Abstract
Background
Neonatal mortality poses a significant public health challenge in sub-Saharan Africa, with congenital heart disease emerging as the leading cause of morbidity and mortality among neonates, especially in countries like Ethiopia. Despite efforts to reduce neonatal mortality rates, Ethiopia continues to experience an increased mortality rate, particularly among neonates with congenital heart disease. This study aims to investigate the incidence and predictors of mortality in this vulnerable population within Ethiopia.
Method
A retrospective cohort study was conducted at an institution, involving 583 randomly selected neonates diagnosed with congenital heart disease. In the current study, the dependent variable was survival status. Data entry utilized EpiData data version 4.6, and analysis was performed using STATA version 16. Probability of death was compared using the log-rank test and Kaplan-Meier failure curve. Significant predictors were identified using bivariable and multivariate Cox regression. Model fitness and proportional hazard assumptions were evaluated using the Cox-Snell graph and Global test, respectively. Associations were assessed by adjusted hazard ratios with 95% confidence intervals.
Results
The study participants were followed for 4844 days. The mortality rate was 9.9%. The incidence density was 11.9 per 1000 person-days of observation. Neonatal sepsis (AHR: 2.24; 95% CI [1.18–4.23]), cyanotic congenital heart disease (AHR: 3.49; 95% CI [1.93–6.28]), home delivery (AHR: 1.9; 95% CI [1.06–3.6]), maternal history of gestational diabetes mellitus (AHR: 1.94; 95% CI [1.04–3.61]), and having additional congenital malformations (AHR: 2.49; 95% CI [1.33–4.67]) were significant predictors for neonatal mortality.
Conclusion and recommendation
The incidence density of mortality was high compared to studies conducted in developed countries. Neonatal sepsis, type of congenital heart disease, place of delivery, maternal history of gestational diabetes mellitus, and having an additional congenital malformation were significant predictors of mortality among neonates with congenital heart disease. Therefore, healthcare providers should pay special attention to patients with identified predictors. Furthermore, the Federal Ministry of Health, stakeholders, and policymakers should collaborate to address this issue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Congenital heart diseases (CHDs) involve major structural abnormalities in the heart or intrathoracic great vessels due to congenital anomalies, which disrupt blood flow through the heart and body [1, 2]. CHDs represent a prevalent category of congenital defects, constituting approximately one-third of all major congenital anomalies [3]. It has two types a cyanotic and cyanotic [4, 5].
Approximately 30–40% of neonates with congenital heart disease (CHD) exhibit symptoms during their first year of life, with 60% of these cases being diagnosed within the first month after birth [6]. The mortality rate of newborns diagnosed with congenital heart disease (CHD) continues to be a pressing global issue. Despite significant progress in medical treatments, newborns with CHD still confront heightened risks of mortality due to the intricate nature of their cardiac abnormalities. Globally, CHD contributes to around 25% of all neonatal deaths related to congenital anomalies, and its incidence is increasing, underscoring a considerable gap in diagnostic capabilities, especially in Africa [1].
As a result, CHD significantly affects the daily routines and financial stability of family members, leading to a decline in their quality of life. Moreover, neonates with heart disease often experience challenges such as malnutrition, infections, and pulmonary hypertension, particularly in low-income countries like Ethiopia, complicating the treatment process further. Altogether, caring for these neonates presents a formidable challenge [7,8,9,10].
Globally, over the last 15 years 1.35 million live births are diagnosed with CHD every year [5]. Majority were born in locations that included Africa with little or no care. Due to this, 90% of newborns with CHD received inadequate medical care [11] and 30% of neonates with CHD die in the neonatal period [3, 12, 13].
In Africa, where only 20 cardiac centers found, the incidence and mortality of neonates with CHD is increased [14, 15] and underreported [11, 14]. In Ethiopia, where the magnitude of neonatal mortality is increased in recent years there is one cardiac center which served for 56 million people [9]. Due to this and other factors the mortality of neonates with CHD in Ethiopia is still unknown [16, 17].
The country has instituted various services such as preconception, essential newborn, antenatal, and postnatal care to alleviate the burden of mortality [18, 19]. Nevertheless, mortality rates remain high, and there is insufficient evidence regarding the incidence and predictors of mortality among neonates with CHD in Ethiopia. Consequently, this study aimed to explore the incidence and predictors of mortality among neonates with CHD in Ethiopia.
Method
Study area and period
This study was conducted at Amhara regional state of Ethiopia. As indicated by the 2020 Health and Health Related Indicators report published by the Ministry of Health (MoH), Amhara region is a home to 82 hospitals, 861 health centers, and 3,565 health posts. Among these hospitals, the University of Gondar, Dessie, Felege-Hiwot, Tibebe-Ghion, Debre Markos, Woldia, Debre Tabor, and Debre Berhan are classified as Comprehensive Specialized Hospitals. The current study was conducted in the above specialized comprehensive hospitals which provide cardiac service.
Study design
Institutional based retrospective cohort study was conducted.
Source population
The source population included all neonates diagnosed with congenital heart disease admitted to the neonatal intensive care units (NICUs) of Comprehensive Specialized Hospitals in the Amhara region.
Study population
Neonates diagnosed with congenital heart disease were specifically chosen for admission to the NICU wards of selected Comprehensive Specialized Hospitals in the Amhara regional state.
Eligibility criteria
All newly diagnosed and admitted neonates with congenital heart disease from January 1st 2018 to December 30th 2022 were included. Infants lacking complete records of the outcome variable were excluded from consideration.
Sample size determination
The sample size required for this study was determined by STATA version 16 (Cox model), based on the following assumptions: a hazard ratio (HR) of 1.4 for the selected covariate of interest (maternal educational status) and a probability of failure (death) of 0.186 from study done in Ethiopia [20], a standard deviation (SD) of 0.4, a margin of error of 5%, and a confidence interval (CI) of 95% to achieve 80% power. Accounting for a 10% non-response rate, the final sample size required for this study was determined to be 587.
Sampling technique and sampling procedure
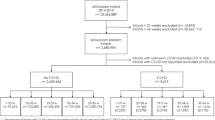
Four specialized hospitals were randomly selected using a lottery method. Study participants were chosen through systematic random sampling, where the sampling interval for each hospital was determined by dividing the number of eligible participants by the allocated sample size. Initial participants were selected using a systematic approach at every 12th interval (calculated as 7176/587 = 12). The first participants were chosen by lottery, and subsequent selections occurred every 12th interval until the desired sample size was achieved (Fig. 1).
Data collection tool and procedure
Data from NICU registration books were collected using a pretested, pre-structured checklist. This checklist encompassed socio-demographic, maternal behavioral and medical, obstetric, and neonatal variables. The retrospective follow-up period was determined by the time from CHD admission to discharge. Data recordings were included based on eligibility criteria. The questionnaire, adapted from literature and guidelines [21,22,23,24,25,26,27,28,29], was prepared in English. The data were collected by healthcare professionals stationed at treatment centers.
Operational definitions
In this study, the date of admission served as the starting point for follow-up, which extended until the infant reached 28 days of life.
Event
death of neonates with congenital heart disease while in the hospital.
Censored
neonates with congenital heart disease who were improved, discharged, or against medical treatment, and referred to others center.
Time scale
days.
Co-morbidities
Newborns with CHD and have other illnesses such as sepsis and under nutrition [30].
Congenital heart defect
Major or minor congenital anomalies defined as anatomical structural and functional defect present at birth which was confirmed by pediatricians or echocardiography [31].
Data quality control
The checklist was pre-tested with 5% of participants outside the study area to ensure data quality. It was then standardized and validated with input from clinical and academic experts. The study’s tool was validated and confirmed reliable.
Data collectors and supervisors, senior BSc nurses with NICU experience, were chosen for each hospital. They underwent one-day training on the study’s objectives, data collection tools, methods, and ethical considerations. Supervisors oversaw the data collection, and spot-checks were conducted by the principal investigator and supervisors to ensure completeness and consistency of the collected data.
Data processing and analysis
After ensuring data completeness and consistency, they were coded and entered into EpiData version 4.6, then exported to STATA version 16 for cleaning and analysis, using summary statistics, text, graphs, and tables.
Multicollinearity was assessed using variance inflation factors. Descriptive statistics, Kaplan-Meier graphs, and log-rank tests were performed. The proportional hazard assumption was tested using Schoenfeld residuals (global test), and model fitness was evaluated with a Cox-Snell residual test. Bivariable and multivariate Cox proportional hazard regression models identified predictors, presenting results with Adjusted Hazard Ratios (AHR) and 95% Confidence Intervals (CI).
Results
A total of 587 neonate’s charts were scrutinized, of which 583 were eligible and complete with a response rate of 99.32%.
Socio-demographic characteristics of mother of neonate
In this study, 310 neonates (53.17%) had mothers aged over 35 years, and 506 (86.79%) were born to married mothers. Furthermore, 404 participants (69.3%) were over seven days old, and 393 (67.41%) were female. Additionally, 418 (83.53%) of the study participants lived in rural areas (Table 1).
Maternal behavioral, medical and obstetric related factors
Among the neonates, 18.18% were born to mothers who consumed alcohol during pregnancy, and 20.24% were born to mothers who chewed khat. 13.35% had mothers with gestational diabetes mellitus, and 6.66% had mothers with cardiac problems. Furthermore, 9.78% were born to HIV-infected mothers, and 22.13% had a family history of congenital heart disease (Table 2).
Most of the mothers of the neonates (506, 86.79%) had two or more pregnancies, 452 (77.53%) experienced spontaneous vaginal births, and 301 (51.63%) had received antenatal care (ANC) follow-up. Additionally, 108 neonates (18.52%) were born to mothers who had a history of neonatal death. Lastly, 165 neonates (28.30%) were born at home (Table 3).
Neonatal related characteristics
Among the newborns with CHD in the NICU, 389 (66.72%) had low birth weight, 260 (44.60%) had neonatal sepsis, 172 (29.50%) had PNA, 320 (54.89%) were born prematurely, and 49 (8.10%) had another cardiac anomaly. The mean birth weight at admission was 2253 g (Table 4).
Overall survival function and probability
The overall mean survival time of neonates admitted to NICU in the study was 8 days with 90.3% (95% CI 0.86–0.93) a SD ± 0.015. This study also showed that the probability of neonatal survival at the 7th and 14th day of hospital stay was 92.5% (95% CI 89 94) SD ± = 0.013) and 82.1% (95% CI: 76–86) SD ± = 0.025), respectively. At the 20 days of hospital stay, the overall survival probability of neonates was 74.1% (95% CI: 65–80) with a standard error of 0.039 (Annex 1).
To assess the survival difference between selected covariates Kaplan Meier graph and log rank test were done. According to both Kaplan Meier graph and log rank test there is survival difference between neonates who had comorbidity and hadn’t; neonates who had comorbidity had low survival rate (Log rank test; Pr > chi2 = 0.0001). Moreover, neonates who had a cyanotic CHD had less survival rate. However, there was no survival difference between neonates (log rank test result; Pr > chi2 = 0.00) (Fig. 2).
Survival status of neonates with CHD
A total of 583 neonates with CHD were followed from admission to 28 days with 4844 person day observations. In the current study, 58 (9.79%) of neonates died and 525 (90.05%) of them censored (Fig. 3). The median survival time was at 7 days (Fig. 4). The overall incidence density rate of mortality was 11.9 [95% CI (9.00–15.00)] per 1000 person days observation. The incidence density rate of mortality among cyanotic CHD neonates and a cyanotic CHD was 37.3 and 6.3 per 1000 person day observations respectively.
Predictors of mortality for neonates with CHD
Before conducting the analysis, multicollinearity, model fitness, and proportional hazard assumption were assessed. The model was fitted and verified using the Cox-Snell graph (Fig. 5), confirming that the assumption was not violated (with a global test value of 0.6337). Additionally, the Variance Inflation Factor (VIF) was found to be 1.06.
In the bivariable Cox regression model, ten variables were considered: gestational diabetic mellitus (GDM), place of delivery, presence of other congenital malformation, sepsis, need for resuscitation during delivery, type of CHD, maternal HIV status, maternal alcohol intake, history of neonatal death, and premature rupture of membrane. These variables were eligible and selected for multivariate analysis. However, only five of them place of delivery, sepsis, type of CHD, presence of other congenital malformations, and GDM remained significant predictors for mortality among neonates with CHD.
Neonates diagnosed with sepsis had a hazard 2.24 times greater than their counterparts [Adjusted Hazard Ratio (AHR): 2.24; 95% CI (1.18–4.23)]. Those with cyanotic congenital heart disease faced a 3.5 times higher risk of mortality [AHR: 3.49; 95% CI (1.93–6.28)] compared to those with non-cyanotic CHD.
Additionally, neonates born at home exhibited a 1.9 times higher hazard of death [AHR: 1.9; 95% CI (1.06–3.6)] than those delivered in a healthcare institution. Neonates with a maternal history of GDM had a mortality rate 1.9 times higher [AHR: 1.94; 95% CI (1.04–3.61)] than their counterparts. Lastly, neonates with other congenital malformations were 2.5 times more likely to die [AHR: 2.49; 95% CI (1.33–4.67)] than those without such malformations (Table 5).
Discussion
The aim of this study was to assess the incidence and predictors of mortality among neonates with CHD in Ethiopia. The mortality rate among neonates with CHD was 9.79%. The mortality incidence among neonates with congenital heart disease was 11.9 [95% CI (0.009–0.015)] per 1000 person-days of observation. Additionally, the median survival time was 7 days.
The Mortality rate revealed in this study was low as compared to studies conducted in Developing countries [23]. However, the rate was high as compared to studies conducted in Taiwan [32]. This variability could be attributed to differences in the quality and availability of cardiac services, as well as disparities in population characteristics. It’s worth noting that the study conducted in developing countries focused solely on critical neonates with CHD.
The incidence reported in this study was in line with studies conducted in China [33, 34], Norway [35] and Turkey [36]. However, it is was higher as compared to studies conducted in Europe [37], Spain [38], Sri lanka [39], united states of America (USA) [40], France [41] and Brazil [42]. Lastly, it low as compared to study done globally [43] and Brazil [44]. The potential explanation might stem from considerable disparities in socioeconomic status, study settings, and the presence of specialized cardiac surgeons and facilities. Furthermore, it could be attributed to variances in neonatal intensive care unit conditions and maternal healthcare service utilization.
Neonates who had neonatal sepsis had more risk for mortality. This finding was in line with studies conducted in Ethiopia, [27, 45,46,47], Indonesia [48] and USA [49]. This might be due to the distinct cardiovascular pathophysiology observed in children with congenital heart disease (CHD) compared to their peers without CHD, the standard diagnosis and management protocols for sepsis cannot be applied directly to this population which underestimates the effect of sepsis.
Neonates with cyanotic congenital heart disease had higher mortality rate. This finding was consistent with a study done in Georgia (69) and Spain (59). This similarity arises from the fact that infants with CHD have critical early difficulties that necessitate emergency care because their aorta pumps mixed blood, which raises the chance of mortality.
Home delivered neonates with CHD had more hazard of mortality. This finding was supported by studies conducted in sub-Sahara Africa [50] and Ethiopia [51]. This could be because newborns delivered at home may not receive immediate resuscitation, thereby increasing the risk of birth complications.
Neonates born to mothers with a history of maternal gestational diabetes mellitus (GDM) exhibited a heightened risk of mortality. This study’s discovery aligns with research conducted in Ethiopia [51]. This correlation may stem from GDM leading to hypoglycemia at birth, respiratory distress, polycythemia, elevated bilirubin levels, and an increased likelihood of preterm birth [52].
Finally, neonates with congenital heart disease (CHD) who also presented with additional congenital malformations faced a higher risk of mortality. This discovery is corroborated by studies conducted in Ethiopia [47] and Finland [53]. This phenomenon could be attributed to the increased mortality risk associated with neonates bearing additional congenital malformations, which consequently impacts the prognosis of CHD adversely.
While this study encompassed a large population and employed a follow-up approach, it encountered several limitations. Firstly, because of its retrospective design, certain maternal predictors like educational and nutritional status were not assessed. Secondly, managing missing values posed a challenge. Lastly, we did not track neonates discharged before 28 days of life, potentially leading to an underestimation of the incidence rate.
Conclusion
The current research revealed higher mortality and incidence density rates compared to studies in developed nations. Additionally, factors such as sepsis, place of birth, maternal history of GDM, other congenital malformations, and type of CHD were identified as significant predictors for neonatal CHD mortality. To address this issue, collaboration between the Federal Ministry of Health, stakeholders, and the Ethiopian Cardiac Association is essential to enhance the quantity and quality of cardiac centers in the country. Moreover, healthcare providers should prioritize neonates with sepsis, additional congenital malformations, and cyanotic CHD. Lastly, reducing home deliveries can be achieved by expanding ANC service coverage.
Availability of data and materials
The data set analyzed during the current study is available from the corresponding author upon reasonable request.
Abbreviations
- ANC:
-
Antenatal Care
- AHR:
-
Adjusted Hazard Ratios
- CHD:
-
Congenital Heart Disease
- CHR:
-
Crude hazard ratio
- GDM:
-
Gestational Diabetes Mellitus
- NICU:
-
Neonatal intensive care units
- USA:
-
United States of America
- VIF:
-
Variance Inflation Factor
References
Liu Y, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455–63.
Yu Z, et al. Congenital heart disease in a Chinese hospital: pre-and postnatal detection, incidence, clinical characteristics and outcomes. Pediatr Int. 2011;53(6):1059–65.
Zikarg YT, Yirdaw CT, Aragie TG. Prevalence of congenital septal defects among congenital heart defect patients in East Africa: a systematic review and meta-analysis. PLoS One. 2021;16(4): e0250006.
Hurisa T, Megersa H, Tsegaye T. Delayed in diagnosis of congenital heart disease and associated factors among pediatric patients in cardiac center Addis Ababa, Ethiopia, 2021/2022 GC. Am J Health Res. 2022;10(3):51–62.
Van Der Linde D, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–7.
Schulkey CE, et al. The maternal-age-associated risk of congenital heart disease is modifiable. Nature. 2015;520(7546):230–3.
Zimmerman MS, et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health. 2020;4(3):185–200.
Jivanji SG, et al. Congenital heart disease in East Africa. Front Pead. 2019;7:250.
Howell HB, et al. Neurodevelopmental outcomes of children with congenital heart disease: a review. Curr Probl Pediatr Adolesc Health Care. 2019;49(10):100685.
Zilla P, et al. Global unmet needs in cardiac surgery. Global Heart. 2018;13(4):293–303.
Saxena A. Status of pediatric cardiac care in developing countries. Children. 2019;6(2): 34.
Glidewell J, et al. Actions in support of newborn screening for critical congenital heart disease—United States, 2011–2018. Morb Mortal Wkly Rep. 2019;68(5):107.
Su Z, et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: an age-period-cohort analysis for the Global Burden of Disease 2019 study. EClinicalMedicine. 2022;43:101249.
Hewitson J, Zilla P. Children’s heart disease in sub-saharan Africa: challenging the burden of disease: children’s heart disease. Sa Heart. 2010;7(1):18–29.
Hoffman JI. The global burden of congenital heart disease. Cardiovasc J Afr. 2013;24(4):141–5.
Tolla MT, et al. Out-of-pocket expenditures for prevention and treatment of cardiovascular disease in general and specialised cardiac hospitals in Addis Ababa, Ethiopia: a cross-sectional cohort study. BMJ Global Health. 2017;2(2): e000280.
Nigussie B, Tadele H. Heart failure in Ethiopian children: mirroring the unmet cardiac services. Ethiop J Health Sci, 2019. 29(1).
Araujo Júnior E, et al. Fetal cardiac evaluation by 3D/4D ultrasonography (STIC): what is its real applicability in the diagnosis of congenital heart disease? SciELO Brasil; 2013. p. III-V.
Nobles-Botkin J, Lincoln A, Cline J. Preconception care resources: where to start. J Midwifery Women’s Health. 2016;61(3):365–9.
Mengistu BA, et al. Incidence and predictors of neonatal mortality among neonates admitted in Amhara regional state referral hospitals, Ethiopia: prospective follow up study. BMC Pediatr. 2020;20(1):142.
Rocha LA, et al. Risk factors for mortality in children with congenital heart disease delivered at a Brazilian tertiary center. Brazilian J Cardiovasc Surg. 2018;33:603–7.
Lopes SAVdA, et al. Mortality for critical congenital heart diseases and associated risk factors in newborns. A cohort study. Arquivos Brasileiros Cardiologia. 2018;111:666–73.
Mat Bah MN, et al. Survival and associated risk factors for mortality among infants with critical congenital heart disease in a developing country. Pediatr Cardiol. 2018;39(7):1389–96.
Tran R, et al. Social determinants of disparities in mortality outcomes in congenital heart disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:472.
Best KE, Rankin J. Long-term survival of individuals born with congenital heart disease: a systematic review and meta‐analysis. J Am Heart Association. 2016;5(6):e002846.
Tankeu AT, et al. Prevalence and patterns of congenital heart diseases in Africa: a systematic review and meta-analysis protocol. BMJ Open. 2017;7(2): e015633.
Tamirat M. Congenital heart defects and associated factors in children with congenital anomalies. Ethiop Med J. 2018;56(4):335–41.
Cheng HH, et al. Outcomes and risk factors for mortality in premature neonates with critical congenital heart disease. Pediatr Cardiol. 2011;32:1139–46.
Polito A, et al. Increased morbidity and mortality in very preterm/VLBW infants with congenital heart disease. Intensive Care Med. 2013;39:1104–12.
Organization WH. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. 2019.
Kohler W, Reinger D IV. Nelson textbook of pediatrics, international edition. 2019;2:324–5.
Yeh S-J, et al. Prevalence, mortality, and the disease burden of pediatric congenital heart disease in Taiwan. Pediatr Neonatology. 2013;54(2):113–8.
Jin X, et al. Incidence and risk factors of congenital heart disease in Qingdao: a prospective cohort study. BMC Public Health. 2021;21(1):1044.
Qu Y, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdong registry of congenital heart disease, China. PLoS One. 2016;11(7): e0159257.
Wik G, et al. Severe congenital heart defects: incidence, causes and time trends of preoperative mortality in Norway. Arch Dis Child. 2020;105(8):738–43.
Çaylan N, et al. Investigation of infant deaths associated with critical congenital heart diseases; 2018–2021, Türkiye. BMC Public Health. 2024;24(1):441.
Khoshnood B, et al. Recent decrease in the prevalence of congenital heart defects in Europe. J Pediatr. 2013;162(1):108–13.
Picarzo JP-L, et al. Congenital heart disease mortality in Spain during a 10 year period (2003–2012). Anales De Pediatría (English Edition). 2018;88(5):273–9.
Gamhewage N, Perera K, Weerasekera M. Effectiveness of newborn pulse oximetry screening for the identification of critical congenital heart disease in a tertiary care hospital in Sri Lanka. Sri Lanka J Child Health. 2021;50(4):699–703.
Lopes S, et al. Mortality for critical congenital heart diseases and associated risk factors in newborns. A cohort study. Arq Bras Cardiol. 2018;111(5):666–73.
Lucron H, et al. Infant congenital heart disease prevalence and mortality in French Guiana: a population-based study. Lancet Reg Health Am. 2024;29.
Rocha LA, et al. Risk factors for mortality in children with congenital heart disease delivered at a Brazilian tertiary center. Braz J Cardiovasc Surg. 2018;33(6):603–7.
Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine. 2020;99(23).
Soares AM. Mortality for critical congenital heart diseases and associated risk factors in newborns. A cohort study. SciELO Brasil; 2018. p. 674–5.
Mekonen HK, Nigatu B, Lamers WH. Birth weight by gestational age and congenital malformations in Northern Ethiopia. BMC Pregnancy Childbirth. 2015;15(1):1–8.
Abebe S, et al. Risk factors associated with congenital anomalies among newborns in southwestern Ethiopia: a case-control study. PLoS One. 2021;16(1): e0245915.
Tesfaye E, Tadele H. Bacterial sepsis among children with congenital heart disease in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Ethiop J Health Sci. 2022;32(3).
Paramita MIP, et al. Predictors of neonatal mortality with congenital heart disease. Bali Med J. 2023;12(1):1114–9.
Ascher SB, et al. Sepsis in young infants with congenital heart disease. Early Hum Dev. 2012;88:S92-7.
Mekonen HK, Nigatu B, Lamers WH. Birth weight by gestational age and congenital malformations in Northern Ethiopia. BMC Pregnancy Childbirth. 2015;15:1–8.
Choi S, et al. How do caregivers of children with congenital heart diseases access and navigate the healthcare system in Ethiopia? BMC Health Serv Res. 2021;21:1–7.
Domanski G, et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC Pregnancy Childbirth. 2018;18(1):367.
Rosa RCM, et al. Congenital heart defects and extracardiac malformations. Revista Paulista Pediatria. 2013;31:243–51.
Acknowledgements
We greatly acknowledge Debre Berhan University, data collectors and supervisors.
Funding
The author didn’t receive any fund for this study.
Author information
Authors and Affiliations
Contributions
AA, SS, and FA conceived the idea and were involved in data collection, entry, analysis, and drafting. MT, GN, EG, LAM, WZT, WA, MTG, MA and BG contributed to manuscript preparation and the revision of the final version. All authors have reviewed and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We adhered to the Declaration of Helsinki guidelines. Ethical approval was granted by the Institutional Review Board of Asrat Woldeyes Health Sciences campus Debre Berhan University. Approval was obtained from each hospital, and letters of permission and informed consent were obtained from the medical directorate of each hospital on behalf of the neonates. Measures were taken to ensure the confidentiality and anonymity of the data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ayfokru, A., Shewasinad, S., Ahmed, F. et al. Incidence and predictors of mortality among neonates with congenital heart disease in Ethiopia: a retrospective cohort study. BMC Pediatr 24, 559 (2024). https://doi.org/10.1186/s12887-024-05023-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-05023-3