Abstract
Background
Drug-resistant epilepsy is defined as failure of seizure control in spite of using 2 or 3 proper antiepileptic drugs in appropriate time. Mineral elements play important roles in neuronal function; it is believed that mineral deficiency may lead to complications through seizure management. In the present study, serum levels of zinc (Zn), copper (Cu), magnesium (Mg), calcium (Ca), and 25-hydroxy vitamin D (Vit D) in drug-resistant-epilepsy (DRE) patients were evaluated and compared with the controlled patients.
Methods
In this cross-sectional study, epileptic patients were included and categorized into two groups of DRE and well-controlled patients. Patients’ serum samples were analysed to evaluate Zn, Cu, Mg, Ca, and Vit D levels. The primary objective was comparison of serum levels of different trace elements between the groups.
Results
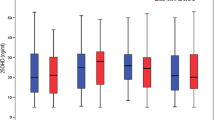
Sixty-four epileptic children including 33 DRE and 31 well-controlled children entered the study. The DRE children showed a significantly earlier onset of disease compared to the other group (p = 0.014). Comparing the frequency of developmental delay between the groups, the results showed this complication was significantly more frequent in the DRE group (p < 0.001). Concerning serum elements, the results showed a significantly higher concentration of Zn in the well-controlled group than the DRE group (p = 0.007). On the other hand, no significant differences were observed between the groups regarding the means of Vit D, Ca, Cu, and Mg levels (p > 0.05).
Conclusion
The results of the present study delineated that drug-resistant epilepsy patients had earlier onset of disease and were at higher risk of neurodevelopmental delay compared with well-controlled-epilepsy patients. A significant lower serum levels of Zn were also observed in drug-resistant-epilepsy patients. This finding may suggest the role of zinc supplementation in help to better control of drug-resistant seizures, as well as, the importance of serum zinc monitoring in epileptic patients.
Similar content being viewed by others
Introduction
Epilepsy is one of the most frequent neurological disorders associated with abnormal electrical currents. This disorder is defined as recurrent occurrence of unprovoked seizures; if two or more unprovoked seizures happen at intervals more than 24 h the diagnosis of epilepsy is done. Totally 51 million people around the world are suffering from this disease, and about 3–4% of children experience at least an epileptic attack in their lifetime [1,2,3]. Although the etiology of disease remains unknown in some cases, several factors such as structural, infectious, immunological, metabolic, or genetic disturbances are involved [4, 5]. Depending on the type and duration of seizures, epilepsy can cause neuronal death, neuronal injury, and alteration of neuronal network, resulting in long term consequences [6].
The first generation or new antiepileptic drugs like carbamazepine, clonazepam, phenobarbital, valproic acid either as monotherapy or multi-drug regimens are prescribed for children to manage their epileptic seizures [7]. Drug-resistant epilepsy (DRE) is an epilepsy that shows resistance to treatment despite the use of 2 or 3 properly chosen antiepileptic drugs with appropriate dosage and duration. Moreover, in the definition of DRE, the frequency of seizures and patient’s follow-up period are considered as the important factors [3]. As, based on Berg and Shinnar definition, DRE is diagnosed when more than one seizure attack is occurred per month for 18 months and no seizure free period is existed for more than 3 consecutive months [3]. DRE problem may relate to advanced damages in structure and microvasculature of nervous system. In addition, increased levels of oxidative stress and inflammatory agents can affect function of drug transporters resulting in DRE [8]. Other mechanisms like alterations in neural sprouting, neurogenesis, voltage-gated ion channels, neurotransmitter receptors and their sensitives, as well as epileptogenesis pattern may be involved [9]. It was reported that about 20% of patients are drug resistant and continue to experience seizure attacks despite using antiepileptic drugs with proper dose and duration [7, 10]. To manage this complication, the number of antiepileptic drugs, the frequency of seizure, and the patient’s follow-up should be considered as the important factors [3].
Evidence has shown the potential role of minerals and micro-nutrients deficiencies on nervous system disorders through affecting voltage and transmitter-gated channels, signal transduction, as well as enzymatic and biological activities [11, 12]. On the other hand, the possible antiepileptic effects of several minerals and micro-nutrients like magnesium, vitamin B6, B12, E, and D have been shown [13]. An investigation demonstrated significantly lower levels of serum selenium (Se) and copper (Cu) in well-controlled and drug-resistant epileptic children compared to the age and sex-matched controls [14]. The other study has shown that macro and micro-nutrient intakes were significantly lower in patients with uncontrolled seizures compared with those with controlled seizures [15]. Comparing serum calcium (Ca), magnesium (Mg), zinc (Zn), and Cu concentrations in seizures-controlled and DRE patients, the results of a study demonstrated a significantly lower level of serum Zn in patients who did not respond to the antiepileptic treatments [16]. This finding was confirmed by the other investigation, and the authors revealed a significant relationship between low levels of serum Zn and intractable epilepsy in 35 patients aged 6 months to 15 years [3].
The prevalence of epilepsy in Iran is still high (1%) [17], and further investigations are needed to assess the etiology or management of DRE. As literatures have shown the role of trace elements on neuronal function; it is supposed that mineral deficiency may lead to neurological complications through resistance to treatment. Hence, by the present study we aimed to compare serum levels of Zn, Cu, Mg, Ca, and 25-hydroxy vitamin D (Vit D) in the well-controlled and DRE patients to show any potential role of trace elements on DRE occurrence.
Materials and methods
A cross-sectional study was conducted at Ali Asghar Children’s Hospital which is affiliated with the Iran University of Medical Sciences (Tehran, Iran). All epileptic children aged 1–17 years who attended our center from 2019 to 2021 entered the study. According to literature [3], patients with at least a seizure attack during recent six months, despite consuming two or more antiepileptic drugs for a year were considered as the DRE group. The other patients with controlled seizure during the treatment period were included in the study as the well-controlled group. Exclusion criteria were symptomatic seizures due to nervous system infections, neurodegenerative diseases, metabolic diseases, structural complications (like tumors); symptomatic Cu, Ca, or Zn deficiency; a positive history of systemic disorder affecting trace elements values (such as Wilson disease, chronic renal or hepatic diseases, malnutrition and acrodermatitis enteropathica); taking supplementation or drugs containing Zn, Cu, Mg, and Ca during recent six months.
All participants’ demographic and clinical data were extracted from the medical records and gathered in a checklist. These data included age, sex, familial history of epilepsy, consumed medicines, type of seizure, treatment duration, and the time of the first seizure. Other demographic characteristics such as geographic area and socioeconomic status were identical between the groups. A dietary intake history was also taken. Accordingly, all participants were on balanced diet and none of them had dietary restriction for medical purposes.
Evaluating serum levels of elements, including Zn, Cu, Mg, Ca, and Vit D, 6 ml of peripheral venous blood was drowned, centrifuged, stored at -70 o C, and sent to the laboratory. The levels of Zn, Cu, Ca, and Mg were measured using the Atomic absorption spectrophotometry method. Vit D measurement was also carried out using a Radioimmune assay kit (IDS Co., United Kingdom). Based on the evidence [18,19,20,21,22], normal ranges of elements were considered as follows (Table 1).
Sample size
According to the results of a former investigation performed by Prasad et al., [16] the means of Zn levels in the controlled epilepsy and drug-resistant groups were 10.38 ± 2.99 and 12.62 ± 3.30.
µg/dl. Using the following sample size formula, considering α error 0.05, and the power of 80%, the sample size was estimated at 32 in each group.
S1 = 2.99; S2 = 3.30
X1 = 10.38; X2 = 12.62
Z1-α/2 = 1.96
Z1-β = 0.85
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Science (SPSS Inc., Chicago, USA), version 27.0. The quantitative variables in the study are presented in mean ± SD (variables with normal distribution) or median and interquartile range (variables without normal distribution). Qualitative variables are also shown in number (%). The Chi-square test was used to compare qualitative variables like sex, familial history, developmental delay, used drugs, and type of seizure between the groups. The Independent T-test was implemented to analyze the quantitative variables (patients’ ages and serum elements) between the groups. Variables without normal distribution (age at onset of the epilepsy and treatment duration) were also analyzed using the Mann-Witney test. The correlations between treatment duration and serum elements were reported using the Spearman correlation. A p-value less than 0.05 was considered the significant level.
Ethical consideration
The present study was approved based on the Helsinki Declaration by the Ethics Board of the Iran University of Medical Sciences (ID Code: IUMS;2854;1395.4.26). Before enrolment, parents or legal guardians of children were informed about the project, and then they were asked for written consent. The participants were assured about their right to discontinue and exit from the study. No cost was imposed, and all data were considered confidential.
Results
Sixty-four epileptic children including 33 (52%) DRE and 31 (48%) well-controlled epilepsy children entered the study. Of all participants, 36 (56%) were male. Myoclonic and Tonic-clonic were the most common types of seizures in the DRE and well-controlled patients, respectively. The physical examination of patients did not reveal any signs of inadequate dietary intake such as malnutrition or acrodermatitis enteropathica.
Concerning the consumed drugs, 21 subjects of the well-controlled group used a single-drug regimen as follows; 6 patients took Phenobarbital (5 mg/kg/day), 5 patients took Levetiracetam (20–40 mg/kg/day), 5 patients took Valproate sodium (20 mg/kg/day), 4 patients took Carbamazepine (20 mg/kg/day), and 1 took Topiramate (5 mg/kg/day). The other patients in the well-controlled group used multiple drugs, of them 9 received two and 4 received three drugs. The duration of treatment was one to two years. Additionally, all patients in the DRE group consumed a high dose of multiple-drug regimen depending on seizure type; 22, 8, and 3 patients used three, four, and five drugs, respectively. The most common combinations were Valproate sodium (20 mg/kg/day), Clobazam (1 mg/kg/day), and Topiramate (7–9 mg/kg/day), followed by Phenobarbital (5 mg/kg/day), clobazam (1 mg/kg/day), and Levetiracetam (40 mg/kg/day), as well as Vigabatrin (100 mg/kg/day), Levetiracetam (40 mg/kg/day), and Phenobarbital (5 mg/kg/day). The multiple-drug regimen in this group of patients varied in terms of medications and treatment duration (longer than a year).
As comparative data are shown in Table 2, the DRE children showed significantly earlier onset of disease compared to the other group (p = 0.014). The treatment period in the DRE group was more prolonged compared to the treatment duration in the well-controlled group (p < 0.001). The majority of DRE children (75%) showed neurodevelopmental delay. Comparing the frequency of developmental delay between the groups, the results showed this complication was significantly more frequent in the DRE group (p < 0.001). More children in the DRE group consumed multiple-drug regimens compared to the patients in the well-controlled group (71.70% vs. 28.30%; p < 0.001). The mean age of DRE children was higher than the well-controlled subjects but the difference was not statistically significant(p = 0.990). No differences were also observed between the groups regarding participants’ genders (p = 0.512) and seizure types (p = 0.106). More patients in the well-controlled group had a familial history of epilepsy; however, the difference between the groups was not statistically significant (p = 0.237). Detailed data are presented in Table 2.
Concerning serum elements, the results showed a significantly higher concentration of Zn in the well-controlled group than the DRE group (p = 0.007). On the other hand, no significant differences were observed between the groups regarding to means of Vit D, Ca, Cu, and Mg levels (p > 0.05). Data are shown in Table 3.
Table 4 shows the correlations between the length of the treatment period and serum elements in both groups. According to the results, no statistically significant correlations were observed between the duration of the treatment period with serum levels of Zn, Ca, Mg, Cu, and Vit D in the studied groups (p > 0.05).
Discussion
Literatures have shown no significant improvement of seizure attacks in one-fifth to one-fourth of epileptic patients despite use of 2–3 antiepileptic drugs [3, 23]. The present study assessed the possible relationships between drug resistance in epileptic patients and concentrations of serum trace elements.
The results of present study showed that the mean of serum Zn in DRE patients was significantly lower than the other group. These findings may suggest the role of zinc supplementation in better control of drug-resistant seizures, as well as, the importance of serum zinc monitoring in patients who have refractory epilepsy. Evidence has demonstrated the modulatory effects of Zn on neural excitability; Zn is a modulator of the glutamic acid decarboxylase and is involved in the production and activity of the gamma-aminobutyric acid (GABA) an inhibitory neurotransmitter [24]. Moreover, the activation of N-Methyl-D-Aspartate (NMDA as an excitatory neurotransmitter) receptors has been shown by low levels of serum Zn [24]. The principal modulatory effects of Zn on NMDA receptors occur through two manners of voltage-dependent and non-dependent channel blockage at various concentrations. Zn binds to the aminoterminal domain of GLUN2 A subunit of NMDA receptors to decrease the probability of ion channel opening through the increment of proton inhibition. Mutation of GLUN2 can alter the sensitivity of NMDA receptors to Zn and results drug-resistant epilepsies [25]. Zn as a cofactor also has inhibitory effects on neuronal T-type calcium channels [14]. Doboszewska et al. reported a high level of Zn in mossy fibers of hippocampus, which this region of brain is critical for epilepsy occurrence and its drug-resistant forms [26]. Excitotoxicity-evoked seizures are resulted from brain inflammation and increased pro-inflammatory cytokines, including IL-1beta. The functions of IL-1beta are diminished by Zn through blocking glutamate receptors [27]. According to Baraka et al. study, proper doses of Zn could diminish seizures by reducing inflammatory and pro-inflammatory factors such as interleukin-1 β and oxidative stress in rats [28]. Furthermore, extracellular signaling effect of Zn exerts via some post-synaptic receptors like protein-coupled receptor 39. Activation of such receptors may increase intracellular calcium release, resulting in upregulation of potassium-chloride (K+-Cl) cotransporters, increase of K+-dependent Cl− efflux in the post-synaptic neurons, promotion of GABA A receptor-mediated cell hyperpolarization, and excitotoxicity inhibition [28]. These mechanisms may explain the modulatory role of Zn in the control of seizure attacks.
Moreover, Zn is a major component of the superoxidase dismutase, an antioxidant enzyme that detoxifies the oxidative radicals which are responsible in neuronal membrane instability and epileptogenesis [3]. In accordance with our findings, Prasad et al. [16] compared serum minerals in epileptic patients with and without response to antiepileptic drugs. Their results showed significantly lower levels of serum Zn in non-responder patients compared to the responder patients (10.38 ± 2.99 vs. 12.62 ± 3.30 µmol/L; p < 0.05); however, considering serum Ca, Mg, and Cu, the differences between the groups were not statistically significant. Kheradmand et al. [3] also showed Zn deficiency was significantly more frequent among patients with DRE compared with patients with controlled epilepsy (70% vs. 20%; p < 0.001). In line with our finding, Elshorbagy et al. [29] confirmed the role of Zn supplementation in the reduction of seizures among patients with idiopathic intractable epilepsy. On the contrary to Zn levels, a significant difference was not observed between the groups concerning serum Cu concentrations (p = 0. 81). Inconsistent with our results, Chakrabarty et al. [14] demonstrated no significant difference in serum Zn concentrations between the two groups of patients with drug-refractory and well-controlled epilepsy (643 ± 149 vs. 607 ± 110; p = 0.13).
Based on the results, no significant differences were observed between the groups regarding serum Ca, Cu, and Mg levels. Concerning the means of Vit D level, no difference was also observed between the groups. Seasonal-related variables were not considered in the study because the samples were collected over a long period and the seasonal conditions were the same for both groups. Our finding may also associate with no notable seasonal variation in our geographic area, affecting patients’ Vit D status [30]. On the other hand, evidence has revealed the role of several factors (seasonal change, duration of sunlight exposure, duration of UV exposure, physical activity, pigments of skin) on serum Vit D levels [30, 31].
According to the results of the present study, DRE patients showed significantly earlier onset of disease and longer treatment period compared with the well-controlled group. Compatible with our findings, other investigations [32, 33] showed early onset of seizure as a significant risk factor for DRE; Karaoğlu et al. showed the age at onset of seizure in DRE case was significantly lower than drug-responsive subjects (1.85 vs. 4.92 years; p < 0.001). Yilmaz et al. also revealed age of epilepsy onset < 12 month in 54.5% of drug resistant and 28.4% of drug responsive epilepsy patients (p < 0.001).
Comparing the frequency of developmental delay between the groups, our results showed this complication was significantly more frequent among DRE patients. Consistent to our results, Karaoğlu et al. [32] showed a significant correlation between motor and mental abnormalities with development of DRE in 200 epileptic children. The other study by Gavrilovic et al. [34] demonstrated that the mean of cognitive score in 103 DRE patients was significantly lower than 52 drug-responsive-epilepsy cases (p < 0.001).
Compatible with other investigations [9, 35], the results showed significantly more children in the DRE group consumed multiple-drug regimens compared to the patients in the well-controlled group. Prescribing more medications in this group of patients help to act against more than one target-based epileptogenic mechanisms [36]. Evidence has confirmed that DRE patients use three or more medications, excluding diazepam, while drug-responsive-epilepsy patients receive less than three antiepileptic drugs. DRE patients added or switched alternative drugs 3.6 to 3.7 times more than their non-refractory counterparts [35].
According to the results, we could not find any significant correlations between the duration of treatment period with serum levels of Zn, Ca, Mg, Cu, and Vit D in the studied groups. It seems several factors like prescribed drugs, consuming supplements, duration of disease, and treatment period may be involved factors [37].
In summary, the results showed early onset of disease and risk of developmental delay in DRE patients. Moreover, lower serum Zn levels may be observed in DRE compared with controlled epilepsy. Accordingly, monitoring of this trace element seems beneficial and should be considered. On the other hand, it should be noticed that the present study had several limitations regarding small sample size, and including few variables. For instance, data on patients consuming different drugs were insufficient to show possibly statistical correlations between the drugs and serum elements levels. Although all included patients were on a well-balanced diet consisting of vegetables, meat and/or dairy foods, which had sufficient dietary diversity of micronutrients, the quantitative analyses regarding trace elements in their diet were not performed. Additionally, this is a cross-sectional study, and the causality cannot be confirmed. Designing future investigations with a larger samples size and considering more variables like different demographic characteristics, life style, nutritional habits, seasonal factors, types of neurodevelopmental abnormalities (in motor or cognitive domains), para clinic findings, the history of status epilepticus, neonatal, or febrile seizures, as well as detailed data regarding consumed drugs and the rout of usage could provide more informative data.
Conclusion
The results delineated that drug-resistant-epilepsy patients had earlier onset of disease and were at higher risk of neurodevelopmental delay compared with well-controlled epilepsy patients. A significant lower serum levels of Zn were also observed in drug-resistant-epilepsy patients. This finding may suggest the role of Zn supplementation in help to better control of drug-resistant seizures, as well as, the importance of serum zinc monitoring in epileptic patients. Further investigations are needed to determine the potential cut-off serum zinc level for drug-resistant epilepsy. Furthermore, a succeeding study is suggested to measure trace elements levels before and after antiepileptic treatment to show possible relationship between trace elements levels and drugs.
Data availability
The datasets related to our study are available from the corresponding author upon reasonable request.
Abbreviations
- Cu:
-
Copper
- Zn:
-
Zinc
- Mg:
-
Magnesium
- Ca:
-
Calcium
- Vit D:
-
25-hydroxy vitamin D
- DRE:
-
Drug-resistant-epilepsy
References
Heydari Y, Bozzi Y, Pavesi L. Decoding epileptic seizures: exploring in Vitro approaches to unravel pathophysiology and propel future therapeutic breakthroughs. Biomedical Mater Devices. 2024:1–13.
Mesraoua B, Brigo F, Lattanzi S, Abou-Khalil B, Al Hail H, Asadi-Pooya AA. Drug-resistant epilepsy: definition, pathophysiology, and management. J Neurol Sci. 2023:120766.
Kheradmand Z, Yarali B, Ahad Z, Pourpak Z, Shams S, Ashrafi MR. Comparison of serum zinc and copper levels in children and adolescents with intractable and controlled epilepsy. IJCN. 2014;8(3):49.
Falco-Walter J, editor. Editor Epilepsy—definition, classification, pathophysiology, and epidemiology. Seminars in neurology. Thieme Medical Publishers, Inc.; 2020.
Anwar H, Khan QU, Nadeem N, Pervaiz I, Ali M, Cheema FF. Epileptic seizures. Discoveries. 2020;8(2).
Minardi C, Minacapelli R, Valastro P, Vasile F, Pitino S, Pavone P et al. Epilepsy in Children: From Diagnosis to Treatment. 2018:39.
Rosati A, De Masi S, Guerrini R. Antiepileptic drug treatment in children with epilepsy. CNS Drugs. 2015;29:847–63.
Suwarba IGNM, Mahalini DS, Widyakusuma IGNAJ. The relationship between hair zinc levels and drug-resistant Epilepsy in Children at Sanglah Hospital. Open Access Maced J Med Sci. 2021;9(B):996–1000.
Potruch A, Khoury ST, Ilan Y. The role of chronobiology in drug-resistance epilepsy: the potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure. 2020;80:201–11.
Guery D, Rheims S. Clinical management of drug resistant epilepsy: a review on current strategies. NEUROPSYCH DIS TREAT J. 2021:2229–42.
Abdelbasir KA, Hassan MH, Mahmoud MH, Bakry AH. Role of minerals in Childhood Epilepsy. SVU-IJMS. 2023;6(1):337–50.
Asadi-Pooya AA, Simani L. Clinical trials of vitamin-mineral supplementations in people with epilepsy: a systematic review. Clin Nutr. 2021;40(5):3045–51.
Arulsamy A, Shaikh MF. Micronutrients and epilepsy. Role of Micronutrients in Brain Health. Springer. 2022:109 – 29.
Chakrabarty B, Dogra AS, Toteja G, Pandey R, Paul VK, Gulati S. Serum trace elements in children with well-controlled and drug refractory epilepsy compared to controls: an observational study. Neurol India. 2022;70(5):1846–51.
Ismail RS, Kishk NA, Rizk HI, El-Kholy T, Abd El-Maoula LM, Ibrahim El-Desoky O, et al. Nutritional intake and its impact on patients with epilepsy: an analytical cross-sectional study. Nutr Neurosci. 2022;25(9):1813–22.
Prasad D, Shaheen U, Satyanarayana U, Surya Prabha T, Jyothy A, Munshi A. Association of serum trace elements and minerals with genetic generalized epilepsy and idiopathic intractable epilepsy. Neurochem Res. 2014;39:2370–6.
Asadi-Pooya AA, Simani L. Epilepsy syndromes in Iran: a systematic review. Acta Neurol Scand. 2021;143(5):475–80.
Yilmaz K, Dursun I, Dusunsel R, Gunduz Z, Yel S. Niveles De magnesio en niños con enfermedad renal crónica estadios 1–3. REV NEFROL DIAL TRAS. 2023;41(1):36–41.
Yu L, Yousuf S, Yousuf S, Yeh J, Biggins SW, Morishima C, et al. Copper deficiency is an independent risk factor for mortality in patients with advanced liver disease. Hepatol Commun. 2023;7(3):76.
Mosayebi Z, Sagheb S, Mirzendedel M, Movahedian AH. Serum vitamin D Deficiency in NICU hospitalized neonates and its association with neonatal outcomes. JFRH. 2021;15(2):99.
Atay O, ASILSOY S, Sirin S, ATAKUL G, AL S, KANGALLI BOYACIOGLU O et al. The Effect of Nutrition and micronutrients on children with atopic dermatitis. Asthma Allergy Immunol. 2023;21(2).
Huang K, Malloy P, Feldman D, Pitukcheewanont P. Enteral calcium infusion used successfully as treatment for a patient with hereditary vitamin D resistant rickets (HVDRR) without alopecia: a novel mutation. Gene. 2013;512(2):554–9.
Wojciak RW, Mojs E, Stanislawska-Kubiak M, Samborski W. The serum zinc, copper, iron, and chromium concentrations in epileptic children. Epilepsy Res. 2013;104(1–2):40–4.
Eissa MA, Abdulghani KO, Nada MA, Elkhawas HM, Shouman AE, Ahmed NS. Serum zinc and copper levels in a sample of Egyptian epileptic children. Egypt J Neurol Psychiat Neurosurg. 2020;56:1–9.
Benarroch E. What are the functions of Zinc in the nervous system? Neurology. 2023;101(16):714–20.
Doboszewska U, Młyniec K, Wlaź A, Poleszak E, Nowak G, Wlaź P. Zinc signaling and epilepsy. Pharmacol Ther. 2019;193:156–77.
Wang B, Fang T, Chen H. Zinc and central nervous system disorders. Nutrients. 2023;15(9):2140.
Baraka AM, Hassab El Nabi W, El Ghotni S. Investigating the role of zinc in a rat model of epilepsy. CNS Neurosci Ther. 2012;18(4):327–33.
Elshorbagy HH, Bassiouny MM, Kamal NM, Azab AA, Ghoneim IA. Study of trace elements and role of zinc supplementation in children with idiopathic intractable epilepsy. J Pediatr Epilepsy. 2016;5(01):026–33.
Heidari B, Mirghassemi MB. Seasonal variations in serum vitamin D according to age and sex. Caspian J Intern Med. 2012;3(4):535.
de Santana KV, Oliver SL, Mendes MM, Lanham-New S, Charlton KE, Ribeiro H. Association between vitamin D status and lifestyle factors in Brazilian women: implications of Sun exposure levels, Diet, and Health. EClinicalMedicine. 2022;47.
Karaoğlu P, Yiş U, Polat Aİ, Ayanoğlu M, Hiz AS. Clinical predictors of drug-resistant epilepsy in children. Turk J Med Sci. 2021;51(3):1249–52.
Yilmaz BS, Okuyaz C, Komur M. Predictors of intractable childhood epilepsy. Pediatr Neuroly. 2013;48(1):52–5.
Gavrilovic A, Toncev G, Boskovic Matic T, Vesic K, Ilic Zivojinovic J, Gavrilovic J. Impact of epilepsy duration, seizure control and EEG abnormalities on cognitive impairment in drug-resistant epilepsy patients. Acta Neurol Belg. 2019;119:403–10.
Singh K. Treatment patterns of antiepileptic drugs and economic outcomes in patients with potential refractory epilepsy in the Texas Medicaid Program 2015.
Perucca E, Perucca P, White HS, Wirrell EC. Drug resistance in epilepsy. Lancet Neurol. 2023.
Sarangi SC, Tripathi M, Kakkar AK, Gupta YK. Effect of antiepileptic therapy on trace elements status in Indian population in a tertiary care hospital from northern India: a cross sectional study. Epilepsy Res. 2014;108(5):917–27.
Acknowledgements
This study was supported by Iran University of Medical Sciences. The authors appreciate their support. The authors also thank to Dr. Zahra Farahani from the maternal, fetal, and neonatal Research Center of Tehran University of Medical Sciences for her kind collaboration.
Funding
This study was supported by the Iran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Dr. A. T. and Dr. L. A. carried out the design, coordinated the study, and participated in most of the experiments. MS. B.P. carried out analysis of data and research. All authors have prepared the manuscript, read, and approved the content of it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the institutional review board of Iran University of Medical Sciences according to the Helsinki declaration. Written informed consent was obtained from the participants’ parents or legal guardians of the minors included in the study.
Consent for publication
Written informed consent for publication of personal or clinical details was obtained from the parents or legal guardians of any participant under the age of 18.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tavasoli, A., Afsharkhas, L. & Parvini, B. Evaluating the serum levels of zinc, copper, magnesium, and 25-hydroxy vitamin D in children with idiopathic drug-resistant epilepsy; a cross-sectional study. BMC Pediatr 24, 518 (2024). https://doi.org/10.1186/s12887-024-04968-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04968-9