Abstract
Background
Although the clinical efficacy and safety of combination of pharmacotherapy and psychotherapy in the treatment of depressive disorders in children and adolescents have been studied, the results remain controversial. This meta-analysis aimed to study the short-term efficacy and acceptability of combined therapy for children and adolescents with depressive disorders.
Methods
We conducted a systematic search in multiple databases for randomised controlled trials (RCTs), up to 31 December 2020, that assessed the combination of pharmacotherapy and psychotherapy against other active treatment options (pharmacotherapy, psychotherapy and placebo combined psychotherapy) in children and adolescents ( ≤ 18 years old) with depressive disorder. This study was registered with PROSPERO (CRD42020196701).
Results
A total of 14 RCTs involving 1,325 patients were included. For the primary and secondary outcomes, there were no statistically significant differences between the compared interventions in terms of remission (odds ratios [OR] = 1.37; 95% confidence interval [CI]: 0.93 to 2.04), acceptability (OR = 0.99; 95% CI: 0.72 to 1.38), efficacy (standardised mean differences = -0.07; 95% CI: -0.32 to 0.19), and suicidality (OR = 1.17; 95% CI: 0.67 to 2.06). Limited evidence showed that the combination of fluoxetine (OR = 1.90, 95% CI: 1.10 to 3.29) or non-selective serotonin reuptake inhibitors (non-SSRI) (OR = 2.46, 95% CI: 1.06 to 5.72) with cognitive-behavioural therapy (CBT) was superior to other active treatment options. Most included trials were rated as ‘some concerns’ in terms of risk of bias assessment.
Conclusion
There is no evidence from the limited available data that all combined therapies are superior to other active treatment options for the acute treatment of depressive disorder in children and adolescents. However, it showed that fluoxetine or non-SSRI pharmacotherapies combined with CBT might be superior to other therapies in short-term. Mixed characteristics (e.g. age) and small sample size of non-SSRI combined therapy may influence the generalisability of the results.
Similar content being viewed by others
Introduction
The observed prevalence of depression is estimated to be 3.2% in children and adolescents in the United States [1]. Depression causes extensive morbidity and mortality in children and adolescents, and depression-related suicide is the third leading cause of death among them [2]. Compared with adults, depressed children and adolescents are more often misdiagnosed and have more frequent suicide ideation and attempts [3]. Depressive disorder in children and adolescents is debilitating and affects family, psychosocial and academic functions [4].
Several kinds of interventions have been used to treat children and adolescents with depression, from pharmacotherapy to psychotherapy. Psychotherapies, especially cognitive-behavioural therapy (CBT) and interpersonal psychotherapy (IPT) are demonstrated to be effective [5]. As for pharmacotherapy, fluoxetine is significantly more efficacious than placebo, whereas some other antidepressants may produce an increased risk of suicidality [6]. Previous studies have shown that antidepressants have minor but significant contributions to the overall efficacy of the combined treatment of depression in adults [7]. However, whether the combination of pharmacotherapy and psychotherapy is more beneficial than pharmacotherapy or psychotherapy alone remains unclear in children and adolescents [8]. Based on our previous findings, fluoxetine combined with CBT was superior to CBT alone but not more effective than fluoxetine alone [9]. The previous network meta-analysis integrated direct evidence with indirect evidence, which indicated considerable heterogeneity and inconsistency, and most results were assessed as having low confidence in the evidence. Therefore, whether the combination of pharmacotherapy and psychological intervention is more efficacious than other active treatment options remain controversial. This meta-analysis aimed to integrate direct evidence and examine the acute treatment phase potential benefits, acceptability, and harms of a combination of pharmacotherapy and psychotherapy for children and adolescents with depressive disorders.
Methods
Search strategy, selection criteria, and risk of bias
The protocol was registered with PROSPERO (CRD42020196701). We updated the literature search of our previous publications [5, 6, 9] to identify trials of the combination of pharmacotherapy and psychotherapy. Further, we searched for eligible published and unpublished randomised controlled trials (RCTs) on PubMed, Embase, CENTRAL, PsycINFO, Web of Science, CINAHL, LiLACS, ProQuest Dissertations, and international trial registers from inception until 31 December 2020 (see details in Appendix 1). Additionally, we searched drug approval agencies websites (such as the FDA), conference proceedings, relevant scientific journals, and related articles or reviews. We contacted the authors to provide incomplete data on the published studies or original data for unpublished studies. Eligible studies included a combination of pharmacotherapy and psychotherapy compared with other active treatment options (pharmacotherapy alone, psychotherapy alone or pill placebo plus psychotherapy) for the treatment of children and adolescents (younger than 18 years old) with a primary diagnosis of depressive disorder (including major depressive disorder [MDD], dysthymia, and other specified types) based on standardised diagnostic criteria (such as Diagnostic and Statistical Manual of Mental Disorders and International Classification of Diseases). Trials involved patients with comorbid mental disorders (e.g. anxiety disorder and attention deficit hyperactivity disorder) were also eligible. However, trials assessing participants with psychotic depression or treatment-resistant depression were excluded, mainly because their treatment responses were different [10]. No restriction was put on language.
All oral medications or alternative treatments within the therapeutic dose range were considered to be eligible. It was considered as a well-structured intervention when psychotherapy was based on a clear manual for therapists or participants. The arm of pill placebo alone or psychotherapy control conditions alone (treatment as usual, no treatment, and waitlist) only were not examined in the meta-analysis. The acute treatment phase was defined as 4-16 weeks. Trials with less than four weeks of treatment were excluded because the benefits of most antidepressants usually begin at least four weeks after commencement. If a study reported data at multiple time points within a predetermined acute phase range or more than 16 weeks, we used eight weeks (or the nearest to eight weeks). The double-blind design is challenging to perform in a psychotherapy trial or a combined trial of psychotherapy [11], therefore the design of blind was unrestricted. Trials in which participants were assessed using physician/parent-rated or self-rated depression scales were all eligible [12]. To reduce heterogeneity between trials, trials with quasi-randomised design and those with a total sample size of less than 10 were excluded.
Two researchers (Y.X. and T.T.) screened the studies, extracted data, and assessed the risk of bias, respectively. Data were extracted using a standardised data collection form. Any discrepancies in data extraction and quality assessment were resolved by consensus and arbitration by senior investigators (X.Z. and P.X.). The risk of bias was assessed using the Cochrane Collaboration’s risk of bias version 2.0 (ROB 2.0) tool [13]. Studies were evaluated in the following six domains: (1) bias arising from the randomisation process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in the measurement of the outcome; (5) bias in the selection of the reported result; and (6) overall bias. Each domain was rated as ‘low risk’, ‘some concerns’, or ‘high risk’.
Outcomes
The primary outcomes included (1) remission (as a dichotomous data), estimated by the total number of patients who met the criteria for remission, was defined as a depressive symptom score below the threshold (e.g. < 28 for Children’s Depression Rating Scale-Revised [CDRS-R]) in the trials; and (2) acceptability, defined as all-cause discontinuation, was measured by the percentage of participants who withdrew from the study for any reason before the end of treatment. Secondary outcomes included (1) efficacy at post-treatment (as continuous data), estimated by the overall change score of the depressive symptom scales (e.g. CDRS-R or Hamilton Rating Scale for Depression scale) completed by self, parents, or clinicians from baseline to the end of treatment; and (2) suicidality, measured by the number of patients reporting suicidal ideation or suicidal attempt/behaviour in the treatment phase.
Where multiple depressive symptom scales were reported in a study, we extracted data from a predefined hierarchy based on psychometric properties and appropriateness for children and adolescents (see details in Appendix 2). Moreover, in a study where different raters reported the scale, we preferred the parent/clinician- rated outcomes to the self-rated ones [14].
Data analysis
We calculated effect sizes and pooled estimates of effect across studies with a random-effects model using the RevMan 5 software (Cochrane Information Management System) due to considerable clinical heterogeneity among trials. We summarised the standardised mean differences (SMD) for continuous data and odds ratios (ORs) for dichotomous data, as well as 95% confidence intervals (CIs). Heterogeneity was assessed using Q and I2 statistics. Statistical significance was set at p < 0.05, and values of 25%, 50%, and 75% displayed low, moderate, and substantial heterogeneity, respectively. Power and Precision (version 4.0) was used to carry out power calculations. When the mean values or standard deviations (SDs) of continuous outcomes were missing, we calculated values by converting the p values, t-values, CIs, or standard errors.
Considering that effectiveness varies depending on the type of medication and psychotherapy used, we conducted three subgroup analyses, these were according to the types of combined pharmacotherapies, types of combined psychotherapies, and types of active treatment options (pharmacotherapy, psychotherapy and placebo combined psychotherapy). We also conducted subgroup analyses regarding (1) mean baseline severity of the depressive disorder (mild vs. moderate to serve, thresholds presented in Appendix 3); (2) treatment duration (treatment ≤ 8 weeks vs. treatment > 8 weeks); (3) conducted country (the USA vs. non-USA); and (4) risk of bias (low risk vs. some concerns vs. high risk). For continuous variables, we performed meta-regression analyses according to the following variables: publication year, mean age, and percentage of female patients. We also conducted sensitivity analyses by excluding some trials from the fundamental analysis for the non-blind design trials, potential publication bias trials, and high-risk trials of ROB 2.0. Potential publication bias was assessed using inverted funnel plots and Egger’s test. The protocol followed the PRISMA recommendations for meta-analysis [15]. All tests were two-sided, and p values less than 0.05 were considered statistically significant.
Results
Selection, inclusion, and characteristics of studies
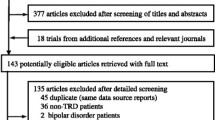
Overall, we searched 11,435 citations, identified the full text of 352 possibly eligible studies, and finally included 14 RCTs (1,325 participants, Fig. 1) published from 1997 to 2020. Six pharmacotherapies (imipramine, fluoxetine, sertraline, omega-3, bupropion, and venlafaxine), three different psychotherapies (CBT, IPT, and psychoeducational psychotherapy [PEP]), and the pill placebo plus psychotherapy were assessed. A total of 567 participants were randomly assigned to combined therapy, 401 to pharmacotherapy alone, 167 to psychotherapy alone, and 190 to pill placebo plus psychotherapy.
The clinical and methodological characteristics of the included studies are shown in Table 1. A total of 13 trials recruited only patients with MDD [8, 16,17,18,19,20,21,22,23,24,25,26,27], and one recruited patients with MDD, dysthymia, and depressive disorder-not otherwise specified [28]. In 10 of the included studies, pharmacotherapies involved selective serotonin reuptake inhibitors (SSRIs), with five studies of fluoxetine, three of sertraline, and two of mixed SSRIs. Additionally, the other four studies included imipramine, venlafaxine, omega-3, and bupropion. Psychotherapy involved CBT in 12 studies, IPT in one, and PEP in one. Seven studies compared combination therapies with pharmacotherapies alone, four trials compared combination therapies with psychotherapies alone, and seven studies compared combination therapies with placebo plus psychological therapies. The average study sample size was 95, ranging from 10 to 327. One trial (7.1%) enrolled only children, 11 (78.6%) enrolled only adolescents, and the remaining two (14.3%) enrolled both children and adolescents. The mean age ranged from 7 to 18 years (mean, 15.2 years old; SD, 2.0), and 753 (56.8%) of the participants were female. Four (28.6%) studies had a multiple-arm design. Nine (64.3%) studies were conducted in the USA and five (35.7%) in other countries, including Australia, Romania, South Korea, and the United Kingdom. The median treatment duration was 12 weeks (interquartile range [IQR], 8–12). Eight trials had a double-blind design; three trials had a single-blinding design, that is, the assessors were blinded to the interventions of patients; and one trial had a non-blinding design with a self-reported scale assessment. There was insufficient information to identify blinding of outcome assessment in two trials also using self-reported scale assessment.
Meta-analysis results for primary outcomes
The results of the meta-analysis for all outcomes are presented in Table 2. Nine studies (939 patients) presented remission data compared combined therapies with pharmacotherapies or psychotherapies alone. The remission did not change substantially with the pooled OR of 1.37 (95% CI = 0.93 to 2.04, p = 0.11) from the random-effects model, and a moderate significant heterogeneity (p = 0.10, I2 = 36%, Fig. 2A) was observed. The statistical power of the effect size was 93%. Thirteen studies (1,276 patients) reported all-cause discontinuation with compared combination therapies with other active treatment options group; the two kinds of interventions did not materially differ, with a pooled OR of 0.99 (95% CI = 0.72 to 1.38, p = 0.98) and no heterogeneity among studies (p = 0.88, I2 = 0%, Fig. 2B). The statistical power of the effect size was 5%.
Meta-analysis results for secondary outcomes
A total of 14 studies (1,276 patients) reported efficacy at short-term post-treatment compared combination therapies with other active treatment options. The overall changes in the score of the depressive symptom scales were not associated with a significant decrease, and the pooled SMD was -0.07 (95% CI = -0.32 to 0.19, p = 0.60) between groups with high and significant heterogeneity (p <0.00001, I2 = 76%, Table 2) among studies. The statistical power of the effect size was 6%. In terms of suicidality, all eight studies (932 patients) presented data on suicidality in combined therapy and other active treatment options. Further, there was no statistically significant effect (OR = 1.17, 95% CI = 0.67 to 2.06, p = 0.58) and no heterogeneity (p = 0.65, I2 = 0%, Table 2). The statistical power of the effect size was 16%.
Subgroup and sensitivity analyses
Owing to significant heterogeneity in remission, various subgroup analyses were conducted to explore potential bias from the type of intervention (Table 3). The combination of fluoxetine (OR = 1.90, 95% CI = 1.10 to 3.29) or non-SSRI pharmacotherapies (OR = 2.46, 95% CI = 1.06 to 5.72) with psychotherapies were superior to other active treatment options; however, CBT was the only psychotherapy available for analysis here. The subgroup analyses in terms of the type of combined psychotherapies and the type of other active treatment options showed no significant difference between interventions (combination therapies vs. other active treatment options), but with significant heterogeneity among studies. Furthermore, we performed subgroup analyses regarding some main baseline characteristics, including mean baseline severity of the depressive disorder, treatment duration, conducted countries, and risk of bias. The results showed that combined therapy was more efficacious than other active treatment options in studies conducted in the USA (OR = 1.90, 95% CI = 1.33 to 2.73) and studies with a ‘high risk’ of bias (OR = 1.89, 95% CI = 1.19 to 3.01). The above subgroup analyses did not show any significant results for acceptability (Appendix 4). Meta-regression analyses showed that none of the continuous modifiers (publication year, mean age, percentage of female patients, and treatment duration) was associated with remission rate or acceptability in the combined treatment compared with other active treatment options (all p > 0.05). Sensitivity analyses for non-blind design studies, potential publication bias trials, and high-risk trials of ROB 2.0 showed that there was no substantial change compared with primary analysis (Appendix 5).
Quality assessment and publication bias
In terms of study quality according to ROB 2.0, 3 (21.4%) out of 14 trials were rated as having a high risk of bias, 8 (57.1%) as having some concerns, and 3 (21.4%) as having a low risk of bias (Appendix 6). The funnel plot and Egger’s tests did not suggest a significant publication bias for all outcomes (all p > 0.05, Appendix 7).
Discussion
This meta-analysis was based on 14 RCTs including 1,325 children and adolescents with depressive disorder randomly assigned to the combination therapy or other active treatment options. To the best of our knowledge, this is the most comprehensive synthesis to assess combination therapies for depressive disorders in children and adolescents in a meta-analysis. There is no evidence from the limited available data that all combined therapies are superior to other active treatment options for the acute treatment of depressive disorder in children and adolescents. However, it showed that fluoxetine or non-SSRI pharmacotherapies combined with CBT might be superior to other therapies in the short-term. Mixed characteristics (e.g. age) and small sample size of non-SSRI combined therapy may reduce the generalisability of the results.
Our findings were similar to those in previous studies involving children and adolescents [29,30,31] as well as adults [32]. However, those reviews also differed from ours in some aspects. One study only analysed CBT combined with newer-generation antidepressants therapies [29], one study included trials with ‘treatment resistant’ participants [30], and another included only patients with MDD [31]. Given the limitations of the data, the authors of these studies cautioned against drawing firm conclusions. The heterogeneity among studies also limited our conclusion, which may be because of the different types of pharmacotherapies and psychotherapies. Further subgroup analysis of types of medications showed that fluoxetine combined with CBT was more efficacious than other active treatment options in terms of remission at post-treatment. It is noticed that, CBT was the only psychotherapy available in these studies. Fluoxetine is one of the most widely studied SSRIs, and CBT is one of the most commonly studied psychotherapies; both of them have shown some effectiveness in the treatment of depressive disorder in children and adolescents [31]. The efficacy of fluoxetine combined with CBT compared with CBT alone has also been demonstrated in our previous network meta-analysis, which integrates direct and indirect evidence [9]. Additionally, the non-SSRIs included in the present study are imipramine [16] and Omega-3 [20]; however, they were only reported in one study each, which may be prone to bias of results. Therefore, limited data may reduce the generalisability of the results of non-SSRI combined therapy. Further, most of these pooled effect sizes were small to medium with some uncertainty, which could be due to the relatively small sample size and wide confidence intervals. Thus, caution should be exercised when explaining the statistical superiority in this study.
It was observed that the dropout prevalence was approximately 23% (ranging from 20% to 27%) among depressed adolescents treated with antidepressants [33]. The most critical factor related to the high rate of dropout was the side effects of medications (such as suicide-related events, mania, skin rash, and headaches) [33]. It has been demonstrated that the optimal dropout rate should not exceed 20% because exceeding this number could indicate questionable representativeness, reliability, and generalisability [34, 35]. No significant difference was observed between all the compared groups with low statistical power or in the subgroup analyses of acceptability. In terms of the included studies, five (35.7%) clinical trials [16, 17, 22, 26] reported dropout rates of more than 20%. Among them, all analysed interventions included pharmacotherapies, psychotherapies, and their combination; two studies [19, 22] had small sample sizes of less than 10 in each intervention group. All of the above five studies included patients older than 12 years. It was previously reported that compared with adults who received pharmacotherapy alone, adults receiving a combination of pharmacotherapy and psychotherapy had a lower dropout rate [36]. Concerning age, it was reported that individuals over the age of 16 had a higher dropout rate than younger patients [37]. However, subgroup analyses of different interventions and the meta-regression of mean age did not show significant results in the present study. Thus, we could not conclude how these potential moderators influence the dropout rate.
Since 2003, the FDA has been stipulating the risk of suicidal thoughts and attempts related to the use of antidepressants for treating children and adolescents with depression in a black box warning [38]. It was reported that patients under the age of 25 treated with antidepressants are more likely to develop suicidal thoughts than older adults [3]. In our analysis, no difference was observed in the rates of suicidal ideation or attempts related to the observed comparisons in children and adolescents with depressive disorder. This result was in line with our previous network meta-analysis [9]. Another study of ours revealed a significantly increased risk for suicide-related outcomes for children and adolescents administered venlafaxine [6]. However, due to the lack of eligible venlafaxine combined therapy data for assessing suicidality in the present study, we could not comprehensively assess the risk of suicide-related outcomes for these related interventions. Moreover, considerable method variability was observed during the data collection and reporting process, making it challenging to extract eligible and homogenous data for meta-analysis. Untreated depressive disorder in children and adolescents is closely related to a high risk of suicide [2], therefore, suicide-related outcomes should be monitored closely regardless of the treatment types.
Our study had several limitations that should be considered when explaining the results. First, relatively few studies have examined the efficacy and acceptability of combined therapy, limiting the comprehensive and systematic assessment of all kinds of psychotherapy and pharmacotherapy combined therapies. Our literature search was as comprehensive as possible, and we also made every effort to search all available unpublished data and contacted authors for additional related data. Although significant publication bias was not observed in the funnel plots and Egger’s test, we cannot eliminate the possibility that some unpublished studies remain missing or that published studies might overvalue the effect size of treatments. Second, substantial heterogeneity was observed in several of the examined comparisons. This may be because of the different characteristics among trials (e.g. various treatment forms, sample size, mean age, sex ratio, disease severity, comorbidity, suicidality or definition of remission across studies). Subgroup and meta-regression analyses of most parameters revealed some potential heterogeneity among the studies. However, the original data were insufficient to perform subgroup analyses or meta-regression for the comorbidity, suicidality and definition of remission. These may limit the generalizability of present results for the real-world clinical populations [39]. Third, children and adolescents with a wide range of 7-18 years old were included in the present study; however, meta-regression analysis of mean age failed to show significant results. Therefore, there was not enough evidence to conclude whether the benefit of each age group could be different. These data should be analysed and contextualised at the individual patient level, without access to individual patient-level data, we cannot be completely confident about the accuracy of information contained in published studies or clinical study reports [40]. Fourth, most included trials were rated as ‘some concerns’ or ‘high risk’ in terms of risk of bias assessment. Subgroup analysis showed that combined therapy was superior to other active treatment options in studies with a ‘high risk’ of bias in terms of remission. Some ‘high risk’ studies did not report the allocation sequence concealed information because it was difficult to conduct a double-blind design in psychotherapy trials, which essentially limited the interpretation of these results. Moreover, sensitivity analyses excluding trials with a ‘high risk’ of bias and with a non-blinded design was conducted, revealing that the results were not substantially different from those of the overall analysis. Fifth, we excluded studies on psychotic depression and treatment-resistant depression, which might overestimate the efficacy of interventions because the most challenging cases were not assessed. Moreover, we could not analyse other outcomes such as the efficacy of long-term follow-up, adverse events, and quality of life, mainly because they were rarely reported in the included studies. These variables are essential for the decision making of clinicians and patients. Sixth, several alternative psychotherapies have not been well studied for depression in young patients; for example, only one study [22] of interpersonal psychotherapy combined with pharmacotherapy met the inclusion criteria of this meta-analysis. Owing to the limited available studies and data of each outcome, many significant outcomes of the meta-analysis were driven by data from the study in March 2004 [26]. Although this study had a large sample size and was well designed, its generalisability was limited because many participants were recruited from advertisements and might not represent patients in clinical practice.
Conclusions
The findings from the present meta-analysis demonstrated that there was no evidence that all combined therapies are superior to other active treatment options in the acute treatment of depressive disorder in children and adolescents. Limited evidence showed that fluoxetine or non-SSRI pharmacotherapies combined with CBT might be superior to other active treatment options. Mixed characteristics (e.g. age) and small sample size of non-SSRI combined therapy may reduce the generalisability of the results. Interventions need to move beyond a ‘one size fits all’ approach to individualising treatment, and clinicians should consider the importance of each outcome, type of medication, and patient preferences. Combined therapies are understudied in this age group, and further studies focusing on the moderators of efficacy and alternative interventions are needed.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, PX and XZ, upon reasonable request.
Abbreviations
- CBT:
-
Cognitive-Behavioural Therapy
- IPT:
-
Interpersonal Psychotherapy
- RCTs:
-
Randomised Controlled Trials
- MDD:
-
Major Depressive Disorder
- ROB:
-
Risk Of Bias version
- CDRS-R:
-
Children’s Depression Rating Scale-Revised
- SMD:
-
Standardised Mean Differences
- ORs:
-
Odds Ratios
- CIs:
-
Confidence Intervals
- SDs:
-
Standard Deviations
- PEP:
-
Psychoeducational Psychotherapy
- SSRIs:
-
Selective Serotonin Reuptake Inhibitors
- IQR:
-
Interquartile Range
References
Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, et al. Prevalence and Treatment of Depression, Anxiety, and Conduct Problems in US Children. J Pediatr. 2019;206:256–67 e3.
Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. The Lancet. 2012;379(9820):1056–67.
Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, et al. Global burden of diseases, injuries, and risk factors for young people's health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2016;387(10036):2383–401.
Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, et al. Global burden of disease in young people aged 10-24 years: a systematic analysis. Lancet. 2011;377(9783):2093–102.
Zhou X, Hetrick SE, Cuijpers P, Qin B, Barth J, Whittington CJ, et al. Comparative efficacy and acceptability of psychotherapies for depression in children and adolescents: A systematic review and network meta-analysis. World Psychiatry. 2015;14(2):207–22.
Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881–90.
Cuijpers P, van Straten A, Hollon SD, Andersson G. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121(6):415–23.
Davey CG, Chanen AM, Hetrick SE, Cotton SM, Ratheesh A, Amminger GP, et al. The addition of fluoxetine to cognitive behavioural therapy for youth depression (YoDA-C): a randomised, double-blind, placebo-controlled, multicentre clinical trial. Lancet Psychiatry. 2019;6(9):735–44.
Zhou X, Teng T, Zhang Y, Del Giovane C, Furukawa TA, Weisz JR, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(7):581–601.
Weissman MM. Teenaged, depressed, and treatment resistant: what predicts self-harm? Am J Psychiatry. 2009;166(4):385–7.
Del Giovane CCS, Cipriani A. Combining Pharmacological and Nonpharmacological Interventions in Network Meta-analysis in Psychiatry. JAMA Psychiatry. 2019;0574.
Boutron IGL, Estellat C, Moher D, Hróbjartsson A, Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 2007;4(2):e61.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Cuijpers PLJ, Hofmann SG, Andersson G. Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin Psychol Rev. 2010;30(6):768–78.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535.
Bernstein GA, Borchardt CM, Perwien AR, Crosby RD, Kushner MG, Thuras PD, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3):276–83.
Clarke G, Debar L, Lynch F, Powell J, Gale J, O'Connor E, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. 2005;44(9):888–98.
Cornelius JR, Bukstein OG, Wood DS, Kirisci L, Douaihy A, Clark DB. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905–9.
Deas D, Randall CL, Roberts JS, Anton RF. A double-blind, placebo-controlled trial of sertraline in depressed adolescent alcoholics: a pilot study. Hum Psychopharmacol. 2000;15(6):461–9.
Fristad MA, Vesco AT, Young AS, Healy KZ, Nader ES, Gardner W, et al. Pilot Randomized Controlled Trial of Omega-3 and Individual-Family Psychoeducational Psychotherapy for Children and Adolescents With Depression. J Clin Child Adolesc Psychol. 2019;48(sup1):S105–S18.
Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al. A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial. Health Technol Assess. 2008;12(14):iii-iv, ix-60.
Gunlicks-Stoessel M, Mufson L, Bernstein G, Westervelt A, Reigstad K, Klimes-Dougan B, et al. Critical Decision Points for Augmenting Interpersonal Psychotherapy for Depressed Adolescents: A Pilot Sequential Multiple Assignment Randomized Trial. J Am Acad Child Adolesc Psychiatry. 2019;58(1):80–91.
Iftene F, Predescu E, Stefan S, David D. Rational-emotive and cognitive-behavior therapy (REBT/CBT) versus pharmacotherapy versus REBT/CBT plus pharmacotherapy in the treatment of major depressive disorder in youth; a randomized clinical trial. Psychiatry Res. 2015;225(3):687–94.
Kim SM, Han DH, Lee YS, Renshaw PF. Combined cognitive behavioral therapy and bupropion for the treatment of problematic on-line game play in adolescents with major depressive disorder. Computers in Human Behavior. 2012;28(5):1954–9.
Mandoki MW, Tapia MR, Tapia MA, Sumner GS, Parker JL. Venlafaxine in the treatment of children and adolescents with major depression. Psychopharmacol Bull. 1997;33(1):149–54.
March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292(7):807–20.
Riggs PD, Mikulich-Gilbertson SK, Davies RD, Lohman M, Klein C, Stover SK. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026–34.
Melvin GA, Tonge BJ, King NJ, Heyne D, Gordon MS, Klimkeit E. A Comparison of Cognitive-Behavioral Therapy, Sertraline, and Their Combination for Adolescent Depression. J Am Academy Child Adolescent Psychiatr. 2006;45(10):1151–61.
Dubicka B, Elvins R, Roberts C, Chick G, Wilkinson P, Goodyer IM. Combined treatment with cognitive-behavioural therapy in adolescent depression: meta-analysis. Br J Psychiatry. 2010;197(6):433–40.
Hetrick SE, Cox GR, Merry SN. Treatment-resistant depression in adolescents: is the addition of cognitive behavioral therapy of benefit? Psychol Res Behav Manag. 2011;4:97–112.
Cox GR, Callahan P, Churchill R, Hunot V, Merry SN, Parker AG, et al. Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents. Cochrane Database Syst Rev. 2014;(11):CD008324.
von Wolff A, Hölzel LP, Westphal A, Härter M, Kriston L. Combination of pharmacotherapy and psychotherapy in the treatment of chronic depression: a systematic review and meta-analysis. BMC Psychiatry. 2012;12:61.
Rohden AI, Benchaya MC, Camargo RS, Moreira TC, Barros HMT, Ferigolo M. Dropout Prevalence and Associated Factors in Randomized Clinical Trials of Adolescents Treated for Depression: Systematic Review and Meta-analysis. Clin Ther. 2017;39(5):971–92 e4.
DiFrancesco R, Rosenkranz SL, Craft J, Morse GD. Tutorial reduces protocol deviations in multicenter ACTG trials with pharmacology endpoints. HIV Clin Trials. 2006;7(4):203–9.
Nash JND. Antidepressants. Psychiatry. 2007;6:289–94.
Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet. 2013;381(9864):375–84.
Danielle T, Maud E, Jean-Yves FJPCH. Adherence to treatment in adolescents. 2008;1:19–24.
Administration UFaD. Suicidality in children and adolescents being treated with antidepressant medications. US Food and Drug Administration. Oct. 2004;15.
McCulloch A, Kroll L, Glass J, Dubicka B. A systematic review of the characteristics of adolescents with major depressive disorder in randomised controlled treatment trials. The. Eur J Psychiatry. 2021.
Cipriani A, Tomlinson A. Providing the most appropriate care to our individual patients. Evid Based Ment Health. 2019;22(1):1–2.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Key Research and Development Program of China (Grant No. 2017YFA0505700 to PX), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Grant No. 2019PT320002 to PX), the National Natural Science Foundation of China (Grant No. 81820108015 to PX), the National Natural Science Foundation of China (Grant No. 81873800 to XZ), the High-level Talents Special Support Plan of Chongqing (Grant No. T04040016 to XZ), the institutional funds from the Chongqing Science and Technology Commission (Grant No. cstc2020jcyj-jqX0024 to XZ).
Author information
Authors and Affiliations
Contributions
YX and XZ conceived the study. YX drafted the manuscript. YX, TT,XL and JL assisted in the design and revision. YX, LF, XL, YJ and KD participated in the search strategy development. YX and XZ designed the statistical analysis; PX and PC were the guarantors. YX and PC contributed equally to this study. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiang, Y., Cuijpers, P., Teng, T. et al. Comparative short-term efficacy and acceptability of a combination of pharmacotherapy and psychotherapy for depressive disorder in children and adolescents: a systematic review and meta-analysis. BMC Psychiatry 22, 139 (2022). https://doi.org/10.1186/s12888-022-03760-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-03760-2