Abstract
Background
Reducing the disease burden of major depressive disorder (MDD) is of major public health relevance. The prevention of depression is regarded as one possible approach to reach this goal. People with multiple risk factors for MDD such as chronic back pain and subthreshold depressive symptoms may benefit most from preventive measures. The Internet as intervention setting allows for scaling up preventive interventions on a public mental health level.
Methods
This study is a multicenter pragmatic randomized controlled trial (RCT) of parallel design aiming to investigate the (cost-) effectiveness of an Internet- and mobile-based intervention (IMI) for the prevention of depression in chronic back pain patients (PROD-BP) with subthreshold depressive symptoms. eSano BackCare-DP is a guided, chronic back pain-specific depression prevention intervention based on cognitive behavioral therapy (CBT) principles comprising six weekly plus three optional modules and two booster sessions after completion of the intervention. Trained psychologists provide guidance by sending feedback messages after each module. A total of 406 patients with chronic back pain and without a depressive disorder at baseline will be recruited following orthopedic rehabilitation care and allocated to either intervention or treatment-as-usual (TAU). Primary patient-relevant endpoint of the trial is the time to onset of MDD measured by the telephone-administered Structured Clinical Interview for DSM (SCID) at baseline and 1-year post-randomization. Key secondary outcomes are health-related quality of life, depression severity, pain intensity, pain-related disability, ability to work, intervention satisfaction and adherence as well as side effects of the intervention. Online assessments take place at baseline and 9 weeks as well as 6 and 12 months post-randomization. Cox regression survival analysis will be conducted to estimate hazard ratio at 12-month follow-up. Moreover, an economic analysis will be conducted from a societal and public health perspective.
Discussion
This is the first study examining an IMI for depression prevention in a sample of chronic pain patients. If this implementation of a depression prevention IMI into orthopedic aftercare proves effective, the intervention could be integrated into routine care with minimal costs and extended for use with other chronic diseases. Results will have implications for researchers, health care providers and public health policy makers.
Trial registration
The trial is registered at the WHO International Clinical Trials Registry Platform via the German Clinical Studies Trial Register (DRKS): DRKS00007960. Registered 12 August 2015.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is related to high disease burden for both people affected and society [1]. In a recent literature review investigating global variation in the prevalence and incidence of MDD, a global point prevalence of 4.7%, a lifetime prevalence between 10 and 15% and a global incidence of 3.0% have been reported [2, 3]. It is estimated that existing psychological and pharmacological treatments have the potential to avert only 36% of the burden of MDD, and only when assuming perfectly efficient provision of existing treatments in terms of coverage, patient compliance, an d clinician competence [4, 5]. Thus, we are either in need of more powerful interventions for treating depression or we should aim at diminishing the likelihood of developing depression in the first place, highlighting prevention of depression as a promising approach. Recent research suggests that psychological preventive interventions such as cognitive behavioral therapy (CBT) or interpersonal psychotherapy have the potential to prevent a clinically significant number of new depression cases [6]. A meta-analysis of 32 randomized controlled trials (RCTs) reported a reduced incidence rate for MDD of 21% (incidence rate ratio = 0.79, 95% confidence interval: 0.69–0.91) when comparing psychotherapy-based preventive interventions with usual care or wait list conditions.
While the effectiveness of preventive interventions seems sufficiently documented, it remains challenging to identify target populations that benefit most from preventive measures [7]. According to Cuijpers and colleagues [8] two factors need be taken into account: the “impact” and the “effort” of preventive measures. An adequate “impact” means that prevention must lead to a substantial reduction of total disease burden. Therefore, a substantial proportion of new cases must be prevented if assembled risk indicators are fully blocked. A reasonable level of “effort” is primarily defined as a low number needed to be treated (NNT) to prevent one new case of MDD. Additionally, persons at risk should be easily identifiable and interventions should not only be cost-effective but also low priced to allow for their implementation at a population level.
From this viewpoint, chronically medically ill patients appear to be a meaningful target population for the prevention of MDD, given the substantially increased prevalence for MDD in this population compared to the general population [9, 10]. In addition, comorbid MDD in medically ill patients is associated with numerous negative implications such as problems in the physician-patient relationship, increased risky health-related behaviors, higher medical symptom burden, medical complications, lowered quality of life and increased mortality [11, 12]. Within the group of medically ill persons, back pain is one of the most common conditions [10, 13] and is associated with a two to three-fold increased risk for MDD [14]. In addition, depression is one of the core predictors of persistent pain symptoms, increased pain related disability, and poor treatment outcomes, and is associated with increased morbidity and health care costs as well as diminished quality of life [11, 12, 15–17].
The benefits of prevention can be multiplied by focusing on patients who already show some depressive symptoms due to several reasons. First, subthreshold depressive symptoms are an additional risk indicator for MDD [18, 19]. Multiple risk groups have increased specificity for prevention measures which leads to a reduction of NNT (“effort”) [20] and leading to greater cost-effectiveness of preventive interventions. Second, by lowering the NNT, the number of persons who are not in need of a preventive intervention, but receive it, will be reduced. Third, subthreshold depression itself is a considerable disease burden for people affected and for society [21, 22]. A successful preventive intervention will not only reduce the risk of developing MDD but also improve depression symptom severity at all levels of depression, as shown by a recent meta-analysis (pooled effect size g = 0.35, 95%-CI: 0.23–0.47; [23]). Fourth, uptake rates of a depression prevention intervention may be higher in a target population of patients with depressive symptoms, as treatment utilization was found to be associated with severity of baseline depression [24].
The internet is an appropriate prevention medium for scaling up preventive interventions as units of delivery are reasonably priced and can be easily administered [7, 25]. It has several additional advantages as discussed elsewhere [26–28].
In prior studies, Internet- and mobile-based interventions (IMI) have shown to be effective in the treatment of MDD [29, 30] as well as in the treatment of subthreshold depression, indicating their potential to be utilized for preventive interventions [25, 31–34]. Human support (guidance) has repeatedly been shown to have a positive effect on effectiveness of and adherence to IMIs [35, 36].
The proposed study aims to investigate the effectiveness and cost-effectiveness of an IMI (eSano BackCare-DP) to prevent the onset of depression for chronic back pain patients (PROD-BP) with subthreshold depressive symptoms. The study will be embedded into routine orthopedic care in order to examine the intervention’s effectiveness in an unselected sample of all eligible chronic back pain patients (i.e. the implementation will not be limited to a self-selected population of people who are already attracted to depression prevention interventions and the Internet). It is expected that
-
1)
eSano BackCare-DP is effective in preventing the onset of MDD compared to treatment as usual (TAU) over a 12-month follow-up period,
-
2)
eSano BackCare-DP is cost-effective compared to TAU.
-
3)
Compared to TAU, eSano BackCare-DP is superior in terms of (a) depression response, (b) work capacity, (c) quality of life, (d) pain related disability and (e) pain intensity.
Furthermore, the distributions of principal confounders in each group will be explored.
Methods
Study design
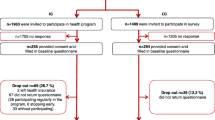
This project is a multicenter randomized controlled clinical trial (RCT) of parallel design comparing the effectiveness and cost-effectiveness of a guided depression prevention IMI with treatment as usual (TAU) (Fig. 1). All participants will receive TAU. Participants of the intervention group will additionally receive the IMI eSano BackCare-DP.
This clinical trial will be conducted and reported in accordance with the CONSORT-supplement for pragmatic RCTs [37] and the guidelines for executing and reporting internet-based intervention research [38]. In order to guarantee data quality and safety, the Clinical Trials Unit Freiburg will perform monitoring visits to the recruitment centres before, during and after completion of the study. Moreover, an independent Data Safety and Monitoring Board (DSMB) has been established. It consists of two experienced scientists and psychotherapists (MHä, MHa) and a statistician (LK) with long-standing experience in clinical trials.
Inclusion and exclusion criteria
Patients who provide written consent will be included in the study if they meet the following criteria: a) age 18 and above, b) presence of chronic back pain assessed by physician diagnosis and participants report on pain chronicity (>6 months), c) sufficient knowledge of German language, d) internet access, e) persistent subthreshold depressive symptomatology (Patient Health Questionnaire (PHQ-9) ≥ 5 in two consecutive screenings within 2–3 weeks. If only one PHQ is over cut-off, a third PHQ will be administered 2 months later.
Patients will be excluded who meet DSM criteria for a) current depressive episode or depressive episode within the last 6 months (following Kupfer [39]), b) current dysthymia, c) current or lifetime bipolar disorder. Additionally patients will be excluded in case of d) participation in ongoing psychotherapy, completed psychotherapy in the past 6 months, or being on a waiting list for psychotherapy (beginning within 3 months), e) currently suicidal or reporting suicidal attempts within the past 5 years. Patients with a diagnosis of any affective disorder will receive information on possible mental health care options, including the offer to take part in a parallel clinical trial for the treatment of depression in patients with chronic back pain and clinical depression [40]. In cases of severe depression, a trained psychotherapist from the study team [HB, SaS, LS] will contact the participant to initiate further actions.
Setting/Recruitment
Recruitment has started in October 2015. Recruitment will continue until the target sample size has been reached. Participants are recruited in the aftermaths of their orthopedic rehabilitation care. In order to increase the representativeness of our sample, we established two comparable recruitment strategies. First, back pain patients from eight orthopedic rehabilitation units are screened at admission and discharge. Clinical staff informs and recommends participation to patients screened positive (personal recruitment). In addition, an information flyer and a patient information form with a detailed description of the study process and information on the intervention are provided. Patients providing their informed consent are contacted by the study team in order to clarify further eligibility criteria by means of an online- and telephone assessment including a telephone administered clinical interview (SCID; [41–43]).
Second, back pain patients from orthopedic rehabilitation units across Germany receive a letter with the same information flyer and study process information (recruitment by letter). Interested back pain patients fill out an online PHQ-9 screening. Positive screened patients (PHQ-9 ≥ 5) providing their informed consent conduct the aforementioned online- and telephone assessment including a second PHQ-9 screening to ascertain persistent depression in line with the first recruitment strategy (second PHQ-9 ≥ 5). A third PHQ-9 screening takes place 2 months afterwards in case of only one PHQ-9 being screening positive. Eligible back pain patients from both recruitment strategies receive an email providing further information and a link referring to the intervention website. All participants are free to seek any additional help during the trial. Trial participants receive 15€ for the completed follow-up telephone assessment.
Randomization
Participants eligible for inclusion will be randomly allocated to one of two groups (intervention or TAU). Randomization and allocation will be prepared in advance by a researcher (SaS) who is responsible for administration of the trial and participants. This researcher will remain blinded to all processes within the intervention. An automated, web-based randomization program (www.sealedenvelope.com) will be used, which features permuted block randomization, variable block sizes of 4,6,8 (randomly arranged), and an allocation ration of 1:1.
Intervention
Intervention condition
The intervention (eSano BackCare-DP) consists of six weekly sessions plus three optional modules and two booster sessions of approximately 45 to 60 min each (see Figs. 2 & 3). Participants can decide when to complete the booster sessions at the end of the last session. They can choose two dates within a timeframe of 3 months following the intervention, and will be reminded to log-in again. Sessions can be repeated as often as desired. All modules consist of information about depression and (chronic) back pain, provided by text, audio, and video, as well as assignments, metaphors and exercises. The content of the intervention is based on cognitive behavioral therapy (CBT) for depression, including elements of psychoeducation, social skills, problem solving, behavioral activation, improving self-care, relaxation and motivation for physical exercises. The intervention is based on prior interventions that have been evaluated in a number of RCTs in different samples [31, 34, 44–46]. We adapted and substantially extended the predecessors to the context of depression prevention and chronic back pain. In order to address chronic back pain patients specifically, psychological pain intervention elements are integrated in every module of the intervention. Moreover, three optional modules are offered, focusing on problems with sleep, partnership/sexuality and returning to the workplace. Emphasis is laid on homework assignments, which are intended to provide practice of learned skills. To enhance patient adherence, interactive elements (quizzes, conditional contents) are implemented. Participants will be asked about adverse events at the beginning of every session with the advice to check whether it is the right time to go on with the session. The intervention platform is provided by Minddistrict (www.minddistrict.com), a company specialized in the provision of web-based health interventions. Access to the platform proceeds through a unique username-password combination and will be available on a 24/7 basis. All transferred data will be secured based on ISO27001 and guidelines NEN7510.
Text message coach
At the beginning of the training, participants are asked if they want to receive daily reinforcing text messages during the 6-week training period. Text message prompts have been shown to be beneficial in internet interventions with positive effects on efficacy and adherence [47–49]. The messages are sent automatically and coordinated with intervention content in order to integrate the learned techniques into daily life of the participants. Message content aims at reminding patients to complete homework assignments, repeating training content, and reinforcing motivation of participants.
Guidance
Trained and supervised (by HB, JL, SP) psychologists (eCoaches) will guide participants during the training by providing a semi-standardized feedback within 2 working days after each completed session, using an eCoach manual. The eCoach manual is standardized to ensure protocol adherence by the eCoaches. All communication between eCoaches and participants runs via the intervention platform. Guidance time spent per participant per intervention will be measured to provide data for the economic analyses of the project. The feedback content will match the participants’ assignments and provide support for treatment adherence. Feedback also includes positive reinforcement to encourage participants to continue with the training. If any further questions arise, participants and eCoaches can contact each other at any time via the intervention platform. eCoaches will also send reminders to participants, who do not complete intervention modules on time.
Control condition
Participants of the control condition will have unrestricted access to TAU. Although national treatment guidelines for low back pain and depression exist [50], TAU following orthopedic rehabilitation care may vary. There is no minimum treatment defined and TAU will not follow a standardized protocol; however, all received types of medical/psychological help during the last 3 months will be monitored with the Trimbos/iMTA questionnaire, to account for all costs associated with psychiatric illness (TIC-P) [51]. Using these data, an accurate description of TAU can be provided.
Sample size/power calculation
The aim of the study is to compare the effectiveness of the intervention against TAU using a cox regression survival analysis with a significance level of 5%. Based on previous studies of depression incidence in chronic back pain patients and subclinical depression, we expect a mean incidence of MDD of 20% in the control group within the 12-months follow-up period [52–55]. Based on a prior conducted trial, we assume an absolute risk reduction of 9% with a respective hazard ratio of 0.522 between treatment and control group at 12 months after randomization [34]. A total of 64 events (event = onset of depression) need to be observed to detect a 47.8% decrease in hazard (hratio = 0.522) of the treatment group relative to the hazard of the control group based on a power of 80%. Accordingly, 406 participants (203 per group) need to be randomized (calculated using Stata/SE 13.1). The inclusion of relevant baseline predictors of depression onset will further increase power (e.g. baseline depression severity). Recruitment will be continued to allow for an expected attrition rate of 20%.
Assessments
Assessments will be conducted at pre-treatment (T0) and at 9 weeks, 6- and 12-months follow-up (T1, T2, T3; see Table 1). All self-report assessments, potentially effect-modifying covariates and demographic variables will be provided using a web-based interface integrated into the intervention platform. Section A (Affective Syndromes) of the SCID [43], the Hamilton Rating Scale for Depression (HAM-D-17 [56]) and the Quick Inventory of Depressive Symptomatology (QIDS-C16; [57]) will be performed via telephone interviews at T0 and T3. Moreover, we translated the SCID-V-RV into German to be able to comment on DSM-IV and -V [58] diagnoses.
Videos promoting the importance of collecting data will be implemented into online assessments to enhance compliance with completing measures. In psychological intervention trials, blinding of study participants and eCoaches is not possible. However, all members of the research team conducting telephone administered outcomes will remain blinded. Therefore, both participants and interviewers will be reminded of the reason and importance of blinding at the beginning of each interview. Moreover, performance bias will be minimized, as the web-based intervention is separated from other health care services.
Procedure on suicidal ideation
The telephone interviews (SCID, HAM-D, QIDS) and questionnaires (PHQ-9) include a suicide screening to identify participants who are currently suffering from suicidal ideation. We will follow a suicide protocol adapted from prior trials [31, 44] if participants score on any suicidality item. Participants who report low suicidal ideation (HAM-D, QIDS or PHQ-9 item score = 1) will receive an email with detailed information on available health services and the advice to seek professional help if symptoms increase. If participants express moderate to high suicidal ideation during the assessment or express any suicidal thoughts or intentions to their eCoach, a trained psychotherapist from the study team [EM, LS, HB, SaS] will contact the participant and initiate further actions.
Outcome measurements
Primary outcome: Time to onset of MDD
To assess the time to onset of MDD within the 12-month follow-up period, the depression related modules of SCID will be part of the telephone assessment [41, 59]. The SCID is a comprehensive, structured interview designed to be used by trained interviewers for the determination of mental disorder diagnoses according to the definitions and criteria of DSM. It enables a reliable, valid and efficient assessment of depressive disorders [59]. Inter-rater reliability of the SCID was reported to be moderate to high and high for inter-rater reliability comparing telephone and face-to-face interviews [60, 61].
Interviewers are trained and weekly supervised by clinical psychologists (LS, EM) and are blinded to randomization condition. After the training period, supervisors and the interviewers assess participants together, with comparison of results as follows: The Inter-Rater Reliability (IRR) for the SCID, measured by Cohen’s kappa and the Intra-Class Correlation for the HAM-D and QIDS. An almost perfect Cohen’s kappa ≥ .81 [62] and an excellent ICC coefficient ≥ .75 [63] are considered as sufficient. Moreover, the interviewers are compared to each other on a random basis to assess the IRR.
Time to onset of MDD will be assessed using life charts. Therefore, life events will be recalled using a calendar method to determine presence of symptoms at each month within the follow-up period. Supervisors are blinded to participants’ group allocation.
Secondary outcomes
Depressive symptoms
Depressive symptoms will be assessed by the self-administered Patient Health Questionnaire (PHQ-9) [64], a telephone-based clinician rating of the Hamilton Rating Scale for Depression (HAM-D-17), and the Quick Inventory of Depressive Symptomatology (QIDS) [56, 57]. The PHQ-9 is a well validated and widely used depression screening instrument [64] and has also been evaluated to be delivered as online-version [65]. The 17-item HAM-D is the most widely used clinician-rated measure of depression severity and as such viewed as the gold standard for the assessment of depression severity. The 16-Item QIDS will be used to further validate the depressive symptom outcome measures. It covers all criterion symptom domains of the DSM for diagnosing a MDD [57]. HAM-D and QIDS are administered to determine depression response [66].
Quality of life
To assess health-related quality of life, the Assessment of Quality of Life (AQoL-6D) will be used, which includes 20 items assessing the following dimensions: independent living, mental health, coping, relationships, pain, and senses [67]. Besides measuring health-related quality of life, the AQoL-6D is suitable for economic evaluations of health programs and has good psychometric properties [67]. It has also shown to be reliable, with a Cronbach’s alpha of .89 [68]. Because this is a relatively new instrument, we additionally will use the EuroQol (EQ-5D-5 L), the most widely used quality of life assessment, as a basis for cost-utility analyses [69]. The EQ-5D measures five health domains of importance to quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The 5-level version includes five levels of response, which has been found to be more discriminative and to reduce ceiling effects compared to the 3-level version [70, 71].
Pain intensity and pain associated disability
Pain intensity and pain-related disability will be measured following the IMMPACT recommendations for core outcome measures for chronic pain clinical trials [72, 73]. Pain intensity will be measured by an 11-point numerical rating scale (0-10) of pain intensity as well as categorical classification of pain intensity (none, mild, moderate, severe). The Oswestry Disability Index (ODI) will be used to assess pain related disability. The ODI is a reliable and valid self-assessment questionnaire including 10 items [74].
Pain self-efficacy
Self-efficacy with regard to pain management will be assessed by using the Pain Self-Efficacy Questionnaire (PSEQ), which is a validated and reliable (internal consistency: α = 0.93) 10 item instrument that assesses self-efficacy expectations related to pain [75].
Work capacity
Work capacity will be assessed using the German version of the Subjective Prognostic Employment Scale (SPE), a validated 3-item self-report questionnaire with high internal consistency (Guttman scaling: rep = .99) [76].
Intervention satisfaction and adherence
Patient satisfaction with the intervention will be measured by using an adaptation of the Client Satisfaction Questionnaire (CSQ-8, German: ZUF-8), optimized for the assessment of client satisfaction with online interventions (CSWIQ-8) [77]. The CSQ-8 is a validated 8 item instrument with high internal consistency (α = 0.93) [78]. The adapted version, validated for the assessment of client satisfaction in web-based interventions, has been shown to have high internal consistency in a range of studies (α = 0.92-0.94) and is associated with treatment adherence and outcome [79–81]. The attrition rate (i.e. percentage of participants who no longer use the intervention assessed by their log in data) will give an estimate of the participants’ intervention adherence.
Side effects of psychotherapy
The German version of the Side Effects of Psychotherapy Inventory (INEP) [82] will be used to measure side-effects of psychotherapy. The INEP consists of 15 items assessing a range of common changes participants may have experienced due to the effects of the preventive intervention in their social and work environments.
Costs
To measure costs, the Dutch cost questionnaire: “Trimbos Institute and Institute of Medical Technology Questionnaire for Costs Associated with Psychiatric Illness” (TiC-P) [51], adapted for the German health care system, will be used [83]. The TiC-P is a widely used self-report questionnaire to measure health care consumption and productivity loss [84]. We further adapted the questionnaire for the population of chronic back pain patients. Participants will register all direct health service uptakes during the last 3 months, e.g. the number of general practice visits, sessions with psychiatrists, and hospital days. In addition, productivity-related costs will also be assessed. This includes the number of ‘work loss’ days (absenteeism), the number of ‘work cut-back’ days (presenteeism), and costs associated with for domestic tasks. Estimated development costs as well as opportunity costs will be included in the economic evaluation.
Covariates
As potentially effect-modifying covariates, demographic variables (gender, age, education), social support and medical variables (prior pain and depression treatments) will be assessed via self-report at baseline. Internet competencies will be assessed by the Internet Affinity Scale [85]. The translated version by Haase and colleagues [86] will be used. Back pain type, severity and chronicity will be extracted from medical records.
Statistical analysis
Clinical analyses
All analyzes will be performed according to the intention-to-treat (ITT) principle. Kaplan-Meier curves and Cox proportional hazard regression analysis will be used to determine differences in time to onset of MDD in weeks between both study conditions over a follow-up period of 12 months. The dependent variable will be time to onset of MDD and treatment condition will be the independent variable. The proportional-hazards assumption will be tested based on the scaled Schoenfeld residuals test. Survival analysis assumes that censoring (i.e., a participant is lost-to-follow-up or completes the follow-up period without experiencing a major depressive episode) is non-informative. Non-informative implies that the reasons why participants drop out of the trial are unrelated to the study condition (i.e., participants in one study arm should not be routinely censored). We will apply the following methods to deal with informative censoring, if necessary: (a) imputation techniques for missing data, (b) sensitivity analyses to illustrate best and worst case scenarios to test the robustness of the base case findings and (c) the use of the drop-out event as a study end point [87]. In addition, the number needed-to-treat (NNT) to prevent one additional event in the intervention group versus the control group will be calculated [88]. Covariates will first be checked whether they are associated with the primary outcome, if not left out of the final analysis.
Secondary outcomes will be analyzed using hierarchical linear modeling. To examine potentially moderating covariates, correlation of covariates and outcome parameter are analyzed using multiple regression models. In addition, per protocol analysis will be performed to investigate the influence of drop-outs on study results. Missing data will be imputed using multiple imputation. Potential confounding factors between source population and study population will be assessed, which enables us to evaluate the external validity of the sample. Finally, characteristics of dropped out participants at follow-up will be inspected and resulting socio-demographic differences between intervention and control group will be described. A significance level of p ≤ .05 will be set for all analyses.
Economic evaluation
Baseline utilities and costs will be compared between both groups and if necessary, statistical techniques will be used to adjust for baseline differences [89]. In the cost-effectiveness analysis, the incremental cost-effectiveness ratio (ICER) will be presented as costs per depression-free year (DFY) gained. DFYs will be based on the number of depression-free weeks up to onset of a major depressive episode within the 12-month follow-up period. The ICER in the cost-utility analysis will be stated as costs per quality-adjusted life year (QALY) gained. Non-parametric bootstrapping (2500 times) will be applied to estimate the robustness of the ICERs and to quantify the uncertainty around the ratios. The bootstrapped replicates of the ICERs will be graphically represented in a cost-effectiveness plane, with effects along the horizontal axis and costs along the vertical axis.
In addition, a cost-effectiveness acceptability curve will be graphed to assess the probability that the intervention is more cost-effective relative to treatment as usual at varying willingness-to-pay (WTP) ceilings.
Discussion
This study will be the first to investigate the effectiveness and cost-effectiveness of a psychological Internet- and mobile-based intervention for the prevention of depression in a chronic pain population. Due to its recruitment strategy from routine medical health care, the entire potential target group can be reached within a naturalistic setting. Results will have implications for researchers, health care providers and public health policy makers.
Conducting IMI-trials commonly involves possible limitations, which we try to overcome using the following measures. First, web-based interventions can have moderate to high drop-out rates [90–93], and drop-out rates can be expected to be even higher in preventive interventions due to the lower symptom burden of participants. We will approach this problem in different ways: a) by focusing on patients with current depressive symptoms b) by providing guidance via eCoaches, which has been shown to have an adherence-facilitating effect [35, 36], and c) by explicitly facilitating at risk participants’ motivation to use the intervention after discharge from orthopedic rehabilitation care. In a prior IMI with diabetes patients, participants completed an average of 78.3% of all sessions [44]. In a sample of subthreshold depressed patients, participants completed an average of 82.2% [31]. These results correspond to findings from a recent meta-analysis on adherence to internet-based CBT (ICBT) [94]. Van Ballegooijen and colleagues concluded that adherence to guided ICBT could be equal to adherence to face-to-face CBT. Participants do not necessarily have to complete all sessions to benefit from IMIs. They may also stop the treatment because they have recovered [95] or experienced improvement in symptoms, thereby reducing the likelihood of developing depression. These cases would represent a prevention success rather than a treatment drop-out [90].
A second limitation of most Internet- and mobile-based trials to date (including those mentioned above) are their highly selective online recruitment strategies. This may explain the promising results concerning drop-out rates, as participants already connected to the Internet comprised the intervention groups. As a down-side, however, those recruitment strategies lead to a lack of external validity [27, 96, 97]. In our study, we address this problem through the integration of the intervention into routine care. Thereby, the entire potential target group will be offered the opportunity to take part in the preventive intervention. The two different recruitment strategies will allow for analyses on different dissemination and implementation strategies of IMIs into routine healthcare. Thus, we can estimate what kind of patients, and to which extent, make use of the offer to take part in a preventive IMI within the whole group of chronic back pain patients.
Third, IMIs can have negative side effects [98–100]. For this reason, we followed the key recommendations of Rozental and colleagues [98]. We increased the flexibility of the treatment schedule by giving participants the possibility of delay at the beginning of each session, and increased flexibility of therapist contact for patients. Additionally, we prolonged treatment duration by adding two booster sessions after the main treatment modules. Furthermore, negative side effects of treatment will be assessed on a regular basis and reasons for drop-out from intervention will be assessed.
The specific strengths of this study are the following: a) Prevention studies are regularly methodologically limited because they lack a diagnosis at baseline and/or follow-up [6]. By carrying out the SCID prior to study start and at 12-months follow-up a high content validity can be ensured. b) With a target sample of 406 participants, the study will be optimally powered, overcoming the small scale trial limitations of most prior prevention studies [6, 20]. Following the ITT principle contributes to reducing overestimation of clinical effectiveness. c) The intervention is specifically tailored to the special needs of the target group of chronic back pain patients. This has been discussed as having an uptake and adherence facilitating effect [27]. We aim to further facilitate adherence through the integration of the intervention into patients’ routine healthcare, which enables clinicians to inform participants about the characteristics and effectiveness of IMIs. This may have a positive impact on their acceptance [101]. d) Using the internet as the medium for prevention might allow for scaling up of preventive interventions on a public mental health level. e) The direct implementation of the intervention into the health-care system increases external validity in contrast to prior RCTs [31, 102].
High prevalence rates underscore that the integration of depression prevention into curative care systems for the medically ill is one of the major emerging global health challenges. If this study - the first of its kind – shows to be effective, the intervention could be implemented into general (chronic) back pain and mental health treatment protocols as well as adapted to other chronically ill patient groups, thus helping to reduce the disease burden of depression for both affected persons and society. Thus, the results of this study will be of major public health relevance.
Abbreviations
- CBT:
-
Cognitive behavioral therapy
- CONSORT:
-
Consolidated Standards of Reporting Trials
- DFG:
-
Deutsche Forschungsgemeinschaft (German Research Foundation)
- DFY:
-
Depression-free years
- DRKS:
-
Deutsches Register Klinischer Studien (German clinical studies trial register)
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- DSMB:
-
Data safety and monitoring board
- ICBT:
-
Internet-based cognitive behavioral therapy
- ICC:
-
Intra-class correlation
- ICD:
-
International classification of diseases
- ICER:
-
Incremental cost-effectiveness ratio
- IMI:
-
Internet- and mobile-based intervention
- IMMPACT:
-
Initiative on methods, measurement and pain assessment in clinical trials
- IRR:
-
Inter-rater reliability
- ISO:
-
International organization for standardization
- ITT:
-
Intention-to-treat
- MDD:
-
Major depressive disorder
- NEN:
-
Nederlandse Norm (Dutch Norm)
- NNT:
-
Number needed to be treated
- PROD-BP:
-
Prevention of depression in back pain patients
- QUALY:
-
Quality-adjusted life year
- RCT:
-
randomized controlled trial
- SCID:
-
Structured Clinical Interview for DSM
- TAU:
-
Treatment as usual
- WHO:
-
World health organization
- WTP:
-
Willingness-to-pay
References
World Health Organization. The global burden of disease: 2004 update [Internet]. Geneva: WHO Press; 2008. [cited 2014 Sep 5]. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43:471–81.
Lépine J-P, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7:3–7.
Chisholm D, Sanderson K, Ayuso-Mateos JL, Saxena S. Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. Br J Psychiatry. 2004;184:393–403.
Andrews G, Sanderson K, Corry J, Lapsley HM. Using epidemiological data to model efficiency in reducing the burden of depression. J Ment Health Policy Econ. 2000;3:175–86.
van Zoonen K, Buntrock C, Ebert DD, Smit F, Reynolds CF, Beekman ATF, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol. 2014;43:318–29.
Munoz RF, Cuijpers P, Smit F, Barrera AZ, Leykin Y. Prevention of major depression. Annu Rev Clin Psychol. 2010;6:181–212.
Cuijpers P. Prevention: an achievable goal in personalized medicine. Dialogues Clin Neurosci. 2009;11:447–54.
Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–26.
Härter M, Baumeister H, Reuter K, Jacobi F, Hofler M, Bengel J, et al. Increased 12-month prevalence rates of mental disorders in patients with chronic somatic diseases. Psychother Psychosom. 2007;76:354–60.
Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23.
Baumeister H, Hutter N, Bengel J, Härter M. Quality of life in medically ill persons with comorbid mental disorders: a systematic review and meta-analysis. Psychother Psychosom. 2011;80:275–86.
Patten SB. Long-term medical conditions and major depression in a Canadian population study at waves 1 and 2. J Affect Disord. 2001;63:35–41.
Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, et al. Mental disorders among persons with chronic back or neck pain: results from the world mental health surveys. Pain. 2007;129:332–42.
Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity. A literature review. Arch Intern Med. 2003;163:2433–45.
Miles CL, Pincus T, Carnes D, Homer KE, Taylor SJC, Bremner SA, et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of sub-group analysis within RCTs. Eur J Pain. 2011;15:775.e1–e11.
Baumeister H, Knecht A, Hutter N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders - a systematic review. J Psychosom Res. 2012;73:79–85.
Bertha EA, Balázs J. Subthreshold depression in adolescence: a systematic review. Eur Child Adolesc Psychiatry. 2013;22:589–603.
Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatr Scand. 2004;109:325–31.
Cuijpers P. Examining the effects of prevention programs on the incidence of new cases of mental disorders: the lack of statistical power. Am J Psychiatry. 2003;160:1385–91.
Backenstrass M, Frank A, Joest K, Hingmann S, Mundt C, Kronmuller KT. A comparative study of nonspecific depressive symptoms and minor depression regarding functional impairment and associated characteristics in primary care. Compr Psychiatry. 2006;47:35–41.
Cuijpers P, Smit F, Oostenbrink J, De Graaf R, Ten Have M, Beekman A. Economic costs of minor depression: a population-based study. Acta Psychiatr Scand. 2007;115:229–36.
Cuijpers P, Koole SL, van Dijke A, Roca M, Li J, Reynolds CF. Psychotherapy for subclinical depression: meta-analysis. Br J psychiatry J Ment Sci. 2014;205:268–74.
Alonzo DM, Harkavy-Friedman JM, Stanley B, Burke A, Mann JJ, Oquendo MA. Predictors of treatment utilization in major depression. Arch Suicide Res. 2011;15:160–71.
Sander L, Rausch L, Baumeister H. Effectiveness of internet-based interventions for the prevention of mental disorders: a systematic review. JMIR Ment. Heal. [Internet]. JMIR Mental Health; 2016 [cited 2016 Sep 7];3:e38. Available from: https://mental.jmir.org/2016/3/e38/
Christensen H, Griffiths KM. The prevention of depression using the Internet. Med J Aust. 2002;177(Suppl):S122–5. Australasian Medical Publishing Co.
Cuijpers P, Van Straten A, Warmerdam L, Van Rooy MJ. Recruiting participants for interventions to prevent the onset of depressive disorders: possible ways to increase participation rates. BMC Health Serv Res. 2010;10:181–7.
Calear AL, Christensen H. Review of internet-based prevention and treatment programs for anxiety and depression in children and adolescents. Med J Aust. 2010;192:12–4.
Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:329–42. Elsevier Ltd.
Ebert DD, Zarski A-C, Christensen H, Stikkelbroek Y, Cuijpers P, Berking M, et al. Internet and computer-based cognitive behavioral therapy for anxiety and depression in youth: a meta-analysis of randomized controlled outcome trials. PLoS One. 2015;10:e0119895.
Buntrock C, Ebert D, Lehr D, Riper H, Smit F, Cuijpers P, et al. Effectiveness of a web-based cognitive behavioural intervention for subthreshold depression: pragmatic randomised controlled trial. Psychother Psychosom. 2015;84:348–58.
Spek V, Cuijpers P, Nyklícek I, Smits N, Riper H, Keyzer J, et al. One-year follow-up results of a randomized controlled clinical trial on internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years. Psychol Med. 2008;38:635–9.
Christensen H, Batterham PJ, Gosling JA, Ritterband LM, Griffiths KM, Thorndike FP, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;366:333–41.
Buntrock C, Ebert DD, Lehr D, Smit F, Riper H, Berking M, et al. Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA. 2016;315:1854–63.
Baumeister H, Reichler L, Munzinger M, Lin J. The impact of guidance on Internet-based mental health interventions — A systematic review. Internet Interv. 2014;1:205–15.
Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13:e30.
Moher D, Hopewell S, Schulz KF, Montori V, Peter C, Gotzsche PC, et al. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–e37.
Proudfoot J, Klein B, Barak A, Carlbring P, Cuijpers P, Lange A, et al. Establishing guidelines for executing and reporting internet intervention research. Cogn Behav Ther. 2011;40:82–97.
Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry. 1991;52(Suppl):28–34.
Lin J, Sander L, Paganini S, Schlicker S, Mittag O, Berking M, et al. Effectiveness and cost-effectiveness of a guided internet- and mobile-based depression intervention for in individuals with chronic back pain: protocol of a multi-centre randomised controlled trial. in progress.
Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. Am J Psychiatry. 1997;154:1593–8.
Allen K, Cull A, Sharpe M. Diagnosing major depression in medical outpatients acceptability of telephone interviews. J. Psychosom. Res. 2003;55:385–7.
First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). Washington: American Psychiatric Press; 2002.
Nobis S, Lehr D, Ebert DD, Baumeister H, Snoek F, Riper H, et al. Efficacy of a web-based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: a randomized controlled trial. Diabetes Care. 2015;38:776–83.
Ebert DD, Nobis S, Lehr D, Baumeister H, Riper HM, Auerbach RP, et al. The 6-month effectiveness of Internet-based guided self-help for depression in adults with Type 1 and 2 diabetes mellitus. Diabet. Med. 2016;34(1): 99-107.
Lin J, Lüking M, Ebert DD, Buhrman M, Andersson G, Baumeister H. Effectiveness and cost-effectiveness of a guided and unguided internet-based Acceptance and Commitment Therapy for chronic pain: study protocol for a three-armed randomised controlled trial. Internet Interv. 2014;2:7–16. Elsevier B.V.
Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. 2009;11:e16.
Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12:e4.
Ritterband LM, Gonder-Frederick LA, Cox DJ, Clifton AD, West RW, Borowitz SM. Internet interventions: in review, in use, and into the future. Prof Psychol Res Pract. 2003;34:527–34.
(BÄK) GMA, Physicians NA of SHI, (KBV), (AWMF) A of SMS. National Disease Management Guideline Low back pain - Short Version, 1st edition. Version 5 [Internet]. 2011 [cited 2016 Jan 5]. Available from: http://www.kreuzschmerz.versorgungsleitlinien.de
Hakkaart-van Roijen L, Van Straten A, Donker M, Tiemens B. Trimbos/iMTA questionnaire for costs associated with psychiatric illness (TIC-P). Rotterdam: Institute for Medical Technology Assessment; 2002.
Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79:71–9.
Ormel J, von Korff M, Burger H, Scott K, Demyttenaere K, Huang YQ, et al. Mental disorders among persons with heart disease - results from World Mental Health surveys. Gen Hosp Psychiatry. 2007;29:325–34.
Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine (Phila Pa 1976). 2000;25:2566–71.
Allart-van Dam E, Hosman CMH, Hoogduin CAL, Schaap CPDR. Prevention of depression in subclinically depressed adults: follow-up effects on the ‘Coping with Depression’ course. J Affect Disord. 2007;97:219–28.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. London: American Psychiatric Pub; 2013.
Wittchen H-U, Zaudig M, Gruschwitz S, Fydrich TSKIDI. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. Göttingen: Hogrefe; 1997.
Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II). Clin Psychol Psychother. 2011;18:75–9.
Crippa JAS, de Lima OF, Del-Ben CM, Filho AS, da Silva Freitas MC, Loureiro SR. Comparability between telephone and face-to-face structured clinical interview for DSM-IV in assessing social anxiety disorder. Perspect Psychiatr Care. 2008;44:241–7.
Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–3.
Fleiss JL. The Design and Analysis of Clinical Experiments. New York: Wiley; 1999.
Löwe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord. 2004;81:61–6.
Erbe D, Eichert H-C, Rietz C, Ebert D. Interformat reliability of the patient health questionnaire: Validation of the computerized version of the PHQ-9. 2016.
Riedel M, Möller H-J, Obermeier M, Schennach-Wolff R, Bauer M, Adli M, et al. Response and remission criteria in major depression - a validation of current practice. J Psychiatr Res. 2010;44:1063–8. Elsevier.
Peacock SJ, Richardson J, Day NA, Hawthorne G, Iezzi A, Elsworth G. Construction of the descriptive system for the assessment of quality of life AQoL-6D utility instrument, Research Paper 49. Cent. Heal. Econ. Melbourne: Monash University; 2010.
Allen J, Inder KJ, Lewin TJ, Attia JR, Kelly BJ. Construct validity of the Assessment of Quality of Life - 6D (AQoL-6D) in community samples. Health Qual. 2013;11:61. Life Outcomes.
Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43.
Pan C-W, Sun H-P, Wang X, Ma Q, Xu Y, Luo N, et al. The EQ-5D-5 L index score is more discriminative than the EQ-5D-3 L index score in diabetes patients. Qual Life Res. 2015;24:1767–74.
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5 L compared to the EQ-5D-3 L across eight patient groups: a multi-country study. Qual Life Res. 2013;22:1717–27.
Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21.
Mannion AF, Junge A, Grob D, Dvorak J, Fairbank JCT. Development of a German version of the Oswestry Disability Index. Part 2: sensitivity to change after spinal surgery. Eur Spine J. 2006;15:66–73.
Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11:153–63.
Mittag O, Raspe H. A brief scale for measuring subjective prognosis of gainful employment: findings of a study of 4279 statutory pension insurees concerning reliability (Guttman scaling) and validity of the scale. Rehabilitation. 2003;42:169–74.
Boß L, Lehr D, Reis D, Vis C, Riper H, Berking M, et al. Reliability and validity of assessing client satisfaction in web-based health interventions. J. Med. Internet Res. 2016;18(8):e234.
Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5:233–7.
Thiart H, Lehr D, Ebert DD, Berking M, Riper H. Log in and breathe out: internet-based recovery training for sleepless employees with work-related strain - results of a randomized controlled trial. Scand J Work Environ Health. 2015;41:164–74.
Ebert DD, Berking M, Thiart H, Riper H, Laferton JAC, Cuijpers P, et al. Restoring depleted resources: Efficacy and mechanisms of change of an internet-based unguided recovery training for better sleep and psychological detachment from work. Health Psychol. 2015;34(Suppl):1240–51.
Heber E, Lehr D, Ebert DD, Berking M, Riper H. Web-based and mobile stress management intervention for employees: a randomized controlled trial. J Med Internet Res. 2016;18:e21.
Ladwig I, Rief W, Nestoriuc Y. What are the risks and side effects of psychotherapy? – Development of an inventory for the assessment of negative effects of psychotherapy (INEP). Verhaltenstherapie. 2014;24:252–63. Karger Publishers.
Nobis S, Lehr D, Ebert DD, Berking M, Heber E, Baumeister H, et al. Efficacy and cost-effectiveness of a web-based intervention with mobile phone support to treat depressive symptoms in adults with diabetes mellitus type 1 and type 2: design of a randomised controlled trial. BMC Psychiatry. 2013;13:306.
Bouwmans C, De Jong K, Timman R, Zijlstra-Vlasveld M, Van der Feltz-Cornelis C, Tan S, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res. 2013;13:217.
Papacharissi Z, Rubin AM. Predictors of internet use. J Broadcast Electron Media. 2000;44:175–96.
Haase R, Schultheiss T, Kempcke R, Thomas K, Ziemssen T. Use and acceptance of electronic communication by patients with multiple sclerosis: a multicenter questionnaire study. J Med Internet Res. 2012;14:e135.
Shih W. Problems in dealing with missing data and informative censoring in clinical trials. Curr Control Trials Cardiovasc Med. 2002;3:4. BioMed Central.
Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–5.
Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14:487–96.
Hilvert-Bruce Z, Rossouw PJ, Wong N, Sunderland M, Andrews G. Adherence as a determinant of effectiveness of internet cognitive behavioural therapy for anxiety and depressive disorders. Behav Res Ther. 2012;50:463–8.
Andrews G, Cuijpers P, Craske MG, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS One. 2010;5:e13196.
Melville KM, Casey LM, Kavanagh DJ. Dropout from internet-based treatment for psychological disorders. Br J Clin Psychol. 2010;49:455–71.
Christensen H, Griffiths KM, Farrer L. Adherence in internet interventions for anxiety and depression: systematic review. J Med Internet Res. 2009;11:e13.
Van Ballegooijen W, Cuijpers P, Van Straten A, Karyotaki E, Andersson G, Smit JH, et al. Adherence to internet-based and face-to-face cognitive behavioural therapy for depression: a meta-analysis. PLoS One. 2014;9:e100674.
Clarke G, Kelleher C, Hornbrook M, Debar L, Dickerson J, Gullion C. Randomized effectiveness trial of an Internet, pure self-help, cognitive behavioral intervention for depressive symptoms in young adults. Cogn Behav Ther. 2009;38:222–34.
Kontos E, Blake KD, Chou WYS, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16:e172.
Norman CD, Skinner HA. eHealth Literacy: Essential skills for consumer health in a networked world. J Med Internet Res. 2006;8:e9.
Rozental A, Boettcher J, Andersson G, Schmidt B, Carlbring P. Negative effects of internet interventions: a qualitative content analysis of patients’ experiences with treatments delivered online. Cogn Behav Ther. 2015;44:223–36.
Boettcher J, Rozental A, Andersson G, Carlbring P. Side effects in internet-based interventions for Social Anxiety Disorder. Internet Interv. 2014;1:3–11.
Rozental A, Andersson G, Boettcher J, Ebert DD, Cuijpers P, Knaevelsrud C, et al. Consensus statement on defining and measuring negative effects of Internet interventions. Internet Interv. 2014;1:12–9.
Baumeister H, Nowoczin L, Lin J, Seifferth H, Seufert J, Laubner K, et al. Impact of an acceptance facilitating intervention on diabetes patients’ acceptance of internet-based interventions for depression: a randomized controlled trial. Diabetes Res Clin Pract. 2014;105:30–9.
Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93.
Acknowledgements
We thank Prof. Dr. Dr. Jürgen Bengel, Dr. Kristin Kieselbach and Prof. Dr. Anja Göritz for their support by providing their expertise in the treatment of chronic pain patients. We thank colleagues who were part of the development of prior interventions [44, 46] that partly build the basis of eSano BackCare-DP. Moreover we like to thank our many study assistants for their support in the development of the intervention. We thank Ellen Meierotto for supervising the SCID-interviewers. We thank Yannik Terhorst and Susanne Stollewerk for proofreading and Mary Wyman for language editing of the manuscript. Many thanks go to the Data Safety and Monitoring Board, consisting of Prof. Dr. Martin Hautzinger, Prof. Dr. Martin Härter and Dr. Levente Kriston as well as the Clinical Trials Unit Freiburg for their support in carrying out the study project.
Special thanks go to the cooperating orthopedic rehabilitation facilities Schoen Klinik, Bad Staffelstein; Rehaklinik Sonnhalde, Donaueschingen; RehaKlinikum Bad Säckingen; Städt. Rehakliniken Bad Waldsee; Schwarzwaldklinik Orthopädie - Abteilung Medizinische Rehabilitation, Bad Krozingen; Rheintalklinik Bad Krozingen; REGIO-Reha Tagesklinik, Freiburg and Universitäts- und Rehabilitationskliniken, Ulm as well as the further orthopedic rehabilitation units of the second recruitment strategy.
Funding
The article processing charge was funded by the German Research Foundation (DFG) and the Albert Ludwigs University Freiburg in the funding programme Open Access Publishing. This trial is conducted within the framework of the project “Effectiveness of a guided web-based intervention for depression in back pain rehabilitation aftercare” funded by the German Federal Ministry of Education and Research (grant number: 01GY1330A). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
Not applicable.
Authors’ contributions
LS, DDE and HB initiated this study. LS, JL, SP, SaS, HB and DDE contributed to the design of this study. LS, JL, KS, SP and HB adapted the intervention content and the assessment. CB provided expertise on economic evaluation. LS, SaS, KS and SP are responsible for recruitment. SaS is responsible for randomization and allocation as well as the administration of study participants. LS wrote the draft of the manuscript. HB provided expertise on depression, chronic back pain and psychological pain interventions. All authors contributed to the further writing of the manuscript and approved the final version of the manuscript.
Competing interests
All authors of the manuscript were involved in the development of eSano BackCare-BP or its predecessor versions. A committee of independent scientists has been formed (DSMB) to supervise study-related decisions and prevent any influence of potential conflicts of interest. HB and DE are consultants for several stakeholders (insurance companies, ministries, psychotherapy chambers, companies). DE is part of the GET.ON Institut GmbH, which aims at implementing evidence-based IMIs into routine care.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All procedures are approved by the ethics committee of the Albert-Ludwigs-University of Freiburg, Germany (EK-297/14_150513) and the data security committee of the German Pension Insurance (Deutsche Rentenversicherung; 8022-6-BW). Written informed consent for participation in the study will be obtained from all participants prior to their involvement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sander, L., Paganini, S., Lin, J. et al. Effectiveness and cost-effectiveness of a guided Internet- and mobile-based intervention for the indicated prevention of major depression in patients with chronic back pain—study protocol of the PROD-BP multicenter pragmatic RCT. BMC Psychiatry 17, 36 (2017). https://doi.org/10.1186/s12888-017-1193-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-017-1193-6