Abstract
This study aimed at determining the intra- and inter-rater reliability in ultrasound body composition measurements and investigating the differences between malnourished and non-malnourished infants. Sonographic images for measurements of fat and muscle thickness were compared between 9 malnourished and 9 non-malnourished hospitalized infants. The mean of fat and muscle thickness sums were 12.44 ± 7.58 mm and 28.98 ± 7.18 mm, respectively. The intra- and inter-rater intraclass correlation coefficient were above 0.9 for both measurements, indicating high intra- and inter-rater reliability. Compared to non-malnourished infants, malnourished infants have 45% of fat thickness sum and 71% of muscle thickness sum. Ultrasound measurements of body composition in infants were different between hospitalized malnourished and non-malnourished infants. This approach has the potential to be utilized more broadly, from assessing the nutritional status of critically ill infants in intensive care units to screening for malnutrition in high-risk infant populations.
Similar content being viewed by others
Introduction
Malnutrition affects an estimated 45 million and 149 million children with wasting and stunting affected, respectively [1]. The reliability and predictive value of WHO guidelines for diagnosing malnutrition in infants have been questioned [2]. While the current identification method of malnutrition is anthropometry, body composition such as fat mass and fat-free mass is increasingly recognized as a more sensitive measure of growth and nutritional status [3]. The validated methods for body composition measurements in infants such as air displacement plethysmography and dual energy x-ray absorptiometry require heavy equipment and are expensive [4]. An alternative device is a portable ultrasound, which is non-invasive, easy to utilize at the patient’s bedside, and demonstrated to be safe with good intra- and inter-rater reliability in studies measuring body composition in adults [5]. The objective of this pilot study was to evaluate the feasibility of ultrasound in diagnosing malnutrition in infants, to determine the intra- and inter-rater reliability in ultrasound measurements, and to investigate differences in ultrasound measurements between malnourished and non-malnourished infants.
Methods
We conducted a pilot case-control study at Queen Elizabeth Central Hospital in Blantyre, Malawi from October to December 2022. We recruited hospitalized, malnourished patients under six months old and an equal number of hospitalized, non-malnourished age- (within 30 days of age) and gender-matched controls. Malnutrition was defined as weight-for-age less than − 2 z-score [1, 2]. We excluded infants with gross limb asymmetry or gross abdominal anomaly, or those who were too clinically unstable to be transported to the ultrasound room for measurements.
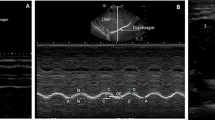
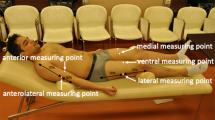
We collected anthropometric measurements in duplicate by each examiner on the right side of the body in accordance with the International Standards for Anthropometric Measurements. Muscle and subcutaneous fat thickness measurement sites [6, 7] were marked on the right side of the body by one operator (MT) and double-checked by one of the other two operators (BN, RB). Prior to starting and during the examination, calm and natural sleep were promoted while infants were in a supine position. Each of the three operators measured each of the measurement sites by ultrasound in duplicate. Measurements were taken using a minimal probe compression technique by placing the transducer on a thick layer of ultrasound gel at a 90° angle to the site of interest. Transverse images were taken in duplicate by a commercial real-time ultrasound system (SonoSite X-PORTE, FUJIFILM SonoSite, Bothell, WA, USA) with a linear array transducer operated at 6 to 13 MHz (HFL38xp, FUJIFILM SonoSite, Bothell, WA, USA). Informed consent was obtained from all subjects and/or their legal guardian. The study was approved by the University of Malawi College of Medicine (P.03/22/3600) and the Liverpool School of Tropical Medicine (22–023) Research Ethics Committees.
To evaluate intra- and inter-rater reliability, the intraclass correlation coefficient (ICC) and 95% confidence interval were calculated based on a two-way random effects model with average measures and absolute agreement (values greater than 0.90 indicates excellent reliability), following guidelines for selecting and reporting intraclass correlation coefficients for reliability research [8]. We examined for normal distribution using the Shapiro-Wilk test. IBM SPSS version 29 (Armonk, NY) was used for all analyses.
Results
Eighteen patients were included in the study (Table 1), and a total of 270 and 216 ultrasound images were used for the inter-rater reliability test of the fat and muscle thickness, respectively. None of the patients were dehydrated during the examination, and only one patient had oedematous limbs over the left side which did not affect our measurements.
The Pearson correlation coefficient for inter-rater reliability was 0.95 and 0.94 for fat and muscle measurements, respectively (Table 2). Modified Bland-Altman plots for (a) the sum of five fat measurements and (b) the sum of four muscle measurements with 95% limits of agreement were constructed to show the observer variability (Fig. 1) [9]. The fat thickness mean was 12.44 ± 7.58 mm with the standard deviation (SD) of observer differences being 1.28 mm and its 95% limits of agreements were between ± 2.51 mm. The muscle thickness mean was 28.98 ± 7.18 mm with SD of observer differences was 3.66 mm and its 95% limits of agreements were between ± 7.17 mm.
The median of absolute measurement differences (ABS) in the sum of five fat measurements was 0.78 mm, which was 6.29% of mean fat thickness sums. The median ABS in the sum of four muscle measurements was 2.53 mm, i.e. 8.74% of mean muscle thickness sums. Figure 2 shows the relative measurement difference (REL) from their mean in (a) fat and (b) muscle thickness at each measurement site (n = 3*18 = 54). The medians of ABS in fat thickness ranged from 0.17 to 0.48 mm at individual measurement site with the medians of REL as 25.54%, 10.63%, 13.99%, 18.35%, and 13.18% at biceps, quadriceps, anterior tibialis, rectus abdominis, and midline abdomen, respectively (Fig. 3). For muscle thickness, the medians of ABS ranged from 0.13 to 2.00 mm. The medians of REL in muscle thickness were 29.49%, 13.15%, 7.51%, and 7.21% at biceps, quadriceps, anterior tibialis, and rectus abdominis, respectively.
The sum of fat thickness and the sum of muscle thickness in the malnourished infants were about 45% and 71% of the values in the non-malnourished infants, respectively (Table 1). Measurements over biceps brachialis had the largest differences between the two groups (36% and 62% of the values in non-malnourished infants) among all measurement sites, followed by measurements over quadriceps.
Discussion
This proof-of-concept study conducted in freely-movable infants under six months old in a resource-limited setting demonstrates that ultrasound measurement of body composition (fat and muscle) is feasible, and has good intra- and inter-rater reliability. Previous research about fat and muscle ultrasound for infants was mainly conducted among neonates or sedated patients in intensive care units [6, 7, 10].
Compared to older age groups, the intra- and inter-rater reliability were less optimal in infants aged under six months, compared to previous studies [10,11,12]. This may be because infants are not able to obey orders and have thinner layers of fat and muscle. The reliability of measurements may be improved by adequate practice for avoiding compression errors such as ensuring the ultrasound gel is thick and can be seen as a dark band in the ultrasound window [10, 11]. Other methods for improving the reliability of ultrasound include using software for identifying fat and muscle tissues [11].
Measurements over biceps brachialis had the largest differences between malnourished and non-malnourished infants, but they also had the largest REL among all measurement sites. In comparison, quadriceps may be a more promising choice for evaluating nutritional status, for having a smaller inter-rater variability and a larger difference among the two groups.
This study was limited by the small sample size, which was due to resource constraints. There is also intra- and inter-observer variability in technique with use of the ultrasounds, but we attempted to minimize the variability by having the same three operators collect measurements for all participants. Furthermore, our control subjects did not have severe acute illness, but their condition relating to their hospitalization could have affected their measurements, which we did not evaluate beyond anthropometry. Despite these limitations, however, our proof-of-concept pilot study revealed that the decrease in fat thickness was more marked in malnourished compared to non-malnourished infants. This study demonstrates the potential for ultrasound body composition to facilitate the early identification of at-risk infants.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
UNICEF / WHO / World Bank. Levels and trends in child malnutrition child malnutrition estimates key findings of the 2021 edition. In. Geneva: WHO. 2021:1–32.
Hoehn C, Lelijveld N, Mwangome M, Berkley JA, McGrath M, Kerac M. Anthropometric criteria for identifying infants under 6 months of age at risk of morbidity and mortality: a systematic review. Clin Med Insights Pediatr. 2021;15:11795565211049904.
Johnson MJ, Beattie RM. Making body composition measurement a part of routine care in children. Clin Nutr. 2018;37(3):763–4.
Andrews ET, Beattie RM, Johnson MJ. Measuring body composition in the preterm infant: evidence base and practicalities. Clin Nutr. 2019;38(6):2521–30.
Perkisas S, Bastijns S, Baudry S, Bauer J, Beaudart C, Beckwee D, Cruz-Jentoft A, Gasowski J, Hobbelen H, Jager-Wittenaar H, et al. Application of ultrasound for muscle assessment in Sarcopenia: 2020 SARCUS update. Eur Geriatr Med. 2021;12(1):45–59.
Brei C, Much D, Heimberg E, Schulte V, Brunner S, Stecher L, Vollhardt C, Bauer JS, Amann-Gassner U, Hauner H. Sonographic assessment of abdominal fat distribution during the first year of infancy. Pediatr Res. 2015;78(3):342–50.
Bertini G, Elia S, Dani C. Using ultrasound to examine muscle mass in preterm infants at term-equivalent age. Eur J Pediatr. 2021;180(2):461–8.
Koo TK, Li MY. A Guideline of selecting and reporting Intraclass correlation coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63.
Jones M, Dobson A, O’Brian S. A graphical method for assessing agreement with the mean between multiple observers using continuous measures. Int J Epidemiol. 2011;40(5):1308–13.
Ng KWP, Dietz AR, Johnson R, Shoykhet M, Zaidman CM. Reliability of bedside ultrasound of limb and diaphragm muscle thickness in critically ill children. Muscle Nerve. 2019;59(1):88–94.
Kelso A, Muller W, Furhapter-Rieger A, Sengeis M, Ahammer H, Steinacker JM. High inter-observer reliability in standardized ultrasound measurements of subcutaneous adipose tissue in children aged three to six years. BMC Pediatr. 2020;20(1):145.
Fivez T, Hendrickx A, Van Herpe T, Vlasselaers D, Desmet L, Van den Berghe G, Mesotten D. An analysis of reliability and accuracy of muscle thickness Ultrasonography in critically Ill children and adults. JPEN J Parenter Enteral Nutr. 2016;40(7):944–9.
Acknowledgements
Thanks to Bright Tsidya for support with ultrasound training and quality control of ultrasound measurements.
Funding
The work was supported by the Malawi-Liverpool-Wellcome Programme Core Award (grant number 206545/Z/17/Z) from the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
MT developed the protocols, supervised by KC. MT, RB, and BN carried out the study, supervised by EM, KC, and PI. MT wrote the first draft of the manuscript, with critical input by PI. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian. The study was approved by the University of Malawi College of Medicine (P.03/22/3600) and the Liverpool School of Tropical Medicine (22–023) Research Ethics Committees.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tai, MF., Bvalani, R., Nkhalema, B. et al. Ultrasound assessment of malnutrition in infancy: a pilot case-control study. BMC Pediatr 24, 2 (2024). https://doi.org/10.1186/s12887-023-04479-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04479-z