3, and the total score ranged from 0 to 27. Depressive symptoms were then categorised as "none or minimal" (0–4), "mild" (5–9), "moderate" (10–14), "moderately severe" (15–19), or "severe" (20–27) [15]. Depressive symptoms were defined as a score of ≥ 5 on the PHQ-9 [8].
Covariates
Age, gender, race (Mexican American; white; black and other), multimorbidity, education level (below high school; high school and college or above), smoking status (former; never and current), drinking status (never; former; light; moderate and heavy) and the poverty income ratio (PIR) were all taken into consideration when determining socio-demographic characteristics. A never smoker is an adult who has never smoked or has smoked fewer than 100 cigarettes in their lifetime; former smokers are individuals who have reported smoking 100 cigarettes in their lifetime but are not currently smokers; and current smokers are individuals who have smoked 100 cigarettes on some days or every day in their lifetime [16]. Never drinkers reported consuming less than 12 drinks; ever drinkers reported having more than 12 drinks in their lives but not in the preceding year; and current drinkers were further categorized as light, moderate, or heavy current drinkers. Heavy current drinkers were defined as women drinking 3 drinks per day and men drinking 4 drinks per day, with 5 or more binge drinking days per month; moderate drinkers were classified as women drinking 2 drinks per day and men drinking 3 drinks per day, with 2 binge drinking days per month. People who drank just a little: did not satisfy the standards outlined above [17]. As a measure of socioeconomic status, the PIR, which is the ratio of total family income to the poverty threshold, was used: low (PIR < 1.35), medium (1.35 ≤ PIR < 3.0), and high (PIR ≥ 3.0) [18]. The presence of diabetes mellitus was defined as the need for the administration of insulin or oral antidiabetic medication treatment. Prediabetes is defined in this study by impaired fasting glucose and impaired glucose tolerance [19]. Body mass index (BMI) was determined by dividing weight in kilograms by the square of height in meters and was classified as underweight (BMI = 18.5), normal weight (BMI = 18.5—24.9), overweight (BMI = 25—29.9), and obese (BMI > 30.0) were the four BMI categories. Multimorbidity, defined as the presence of two or more chronic conditions in a person, has been linked to depression [20, 21]. Cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), asthma, arthritis, cancer, stroke, hypertension, hyperlipidemia, diabetes, and obesity were chosen based on their clinical importance and the availability in NHANES [20]. We used a backward stepwise regression to identify our final model with depression ( ie > 5 on the PHQ9) as our dependent variable. Improvement in the model was assessed using the Akaike Information Criteria (AIC). Two variables, [PIR, education], were excluded due to high collinearity.
Statistical analyses
All statistical analyses were performed with R, version 4.0.5 (R Project for Statistical Computing) using the survey package, version 4 1–1 and with Free Software Foundation statistics software, version 1.3. In all tests, P < 0.05 (2-sided) was considered to indicate statistical significance. The categorical variables were summarized as percent and frequency, while continuous variables were summarized as mean and 95% confidence intervals (CIs). Categorical data were compared using the χ2 test or Fisher's exact test, while continuous data were compared using Student's t-test. The analyses were restricted to participants who had complete data records.
Results
Population characteristics
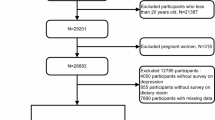
Figure 1 depicts the recruitment and inclusion/exclusion criteria for the study. The study included 6903 representative U.S. participants. Participants with depressive symptoms were younger than those without symptoms (49.0 ± 18.3 years versus 51.7 ± 18.0 years years, P < 0.05) (Table 1). Moreover, participants with increased theobromine intake (> = 43 mg/day) reported more depressive symptoms (34.9% vs 31.3% for low intake group, P < 0.05). In addition, the proportion of patients with depressive symptoms was higher in women and individuals with white ethnicity, lower family income, college or higher education, as well as multimorbidity. Similar differences were noted in never smokers and mild alcohol users.
Multivariate regression analysis
In a multivariate regression model including [gender, age,race, smoke, alcohol, multimorbidity], a higher theobromine intake was associated with increased risk of depression (OR:1.17, 95%CI:1.02–1.34, AIC 319.528; Table 2). A subgroup analysis revealed that in participants aged < 60 years [p < 0.001], without multimorbidity [p < 0.001], obesity [p < 0.05] or cancer [p = 0.002], higher theobromine intake was associated with increased risk for depression (Table 3).
Relationship between theobromine and depression
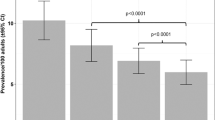
A restricted cubic spline (RCS) was used to furtherly clarify the relationship between theobromine and depression after controlling for possible variables. Figure 2 suggests that theobromine is positively correlated with the prevalence of depression.
Discussion
In this study, we identified an association between increased theobromine consumption and depressive symptoms, even after adjusting for age, sex, race, multimorbidity, smoking status, and alcohol consumption, these relationships were still clearly visible.
Our findings are in the line of previous studies reporting protective effects of theobromine on cognitive function. A wide range of mechanisms have been suggested in the literature such as improved neurotransmission, upregulation of brain derived neurothrophic factors and modulation of calcium and phosphodiesterase homeostasis [22]. Furthermore, experimental findings showed how theobromine is able to cross the blood brain barrier where it regulates the activity of neurotransmitter receptors, such as adenosine receptors, which have been linked to depressive and anxiety states [23, 24]. Other adenosine receptor independent effects were reported such as the reduction of cellular oxidative stress and upregulation of gene expression [PRDX1、PRDX6] [24].
Our findings contrast with previous studies reporting that increased consumption of caffeine, which is also a methylxanthine, is associated with decreased risk of depression [23, 25, 26]. Indeed, a study conducted in the United Kingdom found that unemployed individuals consuming caffeine on a regular basis were more likely to report depresssive symptoms [27]. Although belonging to the same group, pharmacological differences are noted between caffeine and theobromine and may therefore explain the opposite effects on mood but also on blood pressure [24, 25]. Caffeine is metabolised to theobromine in the liver and studies conducted in the rat and in humans, show that theobromine has a higher half-life than caffeine, which is more rapidly degraded [24, 28]. Hence, it is believed that the beneficial effect of caffeine is mediated through its metabolites, such as theobromine [24, 25]. It is also hypothesised that the effects of caffeine are more CNS specific, resulting in symptoms such as alertness, while theobromine exerts its effect primarily via peripheral changes [25]. However, the differences between the two compounds, and their effect on mood stated need to be further explored.
Our subgroup analysis shows that younger participants (i.e. under 60 years old) were more likely to report depression with increased theobromine intake. Previous studies have suggested that young age during pregnancy [especially below 26 years of age] are at increased risk for anxiety and depression [29]. But the pharmacological properties of theobromine may also be affected by recall bias in the elderly, which could not be completely excluded from the questionnaire, and by the effects of oral administration of multiple drugs in the elderly population. Also, our study showed a positive association between theobromine and depressive symptoms in participants without multimorbidity. By showing an association between theobromine consumption and depression, our study further fuels the debate on the role of nutrition in mental health care and particularly in risk groups. For example, previous studies have found polyunsaturated fatty acids (PUFAs), which may affect depression in elderly japanese people [30].
However, some limitations remain. Due to the inability of cross-sectional observational studies to establish causality and directionality, our results should be regarded with caution. In addition, the effect of caffeine could not be investigated due to the data paucity. Furthermore, despite thorough adjustments for confounding, residual confounding cannot be ruled out. In particular, recall bias from older adults cannot be completely excluded.
Conclusion
Our study suggests that theobromine intake is associated with increased risk for depression in adults, highlighting the importance of nutrition on the cognitive function. Finally, further studies are needed to clarify the link between theobromine and mood states.
Availability of data and materials
The National Health and Nutrition Examination Survey (NHANES) data are publically available at https:// wwwn.cdc.gov/nchs/nhanes which is publicly available. Accession number: NHANES 2017–2018.
References
Tchekalarova J, Ivanova N, Nenchovska Z, Tzoneva R, Stoyanova T, Uzunova V, et al. Evaluation of neurobiological and antioxidant effects of novel melatonin analogs in mice. Saudi Pharm J. 2020;28:1566–79. https://doi.org/10.1016/j.jsps.2020.10.004.
Wen Y, Liu C, Liao J, Yin Y, Wu D. Incidence and risk factors of depressive symptoms in 4 years of follow-up among mid-aged and elderly community-dwelling Chinese adults: findings from the China health and retirement longitudinal study. BMJ Open. 2019;9:e29529. https://doi.org/10.1136/bmjopen-2019-029529.
Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, et al. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–88. https://doi.org/10.1016/j.neubiorev.2017.08.019.
Burcu M, Zito JM, Safer DJ, Magder LS, dosReis S, Shaya FT, et al. Association of antidepressant medications with incident type 2 diabetes among medicaid-insured youths. JAMA Pediatr. 2017;171:1200–7. https://doi.org/10.1001/jamapediatrics.2017.2896.
Jackson SE, Smith L, Firth J, Grabovac I, Soysal P, Koyanagi A, et al. Is there a relationship between chocolate consumption and symptoms of depression? A cross-sectional survey of 13,626 US adults. Depress Anxiety. 2019;36:987–95. https://doi.org/10.1002/da.22950.
Pérez-Ara MÁ, Gili M, Visser M, Penninx BWJH, Brouwer IA, Watkins E, et al. Associations of non-alcoholic beverages with major depressive disorder history and depressive symptoms clusters in a sample of overweight adults. Nutrients. 2020;12:3202. https://doi.org/10.3390/nu12103202.
Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2018;226:346–54. https://doi.org/10.1016/j.jad.2017.09.022.
Gao L, Ge W, Peng C, Guo J, Chen N, He L. Association between dietary theobromine and cognitive function in a representative American population: a cross-sectional study. J Prev Alzheimer’s Dis. 2022;9:449–57. https://doi.org/10.14283/jpad.2022.39.
Curti SA, Taylor EN, Su D, Spankovich C. Prevalence of and characteristics associated with self-reported good hearing in a population with elevated audiometric thresholds. JAMA Otolaryngology Head Neck Surg. 2019;145:626–33. https://doi.org/10.1001/jamaoto.2019.1020.
Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. JAMA. 2021;326:1–13. https://doi.org/10.1001/jama.2021.9883.
Li Z, Wang W, Xin X, Song X, Zhang D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. 2018;228:68–74. https://doi.org/10.1016/j.jad.2017.12.004.
Bailey RL, Fulgoni VLR, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr. 2011;94:1376–81. https://doi.org/10.3945/ajcn.111.020289.
Kim K, Melough MM, Vance TM, Noh H, Koo SI, Chun OK. Dietary cadmium intake and sources in the US. Nutrients. 2018;11:2. https://doi.org/10.3390/nu11010002.
Iob E, Frank P, Steptoe A, Fancourt D. Levels of severity of depressive symptoms among at-risk groups in the UK during the COVID-19 pandemic. JAMA Netw Open. 2020;3:e2026064. https://doi.org/10.1001/jamanetworkopen.2020.26064.
Flint KM, Fairclough DL, Spertus JA, Bekelman DB. Does heart failure-specific health status identify patients with bothersome symptoms, depression, anxiety, and/or poorer spiritual well-being? European Heart Journal. Qual Care Clin Outcomes. 2019;5:233–41. https://doi.org/10.1093/ehjqcco/qcy061.
Kim K, Song Y, Oh TJ, Choi SH, Jang HC. Association between iron intake and diabetic peripheral neuropathy in type 2 diabetes: significance of iron intake and the ratio between iron intake and polyunsaturated fatty acids intake. Nutrients. 2020;12:3365. https://doi.org/10.3390/nu12113365.
Rattan P, Penrice DD, Ahn JC, Ferrer A, Patnaik M, Shah VH, et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol Commun. 2022;6:399–410. https://doi.org/10.1002/hep4.1803.
Jenkins GP, Evenson KR, Herring AH, Hales D, Stevens J. Cardiometabolic correlates of physical activity and sedentary patterns in U.S. youth. Med Sci Sports Exerc. 2017;49:1826–33. https://doi.org/10.1249/MSS.0000000000001310.
Linkens AM, Eussen SJ, Houben AJ, Kroon AA, Schram MT, Reesink KD, et al. Habitual intake of dietary advanced glycation end products is not associated with arterial stiffness of the aorta and carotid artery in adults: the Maastricht study. J Nutr. 2021;151:1886–93. https://doi.org/10.1093/jn/nxab097.
King DE, Xiang J, Pilkerton CS. Multimorbidity trends in United States adults, 1988–2014. J Am Board Fam Med. 2018;31:503–13. https://doi.org/10.3122/jabfm.2018.04.180008.
Smith HC, Saxena S, Petersen I. Maternal postnatal depression and completion of infant immunizations: a UK cohort study of 196,329 mother-infant Pairs, 2006–2015. J Clin Psychiatry. 2022;83:20m13575. https://doi.org/10.4088/JCP.20m13575.
Yoneda M, Sugimoto N, Katakura M, Matsuzaki K, Tanigami H, Yachie A, et al. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J Nutr Biochem. 2017;39:110–6. https://doi.org/10.1016/j.jnutbio.2016.10.002.
Mendiola-Precoma J, Padilla K, Rodríguez-Cruz A, Berumen LC, Miledi R, García-Alcocer G. Theobromine-induced changes in A1 purinergic receptor gene expression and distribution in a rat brain alzheimer’s disease model. J Alzheimer’s Dis. 2017;55:1273–83.
Martínez-Pinilla E, Oñatibia-Astibia A, Franco R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front Pharmacol. 2015;6:30. https://doi.org/10.3389/fphar.2015.00030.
Mitchell ES, Slettenaar M, vd Meer N, Transler C, Jans L, Quadt F, et al. Differential contributions of theobromine and caffeine on mood, psychomotor performance and blood pressure. Physiol Behav. 2011;104:816–22. https://doi.org/10.1016/j.physbeh.2011.07.027.
Iranpour S, Sabour S. Inverse association between caffeine intake and depressive symptoms in US adults: data from National Health and Nutrition Examination Survey (NHANES) 2005–2006. Psychiatry Res. 2019;271:732–9. https://doi.org/10.1016/j.psychres.2018.11.004.
Smith AP. Caffeine, cognitive failures and health in a non-working community sample. Hum Psychopharmacol. 2009;24:29–34. https://doi.org/10.1002/hup.991.
Mumford GK, Benowitz NL, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, et al. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur J Clin Pharmacol. 1996;51:319–25.
Dørheim SK, Bjorvatn B, Eberhard-Gran M. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med. 2012;10:152–66. https://doi.org/10.1080/15402002.2012.660588.
Tsujiguchi H, Thi Thu Nguyen T, Goto D, Miyagi S, Kambayashi Y, Hara A, et al. Relationship between the Intake of n-3 polyunsaturated fatty acids and depressive symptoms in elderly japanese people: differences according to sex and weight status. Nutrients. 2019;11:775. https://doi.org/10.3390/nu11040775.
Acknowledgements
Thanks to Figdraw (www.figdraw.com) for technical support (IIUAY3a783). No matter the old village doctors who are going to retire or the young who just set foot on the job, they have no regrets, no conditions and actively participated in the front-line work of epidemic prevention and control in China.
Funding
There is no funding support in this study.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An ethics approval and the consent to participate was not necessary.
Consent for publication
Not applicable.
Competing interests
No competing interests declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Xy., Liu, H., Zhang, Ly. et al. Association between dietary theobromine with depression: a population-based study. BMC Psychiatry 22, 769 (2022). https://doi.org/10.1186/s12888-022-04415-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-04415-y